Published online Sep 6, 2021. doi: 10.12998/wjcc.v9.i25.7512
Peer-review started: February 8, 2021
First decision: June 7, 2021
Revised: June 9, 2021
Accepted: August 4, 2021
Article in press: August 4, 2021
Published online: September 6, 2021
Processing time: 204 Days and 1.4 Hours
The auditory brainstem implant (ABI) is a significant treatment to restore hearing sensations for neurofibromatosis type 2 (NF2) patients. However, there is no ideal method in assisting the placement of ABIs. In this case series, intraoperative cochlear nucleus mapping was performed in awake craniotomy to help guide the placement of the electrode array.
We applied the asleep-awake-asleep technique for awake craniotomy and hearing test via the retrosigmoid approach for acoustic neuroma resections and ABIs, using mechanical ventilation with a laryngeal mask during the asleep phases, utilizing a ropivacaine-based regional anesthesia, and sevoflurane combined with propofol/remifentanil as the sedative/analgesic agents in four NF2 patients. ABI electrode arrays were placed in the awake phase with successful intraoperative hearing tests in three patients. There was one uncooperative patient whose awake hearing test needed to be aborted. In all cases, tumor resection and ABI were performed safely. Satisfactory electrode effectiveness was achieved in awake ABI placement.
This case series suggests that awake craniotomy with an intraoperative hearing test for ABI placement is safe and well tolerated. Awake craniotomy is beneficial for improving the accuracy of ABI electrode placement and meanwhile reduces non-auditory side effects.
Core Tip: The auditory brainstem implant (ABI) is a significantly beneficial treatment to restore hearing sensations for neurofibromatosis type 2 (NF2) patients. However, there is no ideal method in assisting the placement of ABI. The asleep-awake-asleep technique was applied for awake craniotomy and hearing test via the retrosigmoid approach for acoustic neuromas resections and ABI in four NF2 patients. ABI ele
- Citation: Wang DX, Wang S, Jian MY, Han RQ. Awake craniotomy for auditory brainstem implant in patients with neurofibromatosis type 2: Four case reports. World J Clin Cases 2021; 9(25): 7512-7519
