Published online Sep 6, 2021. doi: 10.12998/wjcc.v9.i25.7340
Peer-review started: February 24, 2021
First decision: March 29, 2021
Revised: April 13, 2021
Accepted: July 5, 2021
Article in press: July 5, 2021
Published online: September 6, 2021
Processing time: 188 Days and 12.9 Hours
Pancreatic neoplasms are uncommon in children and in most cases they are benign or have low malignant potential. Pancreatoblastoma and solid pseudopapillary tumor are the most frequent types in early and late childhood, respectively. Complete resection, although burdened by severe complications, is the only curative treatment for these diseases. Pancreatic surgery may result in impaired exocrine and endocrine pancreatic function. However, limited data are available on the long-term pediatric pancreatic function following surgical resection.
To investigate endocrine and exocrine pancreatic function and growth after oncological pancreatic surgery in a pediatric series.
A retrospective analysis of all pediatric patients who underwent surgery for pancreatic neoplasm in our Institution from January 31, 2002 to the present was performed. Endocrine and exocrine insufficiency, auxological and fat-soluble vitamin status (A, D, E and clotting tests) were assessed at diagnosis and at every follow-up visit. Exocrine insufficiency was defined as steatorrhea with fecal elastase-1 < 200 µg/g stool, while endocrine insufficiency was identified as onset of Diabetes or Impaired Glucose Tolerance. Growth was evaluated based on body mass index (BMI) z-score trend.
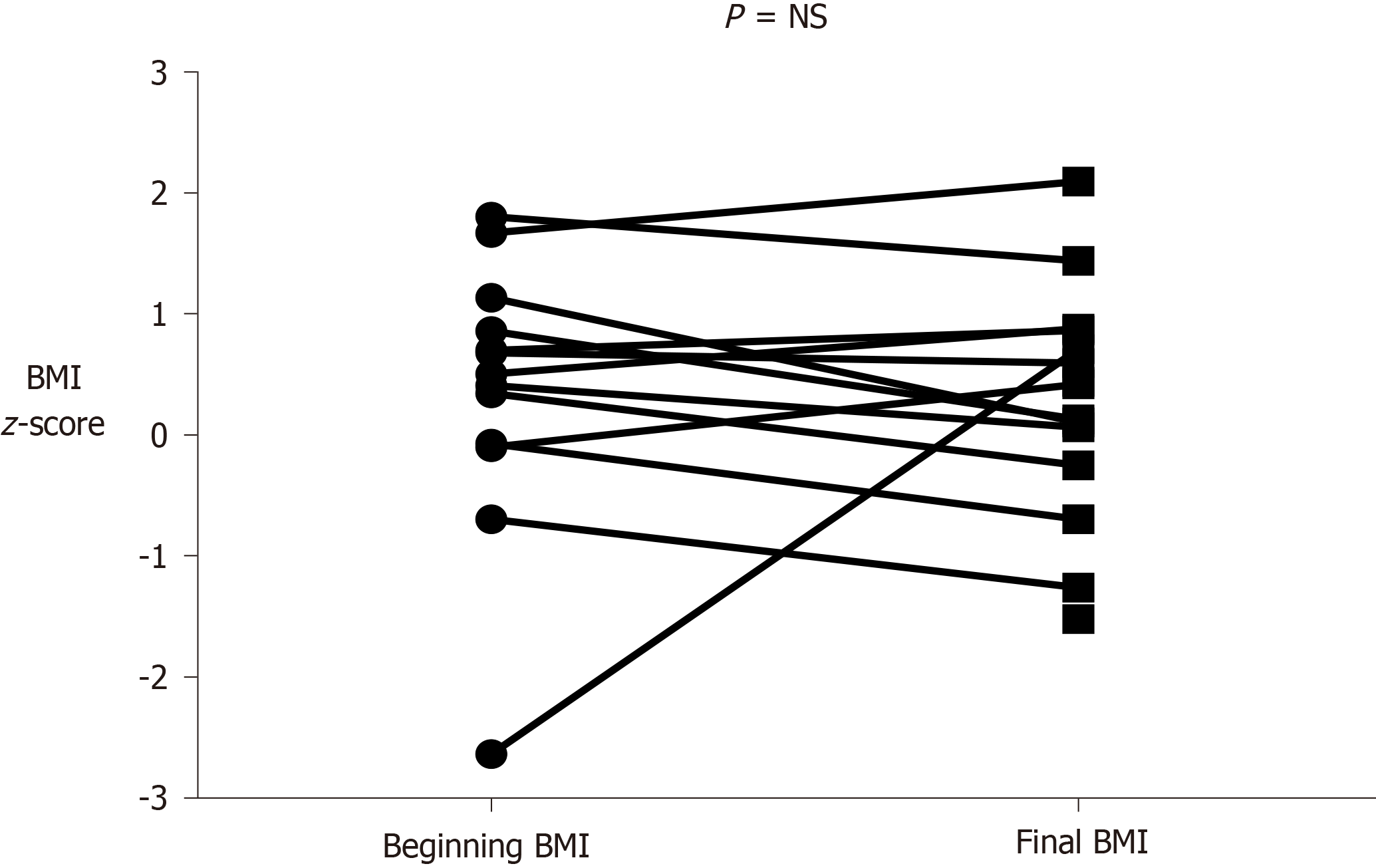
Sixteen patients (12 girls and 4 boys, mean age 10.7 ± 5.3 years), were included. Nine patients (56%) had a neoplasm in the pancreatic head, 4 in the body/tail, 2 in the tail and 1 in the body. Histological findings were as follows: Solid pseudopapillary tumor in 10 patients (62.5%), insulinoma in 2 patients, neuroendocrine tumor in 2 patients and acinar cell carcinoma in 2 patients. The most frequent surgery was pancreaticoduodenectomy (50%). Exocrine failure occurred in 4 patients (25%) and endocrine failure in 2 patients (12.5%). Exocrine insufficiency occurred early (within 6 mo after surgery) and endocrine insufficiency later (8 and 10 years after surgery). Mean BMI z-score was 0.36 ± 1.1 at diagnosis and 0.27 ± 0.95 at the last assessment. Vitamin D was insufficient (< 30 ng/mL) in 8 of the 16 patients during the follow-up period. Vitamins A, E and clotting test were into the normal ranges in all patients.
Careful and long-term monitoring should follow any pancreatic surgery, to recognize and promptly treat exocrine and endocrine pancreatic insufficiency, which can occur after surgery.
