Published online Sep 6, 2021. doi: 10.12998/wjcc.v9.i25.7381
Peer-review started: June 15, 2021
First decision: June 24, 2021
Revised: July 1, 2021
Accepted: July 14, 2021
Article in press: July 14, 2021
Published online: September 6, 2021
Processing time: 76 Days and 18.7 Hours
At present, there is controversy on the role of microvessel density (MVD) in tumors as a prognostic indicator of bladder transitional cell carcinoma (BTCC). However, the MVD in tumors is simply classified based on the expression of several different vascular markers, which has not been related to analytical research on the prognosis of patients with BTCC.
To explore the classification of blood vessels in tumors and studied the relationship between MVD and the prognosis of patients with BTCC.
The tissue mass was detected by tissue microarray and immunohistochemical analysis with monoclonal antibodies against CD31, CD34, CD105, and vascular smooth muscle actin to investigate the MVD in BTCC. The measurement data are expressed as the mean ± SD. The difference between the groups was analyzed by the t-test, the counting data were analyzed by χ2 test. The Kaplan-Meier survival curve was estimated by the product-limit method. The log-rank time-series test was employed to compare the tumor-free survival curves.
The MVD was closely related to the pathological grade, invasive depth, and prognosis of BTCC. Significant differences were found between grade I and grade II, grade II and grade III, superficial and invasive type, and the tumor-free survival group and the recurrence or metastasis group (P < 0.01). Multivariate analysis showed that undifferentiated MVD was an independent prognostic factor for patient survival time. An inverse correlation between undifferentiated tumor MVD and differentiated tumor MVD in BTCC was also shown.
The classification of blood vessels in BTCC could act as an important prognostic indicator and may also be of great significance in the treatment of cancer.
Core Tip: At present, there is controversy on the role of microvessel density (MVD) in tumors as a prognostic indicator of bladder transitional cell carcinoma. In our experimental study, we investigated the MVD in bladder transitional cell carcinoma through tissue microarray and immunohistochemical analyses. By observing the morphological characteristics of blood vessels and the expression of specific markers, we explored the classification of blood vessels in tumors and studied the relationship between MVD and the prognosis of patients.
- Citation: Wang HB, Qin Y, Yang JY. Research on the prognosis of different types of microvessels in bladder transitional cell carcinoma. World J Clin Cases 2021; 9(25): 7381-7390
- URL: https://www.wjgnet.com/2307-8960/full/v9/i25/7381.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i25.7381
Deriving new blood vessels from existing microvessels is an essential process for tumor growth and hematogenous metastasis[1]. Under normal circumstances, the growth of blood vessels in the human body is in a balanced state due to strict regulation and control, and only under certain conditions, such as wound healing, will the physiological proliferation of blood vessels occur. However, in the tumor-bearing state, angiogenesis will be out of control, showing uncontrollable growth. Once the new blood vessels grow into the tumor and form tumor microcirculation, the tumor will grow exponentially and rapidly, reaching hundreds or even thousands of times its original volume in a short time. The quantification of tumor microvessels can provide an indicator of vascular activity, and microvessel density (MVD) is a frequently used indicator to quantify changing tumor microvessels. Recent reports indicated that an increase in MVD was associated with the poor prognosis of patients with several malignant tumors, including breast cancer, prostate cancer, lung cancer, and nasopharyngeal carcinoma[2-8]. Bladder transitional cell carcinoma (BTCC) is the most common bladder malignant tumor, accounting for about 90% of bladder cell carcinomas, but the underlying metastasis mechanism of BTCC is still unclear.
At present, the value of MVD in predicting the prognosis of BTCC is controversial. Several reports showed that MVD was positively correlated with longer survival time and better prognosis of patients[9-13]. However, at the same time, some researchers reported that MVD was inversely related to the survival rate and prognosis of patients[14-16], whereas others did not find a significant correlation between MVD and the survival rate of patients[17,18]. In the past, vascular markers were generally applied to identify the heterogeneity of blood vessels and reveal different aspects and characteristics of tumor vessels. For example, CD34 is expressed in differentiated endothelial cells, whereas CD31 is expressed in both differentiated and undifferentiated endothelial cells[19]. Recently, it was reported that CD105 was superior to CD34 and CD31 in evaluating tumor angiogenesis since it had a greater affinity for activated endothelial cells[6,20]. In addition, in terms of breast cancer and colorectal cancer showing a lower survival rate of patients, some immunological studies have shown that an increase in MVD determined by CD105 staining was an independent prognostic indicator[21-24].
To date, no studies on vascular differentiation related to the prognosis of cancer, especially the prognosis of BTCC, have been conducted. In this experimental study, we investigated the MVD in BTCC through tissue microarray and immunohistochemical analyses. By observing the morphological characteristics of blood vessels and the expression of specific markers, we explored the classification of blood vessels in tumors and studied the relationship between MVD and the prognosis of patients.
Sixty-two patients who underwent bladder cancer surgery (no history of chemotherapy, radiotherapy, or immunotherapy before surgery) and were diagnosed with BTCC by pathology, were assigned to the experimental group, including 47 males and 15 females. The age of the patients ranged from 63.2-86.8 years, with an average of 72.5 years. Eighteen patients without bladder disease were assigned to the control group, including 16 cases with benign prostatic hyperplasia and 2 cases with ureteral cysts, aged 56.8-78.9 years, with an average age of 71.3 years. Patients with other related diseases were excluded. According to the World Health Organization classification criteria, the experimental group was classified based on tumor pathology. Thirteen cases were classified as highly differentiated (Grade I), 16 cases as moderately differentiated (Grade II), and 33 cases as poorly differentiated (Grade III). In terms of clinical stages, based on the UICC-TNM standard, 26 cases were classified as superficial tumors (Tis-T1) and 36 cases as invasive tumors (T2-T4). During the postoperative follow-up period of 14-38 mo, with an average period of 26.5 mo, 38 cases presented with no tumor recurrence or metastasis (tumor-free survival), while 24 cases showed tumor recurrence and five cases showed tumor metastasis (including two deaths).
Three 1-mm tissue blocks were taken from each of the 62 specimens of BTCC and placed into three tissue microarray blocks after combining. The three location blocks of each donor block were randomly sampled from three different parts of the tumor tissue, which avoided necrotic tissue and ensured that they were all tumor cells. The 62 BTCC tissues and the 18 normal bladder tissues without malignant lesions of cases were classified into tissue microarray blocks and routinely sectioned after the tissue microarray blocks were extracted. The specimens in each tissue microarray block were continuously sectioned at a thickness of 4 µm. The remaining BTCC tissue blocks of the 62 cases and the 18 cases of normal bladder tissue without malignant lesions were evaluated by hematoxylin and eosin staining and immunohistochemical analysis.
After the specimen was fixed, and the tissue block was cut vertically and transversely, the bladder tumor tissue was fixed externally, embedded in paraffin, and cut into 4 µm- thick slices. The slice was attached to a glass slide treated with APES. The slice was dewaxed with xylene and dehydrated in a graded series of alcohol (from high concentration to low concentration), washed with running water for 10 min, washed with 0.01 mol/L phosphate buffered saline (PBS) for 5 min, and repaired with antigen in microwave oven for 25 min. After natural cooling, it was washed with PBS for 2 × 3 min. H2O2 was added to incubate for 10 min at room temperature, followed by washing with PBS for 2 × 3 min. Animal serum sealer was added at room temperature for 20 minutes. Then, the sealer was poured out, and the first antibody working solution prepared in diluent was added and incubated overnight (over 18 h) at 4 ℃, followed by washing with PBS for 3 × 3 min. Biotinylated secondary antibody working solution was added and incubated in a wet box at 37 ℃ for 15 min and then washed with PBS for 3 × 3 min. A streptavidin enzyme label was added and incubated in a wet box at 37 ℃ for 15 min, followed by washing with PBS for 3 × 3 min, and developed with DAB. The DAB development was controlled under a microscope, and was stopped with water based on deposition levels before being washed with running water for 10 min. The slide was re-dyed with hematoxylin, turned blue with running water, dehydrated to transparency with a series of alcohol concentrations followed by xylene, and sealed with neutral gum. Positive reactions were observed and counted under a microscope. Cells with brownish-yellow granules in the membrane or cytoplasm were considered positive, while those with no brownish yellow granules were considered negative. MVD was counted under × 200 magnification. All brown-stained cells or cell clusters were counted as one MVD value, and the MVD values in five visual fields were recorded. The multi-visual field averaging method was used to repeatedly count multiple points with a computer pathological graphic analyzer, and the average value was used as the MVD value. The mean value was calculated as the MVD value of one case.
Positive controls were established for each batch of staining (CD31 and CD34 sections of known transitional cell carcinoma of bladder were used as positive controls). MVD counting: MVD was counted under × 200 magnification, and every brown-stained cell or cell cluster was counted as one MVD value. The MVD values in five visual fields were recorded, and the mean value was used as the MVD value of this case.
According to the coloring degree of each high-power visual field and the number of microvessels in the visual field under the optical microscope, the results were judged as follows: When the whole slice was not colored or was pale yellow, it was considered negative, while positive cells were represented by yellow, brown, or tan color. The expression of microvessel number in tumor was detected by the Leica Qwin multimedia pathological image analysis system produced by Leica Company in Germany, and the microvessel value in each visual field was recorded for data analysis.
The experimental results were analyzed by SPSS 13.0 software, and the Kaplan-Meier survival curve was estimated by the product-limit method. The log-rank time-series test was employed to compare the tumor-free survival curves. The Cox proportional hazards model was used to screen the independent prognostic factors. The measurement data are expressed as the mean ± SD. The difference between the groups was analyzed by the t-test, the counting data were analyzed by χ2 test, and P < 0.05 suggested a significant difference.
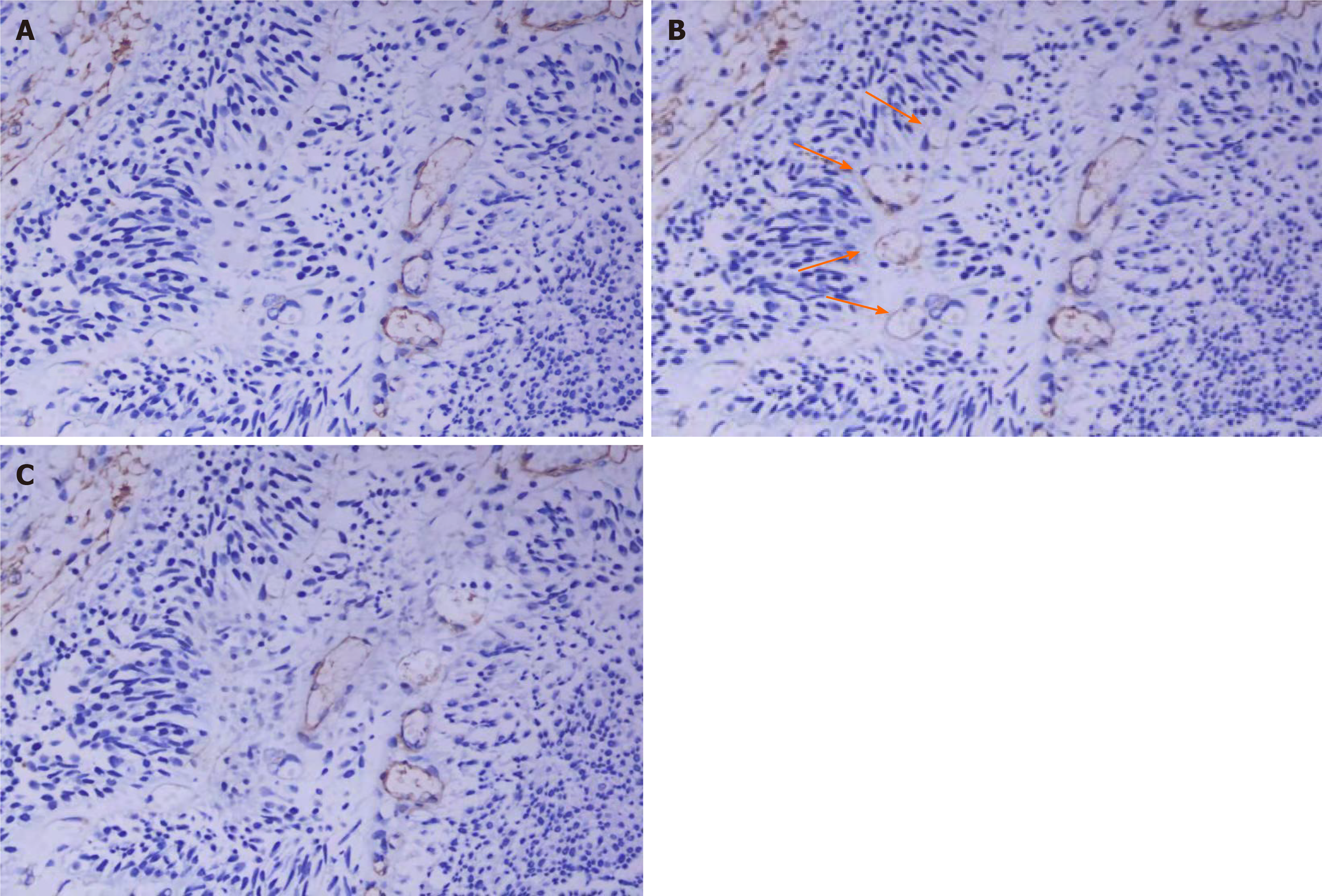
In BTCC, most of the blood vessels stained with the CD31 antibody also stained with the CD34 antibody (Figure 1A and B). However, some blood vessels were only stained with the CD31 antibody and not the CD34 antibody (Figure 1B). Peripheral cell coverage of the blood vessel was determined by staining with the smooth muscle actin (SMA) marker of peripheral cells. The results showed that there were peripheral cells around the blood vessels stained by CD34, while no peripheral cells were observed around the CD31+/CD34- microvessels (Figure 1C).
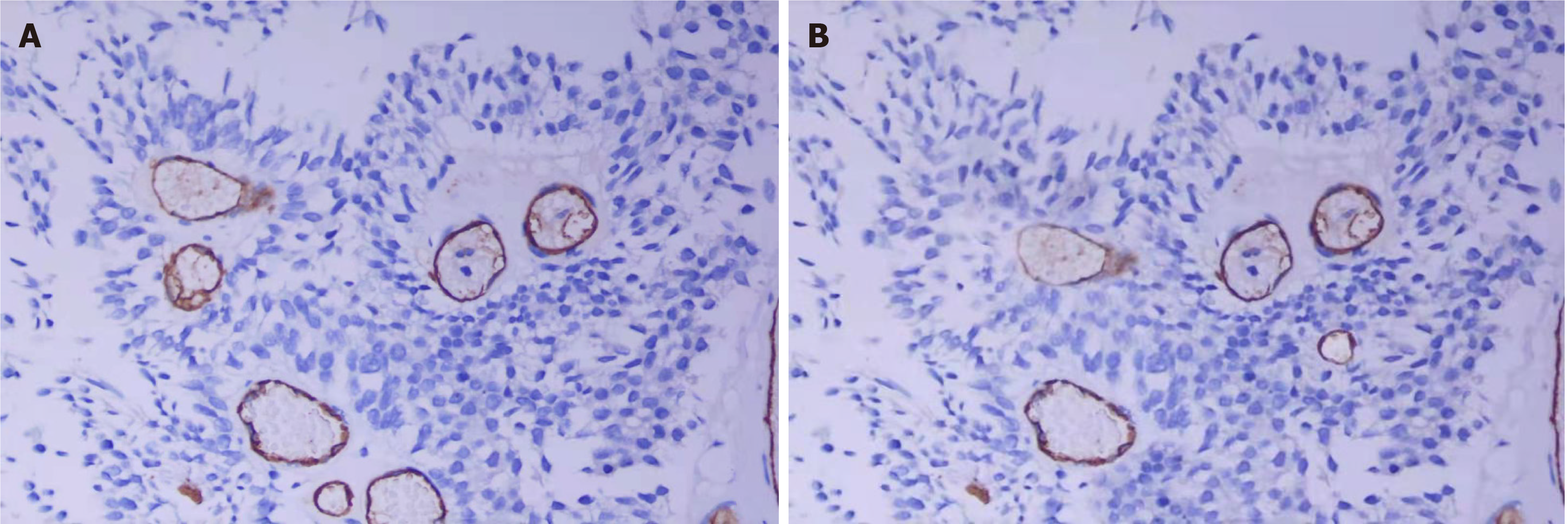
In tumor vessels, CD105 was only expressed in CD34-positive vessels. CD34 and CD105 are almost the same markers for tumor microvessels (Figure 2). The mean values of CD34-MVD and CD105-MVD in the 62 cases of BTCC were 139.7 ± 41.8 and 134.9 ± 36.2, respectively, and there was no difference between CD34-MVD and CD105-MVD (P > 0.05).
The MVD value expressed by the microvessel count is shown in the Table 1. MVD was closely related to the pathological grade, invasive depth, and prognosis of BTCC. Significant differences were found between grade I and grade II, grade II and grade III, superficial and invasive type, and the tumor-free survival group and the recurrence or metastasis group (P < 0.01). CD31 and CD34 were weakly expressed in normal bladder tissue. Undifferentiated vessel density was positively correlated with tumor grade and stage.
| Clinical parameters | Case number | MVD-CD31 | MVD-CD34 | Undifferentiated blood vessel MVD | |
| High | Low | ||||
| Control group | 18 | 18 | 0 | ||
| Pathological grading | |||||
| Grade I | 13 | 117 ± 29.3 | 90 ± 27.5 | 8 | 5 |
| Grade II | 16 | 150 ± 39.8 | 120 ± 28.7 | 6 | 10 |
| Grade III | 33 | 200 ± 43.5 | 160 ± 29.8 | 7 | 26 |
| Clinical stages | |||||
| Superficial type (TIS-T1) | 26 | 120 ± 30.4 | 100 ± 25.5 | 20 | 6 |
| Infiltration type (T2-T4) | 36 | 190 ± 37.7 | 160 ± 32.1 | 13 | 23 |
| Prognosis | |||||
| No recurrence or metastasis | 38 | 130 ±35.6 | 110 ± 34.4 | 26 | 12 |
| Recurrence or metastasis | 24 | 190 ± 45.7 | 160 ± 41.1 | 5 | 19 |
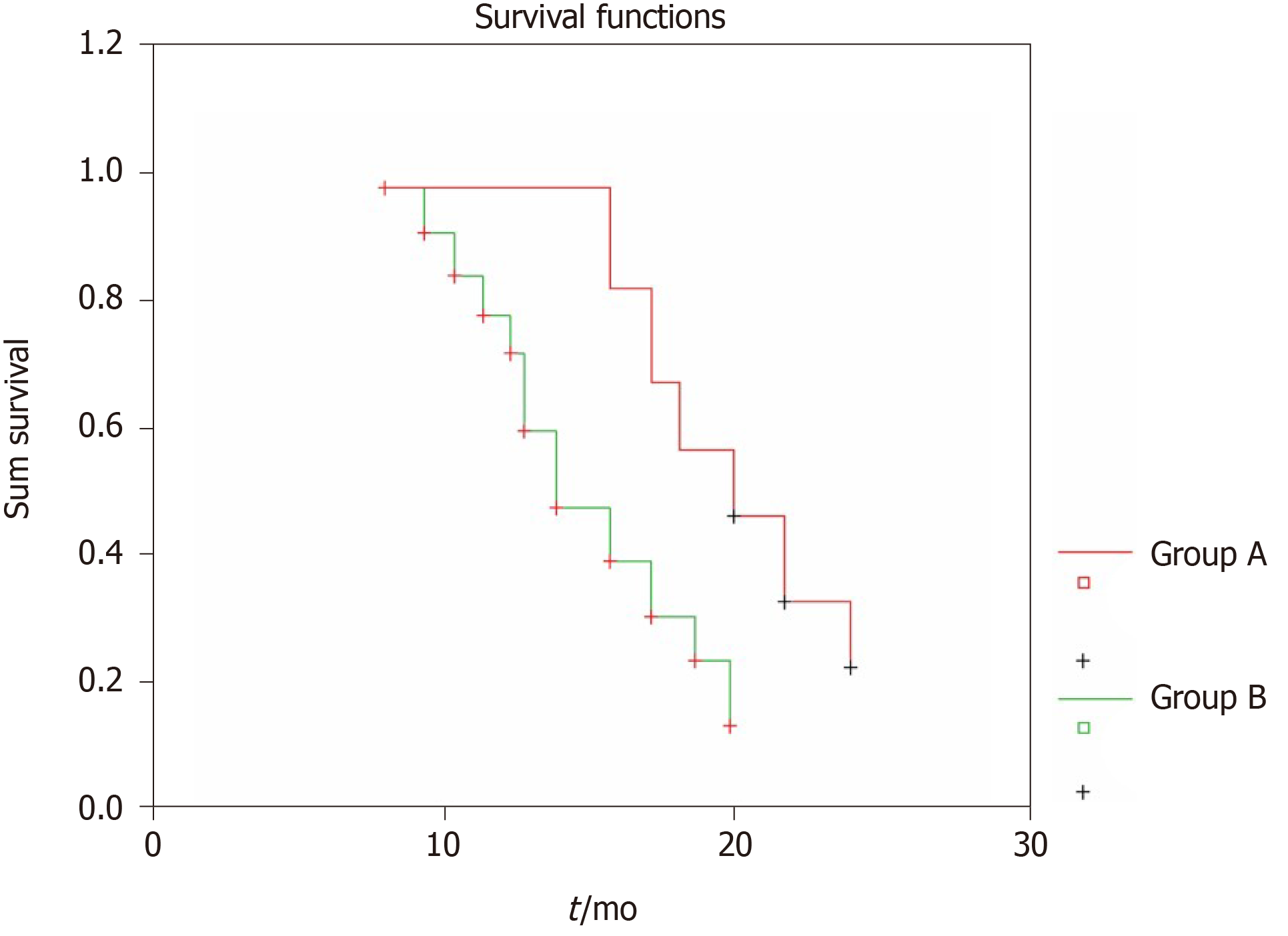
To clearly compare the prognosis of bladder cancer patients with different undifferentiated vessels, we divided all 62 patients into two groups according to the MVD of the undifferentiated vessels in their primary bladder transitional cell tumor tissues. Group A had MVD values lower than the median value and group B had MVD values higher than the median value. The recurrence of the bladder tumor was regarded as the end event, and the Kaplan-Meier tumor-free survival curve of each group was obtained, as shown in Figure 3. After the log-rank time-series test, the difference between the two groups was extremely significant (P = 0.007). The prognosis of the patients with undifferentiated vessels at low MVD values was significantly better than that of the patients with undifferentiated vessels at high MVD values.
Over 90% of malignant urinary tumors are BTCC, which has the biological characteristics of easy invasion and metastasis as well as easy recurrence after surgery[3], and both invasion and metastasis depend upon tumor angiogenesis[4]. This was the first report on the correlation between different types of blood vessels in BTCC and prognosis. The determination of MVD involves counting the microvessels in the part of the tumor with the highest density of tumor blood vessels, which helps to understand the degree of active tumor angiogenesis and the relationship between tumor grading and staging. The MVD value is related to the size of the tumor. The MVD in the experimental group was higher than that in the control group, and there was a significant difference between the two groups. At present, the reagents used to identify the microvessels of tumor tissues are mainly markers of vascular endothelial cells, such as the CD31 antibody, CD34 antibody, and VIII factor-related antibodies, or Von Willebrand factors, which are expressed on most endothelial cells and called markers of returning endothelial cells.
The development of solid tumors goes through the stages of pre-angiogenesis and angiogenesis. In the early stage of angiogenesis, the tumor does not secrete or rarely secretes vascular growth factor, and therefore, the tumor does not induce angiogenesis. As a result, the tumor is maintained at a limited volume and metastasis hardly occurs. When the tumor induces host microvascular proliferation, it enters the angiogenic stage, and the tumor cell population increases rapidly with increased tumor volume, showing potential metastasis tendency.
Two different types of microvessels were observed in BTCC. In BTCC, most of the blood vessels stained with the CD31 antibody were also stained with the CD34 antibody, but some blood vessels were stained with the CD31 antibody and not the CD34 antibody. It has been reported that CD34 was only expressed in differentiated endothelial cells, whereas CD31 was expressed in both differentiated and undifferentiated endothelial cells. We also determined the peripheral vascular cell coverage by staining with the SMA marker of peripheral cells. The results showed that there were peripheral cells around the blood vessels stained with anti-CD34, but there were no peripheral cells around the CD31+/CD34- microvessels (Figure 1C). Therefore, there are two types of microvessels in BTCC, CD34+ microvessels and CD31+/CD34- microvessels. Compared to CD34+, CD31+/CD34- blood vessels displayed some morphological characteristics under light microscopy. They showed no lumens or had small lumens, with thick walls and small shapes. Based on these observations, we confirmed that the CD34-positive vessels were differentiated vessels, whereas the CD31+/CD34- positive vessels were undifferentiated vessels.
Peripheral cells present with different connections to endothelial cells in human BTCC. In the undifferentiated blood vessels, we did not find peripheral cell coverage, whereas in the differentiated blood vessels, we carefully observed that peripheral cells and CD34+ endothelial cells usually appeared in the peripheral parts of the tumors. Thus, when we used peripheral blood vessel coverage to assess the maturity of the blood vessels, undifferentiated blood vessels were noticeably more immature than the differentiated blood vessels. Although it has been reported that SMA and desmin were effective markers of tumor peripheral cells in animal models, we found that desmin was not expressed around the tumor in human BTCC.
Stability and repeatability are very important for determining microvessels with different antibodies by immunohistochemistry. For example, the affinity of most CD31 monoclonal antibodies is lower than that of CD34 monoclonal antibodies, which leads to the smaller number of tumor vessels tested[23-25]. Hence, staining with CD31, in this case, will underestimate the MVD[2]. In our study, the quality of the immunohistochemical staining was highly controlled, so a key difference between CD31 and CD34 staining was found in BTCC. We found two different types of blood vessels by directly staining the blood vessels, namely the undifferentiated blood vessels (CD31+/CD34-) and the differentiated blood vessels (CD34+).
Although we assumed that no blood vessels were stained with CD31-/CD34+, eight blood vessels whose MVD was slightly higher than that of CD31+ also stained as CD34+. This may be an error in blood vessel staining caused by serial sections. For example, sometimes a single blood vessel may be missed in one section, but it appears as two independent blood vessels in another section.
It was reported that peripheral cell coverage could more accurately reflect whether or not microvessels are mature, although it is not the only index[24]. There are also some other molecular markers, such as SMA, desmin, platelet-derived growth factor receptor-H, and NG2, which can all be used to identify peripheral cells. We compared the expression of immune NG2, desmin, and SMA in peripheral cells of BTCC. Many more peripheral cells were labeled as SMA-positive than peripheral cells labeled desmin-positive or NG2-positive, so desmin and NG2 were not effective markers of peripheral cells. It was observed that in the CD34+ blood vessels, the peripheral cell coverage was also different, which was our conclusion when comparing tumor peripheral cells in animal models[25]. However, the relationship between peripheral cells and vascular endothelial cells remains unclear in the prognosis of patients.
In this study, the MVD of undifferentiated vessels in BTCC was quantified for the first time. The MVD of the undifferentiated vessels was positively correlated with the pathological grade of the patients, and the prognosis of the patients was worse. In addition, it was an independent prognostic factor in multivariate analysis. In contrast, the MVD of the differentiated vessels was negatively correlated with the pathological grade of the patients, and the survival rate of the patients was also longer. In each section, the MVD of the undifferentiated vessels was negatively correlated with that of the differentiated vessels.
When the MVD of the undifferentiated vessels was compared with that of the differentiated vessels, it was found that the prognosis of the patients with fewer undifferentiated vessels, but more differentiated vessels, was better than those with more undifferentiated vessels but fewer differentiated vessels.
Interestingly, when CD31 is considered a broad-spectrum vascular marker for staining all blood vessels, higher MVD indicates lower pathological grade and longer survival of patients. Our results also support that the MVD of the differentiated vessels was an important factor affecting prognosis in BTCC. The importance of undifferentiated blood vessels is generally ignored in analyzing BTCC. It was reported that the rapid growth of the tumor did not suggest high vascular density and that the MVD of the tumor may not be high. However, sometimes it was lower than that of normal tissues.
Since Kononen and colleague made the first tissue microarray in 1998[26], tissue microarray has been rapidly popularized worldwide due to its high throughput (large sample capacity), parallel research (uniform test conditions), and high efficiency (greatly reduced test time and reagents). In this experiment, microarray fabrication, slicing, and immunohistochemical staining were completed in only 1 mo. Four markers were detected in 62 samples, and the reagent consumption was much lower compared to the traditional methods. This indicates that tissue microarray technology is an efficient research method, especially suitable for multi-gene research with large sample sizes.
We believe that comparing CD31- and CD34- labeled blood vessels in BTCC by immunohistochemistry cannot show all the circumstances of the tumor vessels, but it can show that a unique CD31+/CD34- blood vessel is closely related to the prognosis of patients. Although undifferentiated blood vessels are considered of great value as the target of targeted therapy, the specific treatment method for this unique type of blood vessel is unclear[27]. Anti-angiogenesis therapy is generally considered to remove the immature or nonfunctional blood vessels to promote the retention of the remaining mature blood vessels[28]. In recent clinical trials, it has been reported in the UK that treatment methods inhibiting tumor cell proliferation and tumor angiogenesis significantly improved the survival rate of patients with urinary system tumors[29,30]. CD31+CD34- vessels may potentially be the target for further therapies in anti-vascular treatment, which undoubtedly will be a great help for the treatment of malignant bladder tumors.
Through this experiment, we quantitatively analyzed two types of MVD in BTCC and compared their relatively different prognostic relationships. The high-density expression of undifferentiated blood vessels can be regarded as an independent prognostic factor for the shorter survival time of patients. In contrast, the high-density expression of differentiated blood vessels can indicate a better prognosis. Our research demonstrates that the vascular structure in BTCC is complex, and in research on angiogenesis regarding this tumor, more detailed studies on vascular classification are needed. Our research is helpful for clinical treatment and undifferentiated blood vessels may be a potential target for vascular treatment. Therefore, in research on anti-angiogenic drugs, drug research targeting undifferentiated blood vessels or differentiated blood vessels or both blood vessels may be a future research direction.
At present, there is controversy on the role of microvessel density (MVD) in tumors as a prognostic indicator of bladder transitional cell carcinoma (BTCC).
The MVD in tumors is simply classified based on the expression of several different vascular markers, which has not been related to analytical research on the prognosis of patients with BTCC.
This study aimed to explore the classification of blood vessels in tumors and studied the relationship between MVD and the prognosis of patients with BTCC.
We investigated the MVD in BTCC through tissue microarray and immunohistochemical analyses. By observing the morphological characteristics of blood vessels and the expression of specific markers, we explored the classification of blood vessels in tumors and studied the relationship between MVD and the prognosis of patients.
Two different types of microvessels in BTCC were identified as undifferentiated (CD31+/CD34-) vessels and differentiated (CD34+) vessels. The MVD of high-grade undifferentiated vessels was positively correlated with a higher tumor grade and shorter survival time of the patients. In contrast, the MVD of high-grade differentiated vessels was positively correlated with lower-grade tumors and longer survival time of the patients. Multivariate analysis showed that undifferentiated MVD was an independent prognostic factor for patient survival time. An inverse correlation between undifferentiated tumor MVD and differentiated tumor MVD in BTCC was also shown.
This was the first report on the correlation between two microvascular types and the prognosis of patients with BTCC. The results showed that the classification of blood vessels in BTCC could act as an important prognostic indicator and may also be of great significance in the treatment of cancer.
Our research is helpful for clinical treatment, and suggests that undifferentiated blood vessels may be a potential target for vascular treatment. Therefore, in research on anti-angiogenic drugs, drug research targeting undifferentiated blood vessels or differentiated blood vessels or both blood vessels may be a future research direction.
Manuscript source: Unsolicited manuscript
Specialty type: Urology and nephrology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Baumann BC, Messing EM S-Editor: Wang JL L-Editor: Filipodia P-Editor: Li JH
| 1. | Cheng WK, Oon CE. How glycosylation aids tumor angiogenesis: An updated review. Biomed Pharmacother. 2018;103:1246-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Miyata Y, Sakai H. Reconsideration of the clinical and histopathological significance of angiogenesis in prostate cancer: Usefulness and limitations of microvessel density measurement. Int J Urol. 2015;22:806-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Gujam FJ, Going JJ, Edwards J, Mohammed ZM, McMillan DC. The role of lymphatic and blood vessel invasion in predicting survival and methods of detection in patients with primary operable breast cancer. Crit Rev Oncol Hematol. 2014;89:231-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Miyata Y, Mitsunari K, Asai A, Takehara K, Mochizuki Y, Sakai H. Pathological significance and prognostic role of microvessel density, evaluated using CD31, CD34, and CD105 in prostate cancer patients after radical prostatectomy with neoadjuvant therapy. Prostate. 2015;75:84-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | Xiao X, Zhou X, Ming H, Zhang J, Huang G, Zhang Z, Li P. Chick Chorioallantoic Membrane Assay: A 3D Animal Model for Study of Human Nasopharyngeal Carcinoma. PLoS One. 2015;10:e0130935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Miyata Y, Sagara Y, Watanabe S, Asai A, Matsuo T, Ohba K, Hayashi T, Sakai H. CD105 is a more appropriate marker for evaluating angiogenesis in urothelial cancer of the upper urinary tract than CD31 or CD34. Virchows Arch. 2013;463:673-679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Şener E, Şipal S, Gündoğdu C. Comparison of Microvessel Density with Prognostic Factors in Invasive Ductal Carcinomas of the Breast. Turk Patoloji Derg. 2016;32:164-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | López JI, Errarte P, Erramuzpe A, Guarch R, Cortés JM, Angulo JC, Pulido R, Irazusta J, Llarena R, Larrinaga G. Fibroblast activation protein predicts prognosis in clear cell renal cell carcinoma. Hum Pathol. 2016;54:100-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Cioca A, Muntean D, Bungardean C. CD105 as a tool for assessing microvessel density in renal cell carcinoma. Indian J Pathol Microbiol. 2019;62:239-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Sharma SG, Aggarwal N, Gupta SD, Singh MK, Gupta R, Dinda AK. Angiogenesis in renal cell carcinoma: correlation of microvessel density and microvessel area with other prognostic factors. Int Urol Nephrol. 2011;43:125-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | López JI, Erramuzpe A, Guarch R, Cortés JM, Pulido R, Llarena R, Angulo JC. CD34 immunostaining enhances a distinct pattern of intratumor angiogenesis with prognostic implications in clear cell renal cell carcinoma. APMIS. 2017;125:128-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Ebru T, Fulya OP, Hakan A, Vuslat YC, Necdet S, Nuray C, Filiz O. Analysis of various potential prognostic markers and survival data in clear cell renal cell carcinoma. Int Braz J Urol. 2017;43:440-454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Mikami S, Oya M, Kosaka T, Mizuno R, Miyazaki Y, Sato Y, Okada Y. Increased vasohibin-1 expression is associated with metastasis and poor prognosis of renal cell carcinoma patients. Lab Invest. 2017;97:854-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Minardi D, Santoni M, Lucarini G, Mazzucchelli R, Burattini L, Conti A, Bianconi M, Scartozzi M, Milanese G, Primio RD, Montironi R, Cascinu S, Muzzonigro G. Tumor VEGF expression correlates with tumor stage and identifies prognostically different groups in patients with clear cell renal cell carcinoma. Urol Oncol. 2015;33:113.e1-113.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Sato M, Nakai Y, Nakata W, Yoshida T, Hatano K, Kawashima A, Fujita K, Uemura M, Takayama H, Nonomura N. Microvessel area of immature vessels is a prognostic factor in renal cell carcinoma. Int J Urol. 2014;21:130-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Cheng SH, Liu JM, Liu QY, Luo DY, Liao BH, Li H, Wang KJ. Prognostic role of microvessel density in patients with renal cell carcinoma: a meta-analysis. Int J Clin Exp Pathol. 2014;7:5855-5863. [PubMed] |
| 17. | Yoo S, You D, Jeong IG, Song C, Hong B, Hong JH, Ahn H, Kim CS. Histologic subtype needs to be considered after partial nephrectomy in patients with pathologic T1a renal cell carcinoma: papillary vs. clear cell renal cell carcinoma. J Cancer Res Clin Oncol. 2017;143:1845-1851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Sullivan HC, Edgar MA, Cohen C, Kovach CK, HooKim K, Reid MD. The utility of ERG, CD31 and CD34 in the cytological diagnosis of angiosarcoma: an analysis of 25 cases. J Clin Pathol. 2015;68:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Lytras D, Leontara V, Kefala M, Foukas PG, Giannakou N, Pouliakis A, Dervenis C, Panayiotides IG, Karakitsos P. Microvessel Landscape Assessment in Pancreatic Ductal Adenocarcinoma: Unclear Value of Targeting Endoglin (CD105) as Prognostic Factor of Clinical Outcome. Pancreas. 2015;44:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Figueiredo CC, Pereira NB, Pereira LX, Oliveira LAM, Campos PP, Andrade SP, Moro L. Double immunofluorescence labeling for CD31 and CD105 as a marker for polyether polyurethane-induced angiogenesis in mice. Histol Histopathol. 2019;34:257-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 21. | Goldiş DS, Sferdian MF, Tarţă C, Fulger LO, Totolici BD, Neamţu C. Comparative analysis of microvessel density quantified through the immunohistochemistry expression of CD34 and CD105 in rectal cancer. Rom J Morphol Embryol. 2015;56:419-424. [PubMed] |
| 22. | Munoz DG, Woulfe JM. Angiogenesis: A new paradigm for Parkinson disease with practical and pathogenic implications. Neurology. 2015;85:1826-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, Jain RK. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91:1071-1121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1259] [Cited by in RCA: 1203] [Article Influence: 85.9] [Reference Citation Analysis (0)] |
| 24. | Wimmer RA, Leopoldi A, Aichinger M, Wick N, Hantusch B, Novatchkova M, Taubenschmid J, Hämmerle M, Esk C, Bagley JA, Lindenhofer D, Chen G, Boehm M, Agu CA, Yang F, Fu B, Zuber J, Knoblich JA, Kerjaschki D, Penninger JM. Human blood vessel organoids as a model of diabetic vasculopathy. Nature. 2019;565:505-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 513] [Article Influence: 85.5] [Reference Citation Analysis (0)] |
| 25. | Seaman S, Zhu Z, Saha S, Zhang XM, Yang MY, Hilton MB, Morris K, Szot C, Morris H, Swing DA, Tessarollo L, Smith SW, Degrado S, Borkin D, Jain N, Scheiermann J, Feng Y, Wang Y, Li J, Welsch D, DeCrescenzo G, Chaudhary A, Zudaire E, Klarmann KD, Keller JR, Dimitrov DS, St Croix B. Eradication of Tumors through Simultaneous Ablation of CD276/B7-H3-Positive Tumor Cells and Tumor Vasculature. Cancer Cell 2017; 31: 501-515. e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 304] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 26. | Fedor HL, De Marzo AM. Practical methods for tissue microarray construction. Methods Mol Med. 2005;103:89-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Korn C, Augustin HG. Mechanisms of Vessel Pruning and Regression. Dev Cell. 2015;34:5-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 211] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 28. | Voss MH, Bhatt RS, Vogelzang NJ, Fishman M, Alter RS, Rini BI, Beck JT, Joshi M, Hauke R, Atkins MB, Burgess E, Logan TF, Shaffer D, Parikh R, Moazzam N, Zhang X, Glasser C, Sherman ML, Plimack ER. A phase 2, randomized trial evaluating the combination of dalantercept plus axitinib in patients with advanced clear cell renal cell carcinoma. Cancer. 2019;125:2400-2408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Ferté C, Koscielny S, Albiges L, Rocher L, Soria JC, Iacovelli R, Loriot Y, Fizazi K, Escudier B. Tumor growth rate provides useful information to evaluate sorafenib and everolimus treatment in metastatic renal cell carcinoma patients: an integrated analysis of the TARGET and RECORD phase 3 trial data. Eur Urol. 2014;65:713-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 30. | Grünwald V, Lin X, Kalanovic D, Simantov R. Early Tumour Shrinkage: A Tool for the Detection of Early Clinical Activity in Metastatic Renal Cell Carcinoma. Eur Urol. 2016;70:1006-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |