Published online Sep 6, 2021. doi: 10.12998/wjcc.v9.i25.7498
Peer-review started: February 2, 2021
First decision: May 11, 2021
Revised: May 24, 2021
Accepted: July 19, 2021
Article in press: July 19, 2021
Published online: September 6, 2021
Processing time: 210 Days and 1.8 Hours
In recent years, targeted therapy and immunotherapy have become important treatment strategies for patients with non-small cell lung cancer (NSCLC). How
We describe a 62-year-old male patient with a right lung adenocarcinoma who harbored an EGFR exon 19 deletion mutation. He received gefitinib combined with six cycles of vinorelbine, cisplatin, and recombinant human endostatin as the first-line therapy. Then gefitinib was administered in combination with recom
This is a rare case of lung adenocarcinoma in a patient with a BRCA2 germline mutation who had long-term benefit from olaparib combination treatment, suggesting that NGS-based genetic testing may render the possibility of long-term survival in NSCLC patients after disease progression.
Core Tip: The clinical evidence for successful off-label use of targeted drugs for lung adenocarcinoma patients following progression on multiple lines of treatment is still lacking now. Herein, we describe the identification of a germline BRCA2 mutation in a lung adenocarcinoma patient. The patient had multiple refractory brain metastases and received olaparib combined with gefitinib and recombinant human endostatin follo
- Citation: Zhang L, Wang J, Cui LZ, Wang K, Yuan MM, Chen RR, Zhang LJ. Successful treatment of refractory lung adenocarcinoma harboring a germline BRCA2 mutation with olaparib: A case report. World J Clin Cases 2021; 9(25): 7498-7503
- URL: https://www.wjgnet.com/2307-8960/full/v9/i25/7498.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i25.7498
Lung cancer is one of the most common malignancies with high morbidity and mortality rates worldwide. In recent years, immunotherapy and targeted therapy have made great progress in non-small cell lung cancer (NSCLC), which is the most common type of lung cancer. However, post-progression effective therapy for NSCLC is still lacking. One highly potential strategy is to identify alternative therapeutic options. Next-generation sequencing (NGS)-based genetic testing, which provides abundant genetic information on cancer including both germline and somatic gene mutations, has resulted in more individualized therapeutic strategies for NSCLC.
BRCA2 is a tumor suppressor gene that encodes a protein involved in the DNA homologous recombination repair (HRR) pathway to maintain genome stability. A BRCA2 germline mutation increases the risks of a variety of malignancies, including a 50%-60% increased risk of breast cancer and a 30% increased risk of ovarian cancer[1]. It is also associated with an increased risk of breast cancer and prostate cancer in males[2,3]. In addition, studies have shown that the HRR genes including BRCA2 may be involved in the tumorigenesis of lung cancer[4]. Multiple clinical studies have con
In September 2019, a 62-year-old Chinese man presented to hospital for treatment because of multiple progression of brain metastases from lung adenocarcinoma with
In March 2017, the patient presented with cough and blood-tinged sputum. He un
No past illnesses were documented.
The patient had no known comorbidities or family history and had a 30-year smoking history.
No abnormal indicators were observed on physical examination. His Eastern Coope
Examinations of serum tumor markers showed that carcinoembryonic antigen, neu
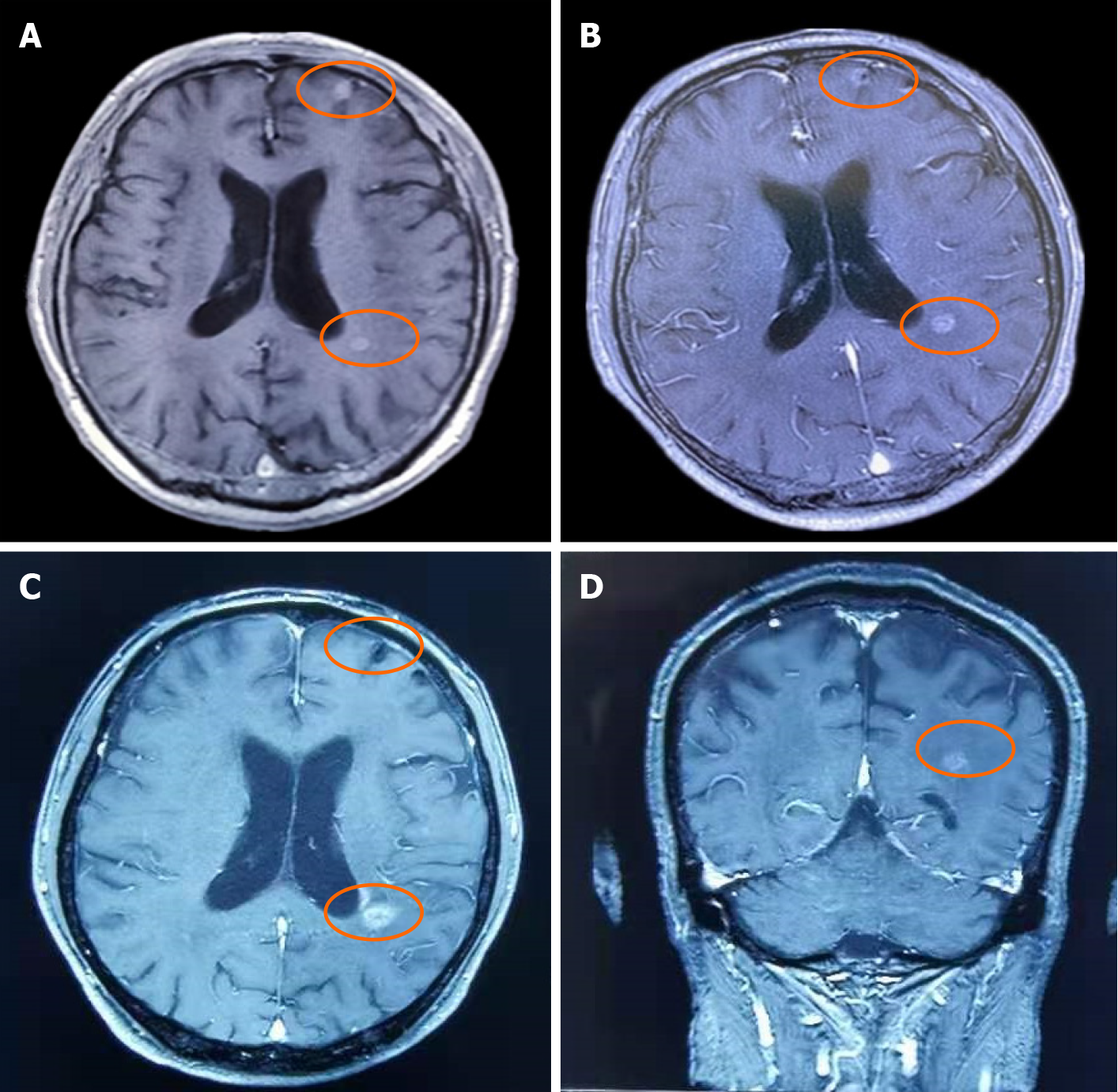
On September 6, 2019, the patient underwent MRI. The long T1 and long T2 signals from nodules of various sizes were found in the supratentorial and infratentorial brain parenchyma, and the largest lesion was located in the left frontal lobe with a diameter of approximately 1.0 cm (Figure 1A). Small patchy and slightly longer T1 and slightly longer T2 signals were observed in bilateral paraventricles and bilateral basal ganglia. The sulcus was not widened and the ventricular system was not dilated.
To determine potential therapeutic methods, the patient underwent genetic testing of 1021 cancer-related genes using peripheral blood (Geneplus-Beijing, Beijing, China) for the second time, and a somatic ASXL1 mutation and the previous germline BRCA2 mutation were identified (Table 1).
| Somatic mutation | ||||
| Gene | Transcript | c.HGVS | p.HGVS | Allele frequency |
| ASXL1 | NM_015338.5 | c.2247C[4>3] | p.V751Lfs*21 | 0.6% |
| Germline mutation | ||||
| Gene | Transcript | c.HGVS | p.HGVS | Homozygous/heterozygous |
| BRCA2 | NM_000059.3 | c.6816_6820delAAGAG | p.G2274Afs*17 | Heterozygous |
A germline BRCA2-mutated right lung adenocarcinoma with focal squamous cell differentiation and multiple brain metastases.
Since September 24, 2019, the patient has been receiving oral olaparib at a dosage of 300 mg twice daily, on the basis of maintenance therapy with gefitinib plus recom
In the course of treatment, the brain metastases were under control and maintained the same size as 2 mo previously. At the end of March 2020, MRI showed a slight re
Olaparib, a PARP inhibitor, has been proven to be effective in patients with BRCA-mutant breast, ovarian, prostate, and pancreatic cancers[5-7,11]. The STUDY19 trial showed that patients with BRCA-mutated ovarian cancer gained great benefit from olaparib[5]. Among patients with HER2-negative metastatic breast cancer and a germ
In this study, the BRCA2-mutated lung adenocarcinoma patient benefited from olaparib combined with gefitinib and recombinant human endostatin with a relatively long survival following progression on multiple lines of treatment. One study found[12] that niraparib (a PARP inhibitor) combined with bevacizumab (an anti-angiogenic drug) significantly improved PFS compared with niraparib alone [median PFS 11.9 mo vs 5.5 mo; adjusted hazard ratio 0.35 (95% confidence interval: 0.21-0.57), P < 0.0001] for platinum-sensitive recurrent ovarian cancer, suggesting that PARP inhibitors combined with anti-angiogenic drugs may increase sensitivity to PARP inhibitors. In this study, the combination of olaparib and an anti-angiogenic drug also led to a good outcome. We observed that the patient had a cavity in the middle of the brain meta
We present a lung adenocarcinoma patient with a BRCA2 mutation who had long-lasting benefit following treatment with olaparib plus gefitinib and recombinant human endostatin. This case provides unequivocal clinical evidence for the off-label use of the PARP inhibitor olaparib in lung adenocarcinoma patients with BRCA mutations after disease progression.
We would like to thank the patient and his family.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gebbia V S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Yuan YY
| 1. | Roy R, Chun J, Powell SN. BRCA1 and BRCA2: different roles in a common pathway of genome protection. Nat Rev Cancer. 2011;12:68-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 898] [Cited by in RCA: 1058] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 2. | Li D, Kumaraswamy E, Harlan-Williams LM, Jensen RA. The role of BRCA1 and BRCA2 in prostate cancer. Front Biosci (Landmark Ed). 2013;18:1445-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Rizzolo P, Silvestri V, Tommasi S, Pinto R, Danza K, Falchetti M, Gulino M, Frati P, Ottini L. Male breast cancer: genetics, epigenetics, and ethical aspects. Ann Oncol. 2013;24 Suppl 8:viii75-viii82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 4. | Lee MN, Tseng RC, Hsu HS, Chen JY, Tzao C, Ho WL, Wang YC. Epigenetic inactivation of the chromosomal stability control genes BRCA1, BRCA2, and XRCC5 in non-small cell lung cancer. Clin Cancer Res. 2007;13:832-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | Hodgson DR, Dougherty BA, Lai Z, Fielding A, Grinsted L, Spencer S, O'Connor MJ, Ho TW, Robertson JD, Lanchbury JS, Timms KM, Gutin A, Orr M, Jones H, Gilks B, Womack C, Gourley C, Ledermann J, Barrett JC. Candidate biomarkers of PARP inhibitor sensitivity in ovarian cancer beyond the BRCA genes. Br J Cancer. 2018;119:1401-1409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 192] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 6. | Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, Delaloge S, Li W, Tung N, Armstrong A, Wu W, Goessl C, Runswick S, Conte P. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N Engl J Med. 2017;377:523-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 2272] [Article Influence: 284.0] [Reference Citation Analysis (0)] |
| 7. | Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R, Nava Rodrigues D, Robinson D, Omlin A, Tunariu N, Boysen G, Porta N, Flohr P, Gillman A, Figueiredo I, Paulding C, Seed G, Jain S, Ralph C, Protheroe A, Hussain S, Jones R, Elliott T, McGovern U, Bianchini D, Goodall J, Zafeiriou Z, Williamson CT, Ferraldeschi R, Riisnaes R, Ebbs B, Fowler G, Roda D, Yuan W, Wu YM, Cao X, Brough R, Pemberton H, A'Hern R, Swain A, Kunju LP, Eeles R, Attard G, Lord CJ, Ashworth A, Rubin MA, Knudsen KE, Feng FY, Chinnaiyan AM, Hall E, de Bono JS. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. N Engl J Med. 2015;373:1697-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1642] [Cited by in RCA: 1750] [Article Influence: 175.0] [Reference Citation Analysis (0)] |
| 8. | Cocco E, Scaltriti M, Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol. 2018;15:731-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 644] [Cited by in RCA: 1005] [Article Influence: 167.5] [Reference Citation Analysis (0)] |
| 9. | Jiang Y, Dai H, Li Y, Yin J, Guo S, Lin SY, McGrail DJ. PARP inhibitors synergize with gemcitabine by potentiating DNA damage in non-small-cell lung cancer. Int J Cancer. 2019;144:1092-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 10. | Kim M, Mun H, Sung CO, Cho EJ, Jeon HJ, Chun SM, Jung DJ, Shin TH, Jeong GS, Kim DK, Choi EK, Jeong SY, Taylor AM, Jain S, Meyerson M, Jang SJ. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat Commun. 2019;10:3991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 431] [Cited by in RCA: 507] [Article Influence: 84.5] [Reference Citation Analysis (0)] |
| 11. | Tacconi EM, Lai X, Folio C, Porru M, Zonderland G, Badie S, Michl J, Sechi I, Rogier M, Matía García V, Batra AS, Rueda OM, Bouwman P, Jonkers J, Ryan A, Reina-San-Martin B, Hui J, Tang N, Bruna A, Biroccio A, Tarsounas M. BRCA1 and BRCA2 tumor suppressors protect against endogenous acetaldehyde toxicity. EMBO Mol Med. 2017;9:1398-1414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 12. | Mirza MR, Åvall Lundqvist E, Birrer MJ, dePont Christensen R, Nyvang GB, Malander S, Anttila M, Werner TL, Lund B, Lindahl G, Hietanen S, Peen U, Dimoula M, Roed H, Ør Knudsen A, Staff S, Krog Vistisen A, Bjørge L, Mäenpää JU; AVANOVA investigators. Niraparib plus bevacizumab vs niraparib alone for platinum-sensitive recurrent ovarian cancer (NSGO-AVANOVA2/ENGOT-ov24): a randomised, phase 2, superiority trial. Lancet Oncol. 2019;20:1409-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 186] [Article Influence: 31.0] [Reference Citation Analysis (0)] |