- URL: https://www.wjgnet.com/2307-8960/full/v9/i25/7512.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i25.7512
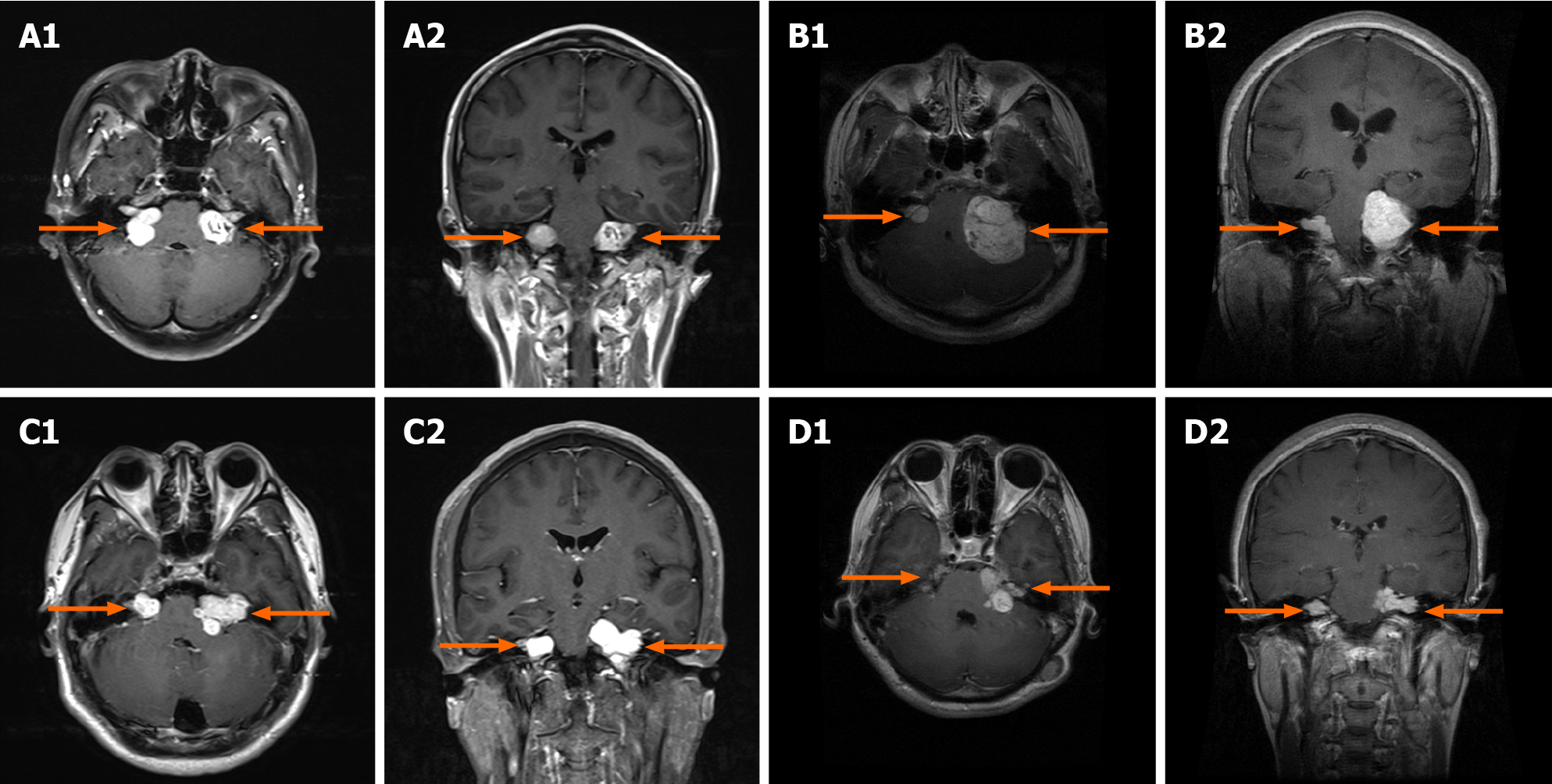
Neurofibromatosis type 2 (NF2) is an autosomal dominant disorder characterized by the development of bilateral vestibular nerve schwannomas. Progressive growth or neurosurgical removal of bilateral tumors often damages the cochlear nerve, causing hearing impairment. The auditory brainstem implant (ABI) guided by evoked audi
Case 1: A 43-year-old female presented with a chief complaint of tinnitus in left ear for 3 years (Table 1).
| Case | Age (yr) | Sex | BMI (kg/m2) | Preoperative hearing status | Comorbidities | Surgical side |
| 1 | 43 | F | 20 | Severe hearing loss in left and mild in right | None | Left |
| 2 | 31 | M | 26 | Complete hearing loss in left ear and profound hearing loss in right | Left-sided lesions at the C3-C4 and C6-C7 intervertebral foraminal areas, 14 years after resection of cervical spinal schwannomas | Left |
| 3 | 38 | M | 25 | Bilateral total deafness | Total deafness | Left |
| 4 | 24 | F | 17 | Complete hearing loss in right ear and severe hearing loss in right ear | Anxiety | Right |
Case 2: A 31-year-old male presented with a chief complaint of visual obscuration for 1 mo.
Case 3: A 38-year-old male presented with a chief complaint of tinnitus in left ear for 12 years, hearing loss in left ear for 4 years, and hearing loss in right ear for 1 year.
Case 4: A 24-year-old female presented with a chief complaint of ptosis in right eye and hearing loss in both ears for 6 years.
Case 1: Patient’s symptom started 3 years ago and had not been a good attention. Her mother was diagnosed with NF2 3 mo ago and the patient performed Magnetic reso
Case 2: Fundus examination was performed by an outside hospital and showed pa
Case 3: 12 years ago, the patient went to another hospital due to tinnitus in left ear. He was diagnosed with neurogenic tinnitus and was administered neurotrophic treatment without improvement. He developed progressive hearing loss in left ear 4 years ago and hearing loss in right ear 1 year ago. He was diagnosed with NF2 based on an MRI scan.
Case 4: Patient’s symptoms started 6 years ago. MRI was performed by an outside hospital and showed bilateral vestibular nerve schwannomas. The patient was diag
Cases 1, 3, and 4: The patient had a free previous medical history.
Case 2: The patient underwent resection of cervical spinal schwannomas 14 years ago.
Case 1: The patient admitted a family history of NF2 associated with mother.
Case 2: The patient’s maternal grandfather, mother and two aunts on my mother’s side were diagnosed NF2 successively.
Cases 3 and 4: The patient denied a family history of NF2.
Case 1: The patient had severe hearing loss in left ear and mild in right ear (Table 1).
Case 2: The patient had complete hearing loss in left ear and profound hearing loss in right ear.
Case 3: The patient had complete hearing loss in both ears
Case 4: The patient had total hearing loss in right ear and severe hearing loss in right ear and suffered from anxiety.
Routine laboratory tests revealed no remarkable abnormality in the four patients.
MRI showed bilateral vestibular nerve schwannomas in four patients (Figure 1).
Cases 1-4: All patients were diagnosed with NF2.
The procedures for the surgery and hearing tests in this case series have been pub
After the patient entered the operating room, electrocardiography, noninvasive blood pressure, body temperature, oxygen saturation and bispectral index (BIS) were monitored. General anesthesia was induced with propofol using a targeted controlled infusion technique. Subsequently, a laryngeal mask airway (LMA) was inserted fo
Once adequate hemostasis was achieved and the electrode array was placed, all the anesthetic agents were discontinued. LMA was removed when the patient’s respi
| Case | Duration of the first phase (min) | The interval of time between discontinuation and eye opening (min) | The interval of time between discontinuation and full cooperation (min) | The plasma propofol awakening concentration (μg/mL) | Duration of hearing test (min) |
| 1 | 336 | 41 | 47 | 0.6 | 56 |
| 2 | 354 | 60 | 65 | 0.6 | 77 |
| 3 | 367 | 65 | 70 | 0.5 | 62 |
Awake craniotomy with well-placed electrodes and a successful intraoperative hearing test was achieved in 3 patients (Cases 1, 2, and 3). It took 41-65 min (mean, 55 min) for the patients to regain consciousness and 47-70 min (mean, 61 min) for them to fully cooperate after anesthetics were discontinued (Table 2). Case 4 was considered uncooperative and in a state of agitation when the plasma concentration of propofol decreased to 0.5 μg/mL and the BIS increased to 85. The patient’s status was not improved after sufentanil 3 μg was administered. Thus, the awake procedure was aborted to ensure the safety of this patient. The ABI electrode was placed with the guidance of EABRs under general anesthesia.
At the end of the hearing test, general anesthesia was induced. The LMA was inserted followed by mechanical ventilation. Anesthesia was maintained with propofol 2.5-3 μg/mL and remifentanil 0.05-0.1 μg/kg/min. Four patients recovered smoothly after the surgery and were transferred to the intensive care unit.
The median duration of surgery in the first asleep stage was 347 min (330-367 min) (Table 2). There were no cardiovascular or respiratory adverse events in any of the 4 patients. No instances of intraoperative seizures, brain swelling or bleeding were observed during the awake phase. Case 3 reported nausea and received ondansetron 4mg and methylprednisolone 40mg without improvement (Table 3). The hearing test showed that the placement of the ABI electrode array over the cochlear nucleus was suboptimal. The position of the electrode was therefore adjusted, and the patient reported no further discomfort. Case 2 reported neck pain, which was considered to be caused by the tumor and surgical history of the cervical spinal cord (Table 3). The pain was relieved with flurbiprofen 50 mg.
| Case | Adverse events during wake-up hearing test | Treatment measures |
| 1 | No event | No event |
| 2 | Neck pain | Flurbiprofen 50 mg |
| 3 | Nausea and vomiting | Ondansetron 4 mg, methylprednisolone 40 mg and adjusting the position of electrode array |
| 4 | Agitation | Aborting the awake surgery |
Case 1: ABI implanted was not switched on because she had functional hearing in her right ear. Cases 2 and 3: They used ABI daily and showed an obvious impro
We successfully performed an asleep-awake-asleep technique for ABI surgery following acoustic neuroma resections via the retrosigmoid approach in three NF2 patients. Awake craniotomy is usually performed to maximize resection of tumors near the eloquent area[3,4]. It can reduce anesthetic interference with brain mapping[5]. Awake craniotomy for posterior fossa surgery reminds a number of significant challenges for the anesthesiologist. Posterior fossa procedure is more commonly performed in the three-quarter prone or prone with jaw adduction and head rotation position, which provide superior surgical exposure. However, it is difficult to get access to the airway and to communicate with patient in this position. Furthermore, sudden alterations of the cardiovascular and respiratory systems may occur during or after brainstem manipulation with surgery in the posterior fossa surgery. Moreover, patients must keep quiet completely during brainstem manipulation.
Shinoura et al[6] published a report in relation to awake craniotomy during vesti
Local anesthesia is the cornerstone of the awake craniotomy technique. The incision for acoustic neuroma resection and ABI surgery was entirely different from an incision for supratentorial tumor resection. On the operative side, the auriculotemporal nerve and cervical plexus were blocked with 0.5% ropivacaine. The anesthesia protocol for the pin sites, the incision, and the dura was similar to the protocol used in awake craniotomy for supratentorial mass lesions[7]. There was no increase in heart rate or blood pressure in any of the four patients during painful phases, and none of the patients experienced headache during the awake hearing test. No signs of cardiovas
A combination of propofol, remifentanil, and sevoflurane was used for general anesthesia in the first asleep phase. The facial, glossopharyngeal and trigeminal nerves were monitored throughout the procedure to assist in the placement of the electrode array and avoid nerve injuries during acoustic neuroma resection. Sevoflurane was limited to 0.5 MAC during tumor resection in all 4 patients[8]. Cranial nerve EMG activity was recorded effectively in all patients.
In this case series, the interval between the discontinuation of the IV infusion and the moment of consciousness recovery was much longer than previously reported in awake craniotomy for supratentorial masses [55 min (41-65 min) vs 14 ± 6 min][9]. This was probably related to the fact that the duration of the first asleep phase was far longer [347 min (330-367 min) vs 98 ± 25 min][9]. The plasma propofol concentration upon awakening would be lower after long-term infusion than after short-term in
Despite receiving prophylactic anti-emetics, case 3 suffered from nausea and vomiting. Under this circumstance, it is worth mentioning that the hearing test showed that the placement of the ABI electrode array over the cochlear nucleus was suboptimal. The patient did not report any discomfort when the position of the elec
Case 4 was uncooperative and agitated when she woke up from general anesthesia. This patient suffered from anxiety before surgery, although great efforts had been made to establish mutual trust. Anxiety might be the main reason for agitation in the awake phase[11]. Since awake craniotomy patients are liable to become anxious and stimulated, their attention and vigilance are critical to the success of the surgery. Therefore, to guarantee safety, the surgeon and anesthesiologist should seriously consider the risk of failure before planning an awake craniotomy for a patient with a severe anxiety disorder[12].
This case series demonstrated that the awake hearing test in ABI might increase the availability of electrodes and decrease patients’ NASEs[2]. Two patients (cases 2 and 3) who used ABI daily showed an obvious improvement in speech recognition scores in lip reading combined with ABIs after 1 year, which was comparable to previously literature[13]. The other two patients (cases 1 and 4) still with functional hearing in the contralateral ear, ABI implanted was not switched on until hearing was lost in the contralateral ear. It has been reported in a previous study that for most patients, hearing will continuously improve after implantation[14]. More studies with long-term follow-up are expected to further investigate the potential benefits of awake craniotomy.
Our experiences suggested that awake craniotomy during ABI placement for NF2 patients was safe and mostly tolerated, and no obvious extra surgical risk was found due to awake craniotomy. This technique can potentially improve the accuracy of electrode positioning in the cochlear nucleus and reduce NASEs during surgery.
Manuscript source: Unsolicited manuscript
Specialty type: Anesthesiology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chan SM, Naganuma H S-Editor: Wu YXJ L-Editor: A P-Editor: Yuan YY
| 1. | Nevison B, Laszig R, Sollmann WP, Lenarz T, Sterkers O, Ramsden R, Fraysse B, Manrique M, Rask-Andersen H, Garcia-Ibanez E, Colletti V, von Wallenberg E. Results from a European clinical investigation of the Nucleus multichannel auditory brainstem implant. Ear Hear. 2002;23:170-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 104] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Zhou Q, Yang Z, Wang Z, Wang B, Wang X, Zhao C, Zhang S, Wu T, Li P, Li S, Zhao F, Liu P. Awake craniotomy for assisting placement of auditory brainstem implant in NF2 patients. Acta Otolaryngol. 2018;138:548-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Kuribara T, Akiyama Y, Mikami T, Kimura Y, Komatsu K, Enatsu R, Tokinaga Y, Mikuni N. Preoperative Prediction of Communication Difficulties during Awake Craniotomy in Glioma Patients: A Retrospective Evaluation of 136 Cases at a Single Institution. Neurol Med Chir (Tokyo). 2021;61:21-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Wang AT, Pillai P, Guran E, Carter H, Minasian T, Lenart J, Vandse R. Anesthetic Management of Awake Craniotomy for Resection of the Language and Motor Cortex Vascular Malformations. World Neurosurg. 2020;143:e136-e148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Zelitzki R, Korn A, Arial E, Ben-Harosh C, Ram Z, Grossman R. Comparison of Motor Outcome in Patients Undergoing Awake vs General Anesthesia Surgery for Brain Tumors Located Within or Adjacent to the Motor Pathways. Neurosurgery. 2019;85:E470-E476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 6. | Shinoura N, Midorikawa A, Hiromitsu K, Saito S, Yamada R. Preservation of hearing following awake surgery via the retrosigmoid approach for vestibular schwannomas in eight consecutive patients. Acta Neurochir (Wien). 2017;159:1579-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Lobo FA, Wagemakers M, Absalom AR. Anaesthesia for awake craniotomy. Br J Anaesth. 2016;116:740-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Nunes RR, Bersot CDA, Garritano JG. Intraoperative neurophysiological monitoring in neuroanesthesia. Curr Opin Anaesthesiol. 2018;31:532-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Deras P, Moulinié G, Maldonado IL, Moritz-Gasser S, Duffau H, Bertram L. Intermittent general anesthesia with controlled ventilation for asleep-awake-asleep brain surgery: a prospective series of 140 gliomas in eloquent areas. Neurosurgery. 2012;71:764-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 10. | Kazama T, Ikeda K, Morita K, Sanjo Y. Awakening propofol concentration with and without blood-effect site equilibration after short-term and long-term administration of propofol and fentanyl anesthesia. Anesthesiology. 1998;88:928-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Kain ZN, Caldwell-Andrews AA, Maranets I, McClain B, Gaal D, Mayes LC, Feng R, Zhang H. Preoperative anxiety and emergence delirium and postoperative maladaptive behaviors. Anesth Analg. 2004;99:1648-1654, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 418] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 12. | Potters JW, Klimek M. Awake craniotomy: improving the patient's experience. Curr Opin Anaesthesiol. 2015;28:511-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Lloyd SKW, King AT, Rutherford SA, Hammerbeck-Ward CL, Freeman SRM, Mawman DJ, O'Driscoll M, Evans DG. Hearing optimisation in neurofibromatosis type 2: A systematic review. Clin Otolaryngol. 2017;42:1329-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 14. | Otto SR, Brackmann DE, Hitselberger WE, Shannon RV, Kuchta J. Multichannel auditory brainstem implant: update on performance in 61 patients. J Neurosurg. 2002;96:1063-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 151] [Article Influence: 6.6] [Reference Citation Analysis (0)] |