Core Tip: There are few published data on pediatric pancreatic neoplasms and, in particular, on the long-term outcome of pancreatic function and growth following surgery. Our findings, based on a retrospective review of 16 patients, showed that the development of exocrine (earlier) and endocrine failure (later) is not rare; careful and long-term monitoring of pancreatic function, can guarantee normal growth during early life.
- Citation: Bolasco G, Capriati T, Grimaldi C, Monti L, De Pasquale MD, Patera IP, Spada M, Maggiore G, Diamanti A. Long-term outcome of pancreatic function following oncological surgery in children: Institutional experience and review of the literature. World J Clin Cases 2021; 9(25): 7340-7349
- URL: https://www.wjgnet.com/2307-8960/full/v9/i25/7340.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i25.7340
Pancreatic neoplasms are uncommon in childhood with an incidence rate, as reported in the United States, of 0.018 cases per 100000 people[1]; in most cases they are benign or have low malignant potential[2]. Pancreatoblastoma and solid pseudopapillary tumor (SPT) are the most common histologies in early and late childhood, respectively[1,3,4]. Endocrine tumors, adenocarcinoma, sarcoma, lymphoma and neuroblastoma rarely occur in children. Prognosis, although better than in adults, is strongly influenced by tumor histology, with excellent survival for SPTs (95%) and lower for neuroendocrine tumors (51%), sarcomas (43%) and acinar cell carcinomas (34%)[5]. Complete surgical resection provides the only curative treatment for patients with pancreatic neoplasms, except for lymphoma[6]. Distal pancreatectomy is the preferred surgical approach for body/tail neoplasms and pancreaticoduodenectomy (PD) for head neoplasms[7-9]. PD, also known as Whipple procedure, is the treatment of choice for pancreatic neoplasms involving the head of the pancreas and the periampullary tract[10-13]. It is, however, a very complex surgery with many complications and a high mortality rate, even in adults[14-17]. Pancreatic resections may result in impaired pancreatic, endocrine and exocrine function[7-9,18-22]. Given the great relevance of pancreatic function on nutritional status and growth in childhood, we planned the present study to assess the long-term outcome of exocrine and endocrine pancreatic function, growth and vitamin status following pancreatic resection for neoplasms in our Institution.
All consecutive patients, 18 years old or younger, affected by pancreatic neoplasms and who had undergone pancreatic surgery between January 31, 2002 to the present were retrospectively reviewed from our Institutional database. Patients with pancreatic neoplasms had been followed since 2002 by a structured multidisciplinary team consisting of pediatric surgeons, pediatric gastroenterologists, oncologists, endocrinologists, radiologists and dietitians.
All patients with pancreatic neoplasms who had undergone pancreatic surgery were considered eligible.
The following outcomes were selected: (1) Rate of exocrine insufficiency, defined as steatorrhea associated with fecal elastase-1 < 200 µg/g stool[23]; (2) Rate of endocrine insufficiency defined as fasting blood glucose ≥ 126 mg/dL (≥ 7.0 mmol/L) or glycated hemoglobin ≥ 6.5% (≥ 48 mmol/L) for the diagnosis of diabetes and fasting blood glucose 100-125 mg/dL (5.6-6.9 mmol/L) or glycated hemoglobin 5.7%-6.4% (39-47 mmol/mol) for the diagnosis of impaired glucose tolerance (IGT)[24]; (3) Growth based on the body mass index (BMI) z-score trend; the growth charts from the CDC (Centers for Disease Control)[25] were used as a reference; and (4) Rate of fat-soluble vitamin deficiency (A, E, D, clotting test).
Our scheduled follow-up after oncological pancreatic surgery is reported in Table 1. Generally, steatorrhea was assessed before discharge and at every follow-up visit; we employed only fecal elastase to follow the trend of exocrine failure.
| T1 | T2 | T3 | |
| Clinical examination (physical examination, symptoms, weight, height, BMI) | Every 4 mo | Every 6 mo | Once a year |
| Routine lab tests (CBC, liver and kidney function tests, amylase, lipase, bilirubin, albumin, etc.) | Every 4 mo | Every 6 mo | Once a year |
| Vitamin dosage (A, D, E, clotting test) | Every 4 mo | Every 6 mo | Once a year |
| Serological markers (according to the underlying histology) (alpha-FP, Chromogranin-A) | Every 4 mo | Every 6 mo | Once a year |
| Assessment of exocrine pancreatic function (fecal elastase) | Every 4 mo | Every 6 mo | Once a year |
| Assessment of endocrine pancreatic function (fasting blood glucose, glycated hemoglobin, C-peptide) | Every 4 mo | Every 6 mo | Once a year |
| Imaging (abdominal US) | Every 4 mo | Every 6 mo | Once a year |
| Surgical, diabetological and oncological consulting | Every 4 mo | Every 6 mo | Once a year |
| Nutritional assessment (3-d recall dietary assessment and diet optimization by dietitians) | Every 4 mo | Every 6 mo | Once a year |
Data relating to the study variables were extracted from medical records.
Categorical variables were summarized as percentage and continuous variables as mean ± SD. The Mann-Whitney U test was used to compare continuous variables and Fisher’s exact test was used to compare frequencies between groups. A P value of < 0.05 was considered to indicate statistical significance. Statistical evaluation and figures were performed using Graph Pad 6 for Windows.
The Ethical Committee of “Bambino Gesù” Children’s Hospital in Rome approved this study. Our Hospital is an Institute authorized by the Ministry of Health Care for research and clinical study. Therefore, to enroll patients in clinical studies all the parents or tutors give their consent by signature of a specific document when the children are admitted to our Hospital. In this way we have the consent in line with the indications of our Ethical Committee when the patients are admitted to the hospital.
Sixteen patients, twelve girls (75%) and four boys (25%) (P = 0.0121), all surviving, fulfilled the study inclusion criteria. Mean age at diagnosis and at the last evaluation were 10.7 ± 5.3 years and 16.6 ± 5.2 years, respectively. Mean follow-up was 5.7 ± 4.3 years. Nine of 16 patients (56%) had a neoplasm in the head of the pancreas (P = NS), and of the remaining 7 patients, 4 had a tumor in the body/tail, 2 in the tail and 1 in the body. Histologies were as follows: SPT in 10 patients (62.5%) (P = NS); the remaining histopathological diagnoses were: Insulinoma in 2 patients, neuroendocrine tumor in 2 and acinar cell carcinoma in the remaining two patients. Mean age at diagnosis was significantly higher in patients with SPT (13.5 ± 2.4 years) than in the others (6.2 ± 5.8 years) (P = 0.0167). Abdominal pain was the most frequent presenting symptom (43.7%). PD was the main surgery performed (50% of patients). Exocrine and endocrine insufficiency occurred in 4 (25%) and in 2 (12.5%) of 16 patients, respectively. Two patients developed both exocrine and endocrine insufficiency and 2 only exocrine insufficiency. All patients with exocrine pancreatic failure required supplementation with pancrelipase, which is still ongoing, while 2 patients showed IGT not requiring insulin therapy. All these patients had pancreatic head tumors treated by PD. Interestingly, exocrine insufficiency occurred within the first 6 mo after surgery, while endocrine insufficiency occurred later (8 and 10 years after surgery). With regard to the growth trend, mean BMI z-score at diagnosis was 0.36 ± 1.1 and at the last follow-up was 0.27 ± 0.95 (P = NS) (see also Figure 1 for details). Vitamins A and E and clotting tests were in the normal ranges in all patients. Vitamin D was found to be insufficient (< 30 ng/mL) at some follow-up visits in 8 of the 16 patients. The main characteristics of our patients are detailed in Table 2.
| No. | Sex | Age | Histology | Symptoms | Tumor site | Type of surgery | Length of follow-up | Exocrine pancreatic failure | Endocrine pancreatic failure | Vitamin D insufficiency |
| 1 | F | 12 | SPT | Weight loss | HEAD | PPPD | 10.4 yr | Yes | Yes | Yes |
| 2 | M | 9.8 | SPT | Occasional diagnosis | BODY | Distal Pancreatectomy | 6.4 yr | No | No | No |
| 3 | F | 17.3 | SPT | AP | HEAD | PPPD | 3.9 yr | Yes | No | Yes |
| 4 | M | 15.5 | Neuroendocrine tumor | AP | HEAD | PPPD | 2.3 yr | No | No | No |
| 5 | F | 6.6 | Acinar cell carcinoma | AP+ weight loss | HEAD | PPPD | 11.5 yr | Yes | Yes | Yes |
| 6 | M | 4.11 | Acinar cell carcinoma | Not available | HEAD | PD | 12.3 yr | Yes | No | Yes |
| 7 | F | 14.3 | SPT | Weight loss | TAIL | Distal Pancreatectomy | 2 yr | No | No | Yes |
| 8 | F | 13.2 | SPT | AP | HEAD | PPPD | 9 mo | No | No | Yes |
| 9 | F | 3 mo | Insulinoma | Hypoglycemia | HEAD | Cephalic Pancreatectomy | 11 yr | No | No | Yes |
| 10 | F | 11 | SPT | AP | BODY/TAIL | Distal Splenopancreatectomy | 2 yr | No | No | No |
| 11 | M | 10 | Neuroendocrine tumor | Screening in Tuberous Sclerosis | BODY/TAIL | Distal Pancreatectomy | 9 yr | No | No | No |
| 12 | F | 15.5 | SPT | No symptoms | BODY/TAIL | Distal Splenopancreatectomy | 2.5 yr | No | No | No |
| 13 | F | 13.8 | SPT | AP | HEAD | PPPD | 10 yr | No | No | No |
| 14 | F | 11.6 | SPT | No symptoms | BODY/TAIL | Distal Splenopancreatectomy | 3.4 yr | No | No | Yes |
| 15 | F | 16.2 | SPT | AP+ weight loss | TAIL | Distal Pancreatectomy | 3.8 yr | No | No | No |
| 16 | F | 7 mo | Insulinoma | Hypoglycemia | HEAD | PPPD | 1 mo | No | No | No |
Pancreatic neoplasms are rare in childhood and the main treatment, regardless of tumor type, is radical resection[21]. The extent of surgical resection needed for complete debulking is related to the neoplasm site. Whenever possible, less extensive resection is advocated; however, a minority of patients will require resection of the pancreatic head and hepatobiliary reconstruction. Proper discussion of the risks and benefits is particularly difficult due to the lack of literature. In particular, few data are available on the long-term outcome of exocrine and endocrine insufficiency, following surgery. Due to the high relevance of normal absorption in children to guarantee the expected growth course, we investigated, in a series of 16 children with pancreatic tumors who had undergone surgery in the last 19 years, the long-term outcome of exocrine/endocrine function, growth and development of fat-soluble vitamin deficiency. Overall, 25% of patients in the present series developed exocrine failure. In adults, the rate of pancreatic exocrine insufficiency after partial pancreatectomy seems to be higher (about 35%)[19]. In Tables 3 and 4 we summarize the main characteristics of the studies that focused on long-term outcome of pancreatic functions following oncological surgery in children[26-37]. The data showed that the prevalence of exocrine insufficiency in pediatric patients ranged from 0%[29] to 83.3%[33]. Vasudevan et al[27] found exocrine failure rate similar to our finding (23%), while Cheng et al[26] found a much lower rate (8.6%). Park et al[34] reported that six of 8 patients experienced mild steatorrhea.
| Ref. | Country | Length of follow-up (yr) | n of cases/ M/F (%) | Mean or median age (yr) | Exocrine insufficiency (%) | Endocrine insufficiency (%) |
| Present | Italy | 5.7 | 16 (25/75) | 10.7 | 25 | 12.5 |
| Cheng et al[26], 2020 | China | 3.1 | 104 (31/69) | 9.9 | 8.6 | 1 |
| Vasudevan et al[27], 2020 | United States | 2.8 | 65 | 13 | 23 | 3 |
| Mizuno et al[28], 2018 | Japan | 30 | 1 (M) | 12 | 0 | 0 |
| Divarcı et al[29], 2017 | Turkey | 3.6 | 5 (0/100) | 15 | 0 | 0 |
| d’Ambrosio et al[30], 2014 | Italy | 2.1 | 5 (40/60) | 7 | 20 | 20 |
| Laje et al[31], 2013 | United States | 6.7 | 6 (17/83) | 15 | 17 | 0 |
| Scandavini et al[32], 2018 | Sweden | 6.6 | 13 (23/77) | 11.4 | 31 | 7,7 |
| Lindholm et al[33], 2017 | United States | 4.7 | 12 (42/58) | 9 | 83.3 | 0 |
| Park et al[34], 2016 | Korea | 10.5 | 18 (25/75) | 10.5 | 75 | 12.5 |
| Muller et al[35], 2012 | France | 4.2 | 216 (44/56) | 8.9 | 6.25 | 0 |
| Speer et al[36], 2012 | United States | 1.4 | 11 (36/64) | 14 | 9 | 0 |
| Yazbeck et al[37], 2010 | Lebanon | - | 1 (F) | 13 | 100 | 0 |
| Onset symptoms (%) | Histology (%) | Type of surgery (%) |
| Abdominal pain (50) | SPT (64) | PD (61) |
| Palpable mass (17) | Pancreatoblastoma (13) | Distal/central pancreatectomy (30) |
| Nausea/emesis (16) | Neuroendocrine tumors (7) | Tumor enucleation (9) |
| Occasional diagnosis (8) | Neuroblastoma (4) | |
| Jaundice (8) | Rhabdomyosarcoma/sarcoma (4) | |
| Diarrhea (1) | Acinar cell carcinoma (4) | |
| Pancreatic islet cells cancer (1) | ||
| Other (3) |
In contrast, endocrine insufficiency, described in 40% of adult patients after partial pancreatectomy[19], was observed only in few pediatric patients. We found endocrine failure in 12.5% and Vasudevan et al[27] found endocrine failure in 3%. No patients with endocrine insufficiency were found in seven surveys[28,29,31,33,35-37]. Cheng et al[26] reported that only one patient developed endocrine insufficiency in 104 and Park et al[34] found one in 8 patients. This finding might reflect the larger functional reserve of the endocrine pancreas in young patients[32]. It is possible that the functional reserve could become insufficient many years after pancreatic resection, as confirmed by our two cases who developed endocrine failure after 8 and 10 years after surgery. The two patients who showed IGT not requiring insulin therapy, did not develop islet cell autoantibodies, so we could exclude the diagnosis of latent autoimmune diabetes in adults[38]. This finding suggests that long-term follow-up is crucial to identify endocrine pancreatic failure.
According to our results, previous studies showed that pancreatic exocrine function can occur early (within 2 mo after surgery) following PD but that it may later revert (from 6 mo to 12 mo)[21,22]. Glucose metabolism tends to remain normal for 1 year after PD, but it can worsen after many years[20,22,39].
The magnitude of the pancreatic resection is the main factor influencing the worsening of pancreatic function over the years. Divarcı et al[29], found no cases of exocrine and endocrine failure in patients who underwent tumor enucleation. Lindholm et al[33] found a rate of exocrine insufficiency closer to that reported in adults; interestingly this study included only patients who had undergone PD, as occurred in adults; pylorus-sparing PD can indeed worsen pancreatic function due to insufficient pylorus contraction with consequent stasis within the pancreatic duct and parenchyma destruction[40,41].
Table 5 shows available data in the literature, including data from the present report, to assess associations between exocrine and endocrine insufficiency with tumor site and type of surgery. It can be seen that 93% of all cases of exocrine insufficiency is associated with PD, confirming the results of Lindholm et al[33].
| Overall | Head (%) | Body/tail (%) | |
| 235 | 149 (63) | 106 (37) | |
| Exocrine insufficiency | 35 | 31 (89) | 4 (11) |
| Endocrine insufficiency | 5 | 4 (80) | 1 (20) |
| Overall | PD (%) | Other surgery (%) | |
| 140 | 106 (76) | 34 (24) | |
| Exocrine insufficiency | 30 | 28 (93) | 2 (7) |
| Endocrine insufficiency | 5 | 4 (80) | 1 (20) |
Surgery did not impact on growth course in our patients according to the finding of a previous study[34]. We can argue that the early identification of pancreatic insufficiency and prompt treatment with enzymes is the main strategy to avoid nutritional deficiency and to ensure adequate growth. Early identification of exocrine insufficiency may also prevent the development of fat-soluble vitamin deficiency. Some patients developed vitamin D insufficiency while vitamin A, E and clotting tests remained in the normal ranges in all patients. It can be argued that the low levels of vitamin D may not be exclusively related to exocrine pancreatic insufficiency, in view of the high prevalence of vitamin D insufficiency in the healthy population[42].
In conclusion, despite the important limitation of the retrospective design of this study that could have led to missed data, these findings highlight digestive and nutritional issues in a context in which they are frequently disregarded. Careful and long-term monitoring should follow any pancreatic surgery in children. The loss of exocrine function can occur early but it seems to have no impact on the growth course if it is promptly treated. The impairment of endocrine function is less frequent and may take many years to develop.
Pancreatic neoplasms are very rare in children and available data in this field are limited. Surgery allows the long-term survival of these patients, even if it could lead to complications such as pancreatic insufficiency. Currently there is little evidence on the onset of pancreatic failure and growth trend in children after pancreatic surgery.
We would like to increase knowledge regarding the evolution of pancreatic function after surgical resection in children with pancreatic neoplasms. Currently there is no scheduled follow-up to monitor the long-term complications of pancreatic surgery and in pediatric age it is essential to immediately diagnose the possible onset of pancreatic insufficiency to ensure adequate growth.
The aim of this study was to evaluate the long-term outcome of pancreatic function after pancreatic surgery in children, identify the incidence of endocrine and exocrine pancreatic insufficiency, fat-soluble vitamin deficiency and failure to thrive.
We retrospectively analyzed all consecutive pediatric patients diagnosed with pancreatic neoplasms who underwent pancreatic surgery in our institution between January 31, 2002 and the present. Patients were followed by a multidisciplinary team that assessed auxological parameters, clinical symptoms, laboratory and radiological tests at each follow-up visit.
Sixteen patients (12 girls and 4 boys, mean age 10.7 ± 5.3 years), were included. The most frequent surgery was pancreaticoduodenectomy (50%). Exocrine failure occurred in 4 patients (25%) within 6 mo after surgery, while endocrine failure occurred in 2 patients (12.5%) 8 and 10 years after surgery, respectively. No statistically significant differences were found in BMI z-score at diagnosis and at the last follow-up. Vitamin D was insufficient (< 30 ng/mL) in 8 of the 16 patients while vitamins A, E and clotting test were into the normal ranges in all patients.
Our study highlights that the development of exocrine and endocrine pancreatic insufficiency after pancreatic surgery is not rare; these potential complications must be adequately identified and treated, as pancreatic enzyme replacement therapy prevents malabsorption and consequent growth failure.
It is essential to identify and establish a standardized follow-up in pediatric patients, organized by a multidisciplinary team including a surgeon, oncologist, gastroenterologist, endocrinologist, radiologist and dietician.
We thank Guglielmi L for editing and reviewing the English language and for biostatistical analysis.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tan CL S-Editor: Gao CC L-Editor: Webster JR P-Editor: Li JH
| 1. | Perez EA, Gutierrez JC, Koniaris LG, Neville HL, Thompson WR, Sola JE. Malignant pancreatic tumors: incidence and outcome in 58 pediatric patients. J Pediatr Surg. 2009;44:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 2. | Jin WW, Lu C, Mou YP, Wang YY, Zhu QC, Xia T. [Early experience of minimal invasive surgery for adolescent with pancreatic head tumor: a report of 15 cases]. Zhonghua Wai Ke Za Zhi. 2020;58:512-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Rojas Y, Warneke CL, Dhamne CA, Tsao K, Nuchtern JG, Lally KP, Vasudevan SA, Hayes-Jordan AA, Cass DL, Herzog CE, Hicks MJ, Kim ES, Austin MT. Primary malignant pancreatic neoplasms in children and adolescents: a 20 year experience. J Pediatr Surg. 2012;47:2199-2204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 4. | Shorter NA, Glick RD, Klimstra DS, Brennan MF, Laquaglia MP. Malignant pancreatic tumors in childhood and adolescence: The Memorial Sloan-Kettering experience, 1967 to present. J Pediatr Surg. 2002;37:887-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 126] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Picado O, Ferrantella A, Zabalo C, Rao K, Thorson CM, Sola JE, Perez EA. Treatment patterns and outcomes for pancreatic tumors in children: an analysis of the National Cancer Database. Pediatr Surg Int. 2020;36:357-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 6. | Crocoli A, Grimaldi C, Virgone C, De Pasquale MD, Cecchetto G, Cesaro S, Bisogno G, Cecinati V, Narciso A, Alberti D, Ferrari A, Dall'Igna P, Spada M, Inserra A. Outcome after surgery for solid pseudopapillary pancreatic tumors in children: Report from the TREP project-Italian Rare Tumors Study Group. Pediatr Blood Cancer. 2019;66:e27519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Papavramidis T, Papavramidis S. Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature. J Am Coll Surg. 2005;200:965-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 536] [Article Influence: 26.8] [Reference Citation Analysis (1)] |
| 8. | Choi SH, Kim SM, Oh JT, Park JY, Seo JM, Lee SK. Solid pseudopapillary tumor of the pancreas: a multicenter study of 23 pediatric cases. J Pediatr Surg. 2006;41:1992-1995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Yu DC, Kozakewich HP, Perez-Atayde AR, Shamberger RC, Weldon CB. Childhood pancreatic tumors: a single institution experience. J Pediatr Surg. 2009;44:2267-2272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 10. | Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 896] [Cited by in RCA: 968] [Article Influence: 50.9] [Reference Citation Analysis (34)] |
| 11. | Herter FP, Cooperman AM, Ahlborn TN, Antinori C. Surgical experience with pancreatic and periampullary cancer. Ann Surg. 1982;195:274-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 125] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Cohen JR, Kuchta N, Geller N, Shires GT, Dineen P. Pancreaticoduodenectomy for benign disease. Ann Surg. 1983;197:68-71. [PubMed] |
| 13. | Michelassi F, Erroi F, Dawson PJ, Pietrabissa A, Noda S, Handcock M, Block GE. Experience with 647 consecutive tumors of the duodenum, ampulla, head of the pancreas, and distal common bile duct. Ann Surg. 1989;210:544-54; discussion 554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 161] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, Talamini MA, Hruban RH, Ord SE, Sauter PK, Coleman J, Zahurak ML, Grochow LB, Abrams RA. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226:248-57; discussion 257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1357] [Cited by in RCA: 1387] [Article Influence: 49.5] [Reference Citation Analysis (34)] |
| 15. | Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann Surg. 1996;223:273-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 547] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 16. | Trede M, Schwall G, Saeger HD. Survival after pancreatoduodenectomy. 118 consecutive resections without an operative mortality. Ann Surg. 1990;211:447-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 783] [Cited by in RCA: 765] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 17. | Nitecki SS, Sarr MG, Colby TV, van Heerden JA. Long-term survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving? Ann Surg. 1995;221:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 416] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 18. | Dall'igna P, Cecchetto G, Bisogno G, Conte M, Chiesa PL, D'Angelo P, De Leonardis F, De Salvo G, Favini F, Ferrari A; TREP Group. Pancreatic tumors in children and adolescents: the Italian TREP project experience. Pediatr Blood Cancer. 2010;54:675-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 19. | Elliott IA, Epelboym I, Winner M, Allendorf JD, Haigh PI. Population-Level Incidence and Predictors of Surgically Induced Diabetes and Exocrine Insufficiency after Partial Pancreatic Resection. Perm J. 2017;21:16-095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Yamaguchi K, Tanaka M, Chijiiwa K, Nagakawa T, Imamura M, Takada T. Early and late complications of pylorus-preserving pancreatoduodenectomy in Japan 1998. J Hepatobiliary Pancreat Surg. 1999;6:303-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 101] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Sato N, Yamaguchi K, Yokohata K, Shimizu S, Morisaki T, Chijiiwa K, Tanaka M. Short-term and long-term pancreatic exocrine and endocrine functions after pancreatectomy. Dig Dis Sci. 1998;43:2616-2621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Ohtsuka T, Yamaguchi K, Chijiiwa K, Kinukawa N, Tanaka M. Quality of life after pylorus-preserving pancreatoduodenectomy. Am J Surg. 2001;182:230-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Frulloni L, Falconi M, Gabbrielli A, Gaia E, Graziani R, Pezzilli R, Uomo G, Andriulli A, Balzano G, Benini L, Calculli L, Campra D, Capurso G, Cavestro GM, De Angelis C, Ghezzo L, Manfredi R, Malesci A, Mariani A, Mutignani M, Ventrucci M, Zamboni G, Amodio A, Vantini I; Italian Association for the Study of the Pancreas (AISP); Bassi C, Delle Fave G, Frulloni L, Capurso IV, Magarini F, Albarello L, Alfieri S, Anti M, Arcidiacono P, Baiocchi L, Berretti D, Boraschi P, Buscarini E, Carroccio A, Celebrano MR, Casadei R, Chilovi F, Conigliaro R, Dall'Oglio L, De Boni M, De Pretis G, Di Priolo S, Di Sebastiano PL, Doglietto GB, Filauro M, Frieri G, Fuini A, Loriga P, Macarri G, Manes G, Massucco P, Milani S, Pasquali C, Pederzoli P, Pietrangeli M, Rocca R, Russello D, Siquini W, Traina M, Veneroni L, Zilli M. Italian consensus guidelines for chronic pancreatitis. Dig Liver Dis. 2010;42 Suppl 6:S381-S406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36 Suppl 1:S67-S74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1255] [Cited by in RCA: 1540] [Article Influence: 128.3] [Reference Citation Analysis (4)] |
| 25. | Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC growth charts: United States. Adv Data. 2000;1-27. [PubMed] |
| 26. | Cheng H, Yang S, Ren Q, Yang W, Han W, Chang X, Zhu Z, Qin H, Wang H. Pancreatectomies for pediatric pancreatic tumors: A single institute experience from 2007 to 2018. J Pediatr Surg. 2020;55:1722-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Vasudevan SA, Ha TN, Zhu H, Heaton TE, LaQuaglia MP, Murphy JT, Barry WE, Goodhue C, Kim ES, Aldrink JH, Polites SF, Leraas HJ, Rice HE, Tracy ET, Lautz TB, Superina RA, Davidoff AM, Langham MR Jr, Murphy AJ, Bütter A, Davidson J, Glick RD, Grijalva J, Gow KW, Ehrlich PF, Newman EA, Lal DR, Malek MM, Le-Nguyen A, Piché N, Rothstein DH, Short SS, Meyers R, Dasgupta R. Pancreaticoduodenectomy for the treatment of pancreatic neoplasms in children: A Pediatric Surgical Oncology Research Collaborative study. Pediatr Blood Cancer. 2020;67:e28425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Mizuno S, Okuda Y, Gyoten K, Koide T, Suzaki M. Long-term functional outcomes after pylorus preserving pancreaticoduodenectomy from childhood through middle age: 30-year follow-up of nutritional status, pancreatic function, and morphological changes of the pancreatic remnant. J Pediatr Surg. 2018;53:1263-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 29. | Divarcı E, Dökümcü Z, Çetingül N, Nart D, Barbet FY, Ergün O, Çelik A. Radical resection of the pancreas should not always be necessary in the surgical management of pancreatic solid pseudopapillary tumor in children. Turk J Gastroenterol. 2017;28:214-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | d'Ambrosio G, del Prete L, Grimaldi C, Bertocchini A, Lo Zupone C, Monti L, de Ville de Goyet J. Pancreaticoduodenectomy for malignancies in children. J Pediatr Surg. 2014;49:534-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Laje P, Bhatti TR, Adzick NS. Solid pseudopapillary neoplasm of the pancreas in children: a 15-year experience and the identification of a unique immunohistochemical marker. J Pediatr Surg. 2013;48:2054-2060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Scandavini C, Valente R, Rangelova E, Segersvärd R, Arnelo U, Permert J, Svensson PJ, Stenman J, Del Chiaro M. Pancreatectomies for pancreatic neoplasms in pediatric and adolescent age: A single institution experience. Pancreatology. 2018;18:204-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Lindholm EB, Alkattan AK, Abramson SJ, Price AP, Heaton TE, Balachandran VP, La Quaglia MP. Pancreaticoduodenectomy for pediatric and adolescent pancreatic malignancy: A single-center retrospective analysis. J Pediatr Surg. 2017;52:299-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 34. | Park HH, Kim HY, Jung SE, Lee SC, Park KW. Long-term functional outcomes of PPPD in children--Nutritional status, pancreatic function, GI function and QOL. J Pediatr Surg. 2016;51:398-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Muller CO, Guérin F, Goldzmidt D, Fouquet V, Franchi-Abella S, Fabre M, Branchereau S, Martelli H, Gauthier F. Pancreatic resections for solid or cystic pancreatic masses in children. J Pediatr Gastroenterol Nutr. 2012;54:369-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Speer AL, Barthel ER, Patel MM, Grikscheit TC. Solid pseudopapillary tumor of the pancreas: a single-institution 20-year series of pediatric patients. J Pediatr Surg. 2012;47:1217-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 37. | Yazbeck N, Muwakkit S, Abboud M, Saab R. Zinc and biotin deficiencies after pancreaticoduodenectomy. Acta Gastroenterol Belg. 2010;73:283-286. [PubMed] |
| 38. | Fourlanos S, Dotta F, Greenbaum CJ, Palmer JP, Rolandsson O, Colman PG, Harrison LC. Latent autoimmune diabetes in adults (LADA) should be less latent. Diabetologia. 2005;48:2206-2212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 229] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 39. | Kim HC, Suzuki T, Kajiwara T, Miyashita T, Imamura M, Tobe T. Exocrine and endocrine stomach after gastrobulbar preserving pancreatoduodenectomy. Ann Surg. 1987;206:717-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 40. | Ohtsuka T, Tanaka M, Miyazaki K. Gastrointestinal function and quality of life after pylorus-preserving pancreatoduodenectomy. J Hepatobiliary Pancreat Surg. 2006;13:218-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 41. | Fang WL, Su CH, Shyr YM, Chen TH, Lee RC, Tai LC, Wu CW, Lui WY. Functional and morphological changes in pancreatic remnant after pancreaticoduodenectomy. Pancreas. 2007;35:361-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 42. | Wimalawansa SJ, Razzaque MS, Al-Daghri NM. Calcium and vitamin D in human health: Hype or real? J Steroid Biochem Mol Biol. 2018;180:4-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |