Published online Aug 26, 2021. doi: 10.12998/wjcc.v9.i24.7237
Peer-review started: April 16, 2021
First decision: April 27, 2021
Revised: May 25, 2021
Accepted: July 5, 2021
Article in press: July 5, 2021
Published online: August 26, 2021
Processing time: 129 Days and 18.6 Hours
Multiple primary cancer refers to more than one synchronous or sequential cancer in the same individual. Multiple primary cancer always presents as solid cancer or acute myeloid leukemia (AML) secondary to lymphoma. Here, we report a rare case of secondary peripheral T-cell lymphoma and AML after Burkitt lymphoma treatment.
A 54-year-old female patient was admitted to our hospital complaining of edema on her left lower limb. Physical examination revealed multiple superficial lymphadenectasis on her neck and pelvis. Color ultrasonography examination showed multiple uterine fibroids and a solid mass at the lower left side of the abdomen. Pathological biopsy revealed Burkitt lymphoma. After three hyper-CVAD (A + B) regimens, she achieved complete remission. Two years later, lymphadenectasis reoccurred. A relevant biopsy confirmed the diagnosis of peripheral T-cell lymphoma, which was accompanied by gastrointestinal invasion and hemocytopenia. Meanwhile, bone marrow examination revealed AML. On the second day of scheduled treatment, she developed gastrointestinal bleeding, peptic ulcers, and hemorrhagic shock and was critically ill. She was then discharged from the hospital due to financial concerns.
This is the first report of secondary peripheral T-cell lymphoma and AML after Burkitt lymphoma treatment with heterochronous and synchronal multiple primary cancers.
Core Tip: Frequently, secondary malignancies are not identified in a timely manner or are misdiagnosed. Literature indicates that secondary malignancies occur sporadically and spread gradually. The survival time for secondary cancer depends on the extent of tumor lesions than the number of tumors. This paper presents a rare case of secondary cancer to provide a complete understanding to the medical staff allowing them to make early diagnoses and initiate rational and integrated treatments to prolong the survival time and improve the prognosis.
- Citation: Huang L, Meng C, Liu D, Fu XJ. Secondary peripheral T-cell lymphoma and acute myeloid leukemia after Burkitt lymphoma treatment: A case report. World J Clin Cases 2021; 9(24): 7237-7244
- URL: https://www.wjgnet.com/2307-8960/full/v9/i24/7237.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i24.7237
Multiple primary cancer (MPC) refers to two or more simultaneous or sequential occurrences of primary malignant tumors in the same individual arising from different sites and/or with different histology or morphology[1]. Depending on the time interval between two cancer occurrences, MPC can be classified as synchronous (occurrence of two cancers detected simultaneously or sequentially within a time interval of ≤ 6 mo) or heterochronous (occurrence of two cancers detected sequentially within a time interval of > 6 mo)[1]. MPCs are always reported as two or more solid cancers[1-4], sometimes as secondary malignancies following nonsolid cancer treatment[1]. However, both lymphoma and leukemia secondary to lymphoma treatment are rare. This report presents a rare case of secondary peripheral T-cell lymphoma not otherwise specified (PTCL-NOS) and acute myeloid leukemia (AML) after treatment for Burkitt lymphoma and a review of the relevant MPC literature to understand the clinical features, pathogenesis, treatment and prognosis of MPC.
A 54-year-old woman was admitted to our hospital with a complaint of edema on her left lower limb on April 7, 2017. The patient showed no signs of abdominal pain, abdominal distension, low fever, night sweats, fatigue, frequent urination, urgent urination, dysuria, abnormal vaginal bleeding or discomfort owing to vaginal discharge.
She was fit and had no history of exposure to chemicals or radiation.
She had no specific personal or family medical history.
Physical examination revealed multiple superficial lymphadenectasis on her neck and a palpable mass of approximately 8 cm × 6 cm on the lower left side of her abdomen.
A blood test for hepatitis B virus (HBV) showed an HBV surface antigen of > 225.00 ng/mL, HBV e antigen of 10.822 PEI U/mL, HBV core antibody 11.082 PEI U/mL and positive HBV pre-S1 antigen.
Pelvic ultrasound examination revealed multiple uterine fibroids, with the largest located at the bottom of the uterus (5.5 cm × 5.4 cm) and a solid mass at the lower left side of the abdomen (7.5 cm × 5.7 cm). Color Doppler echocardiography revealed mild aortic valve regurgitation. No abnormalities were observed in the cardiac morphology, structure and valve activity.
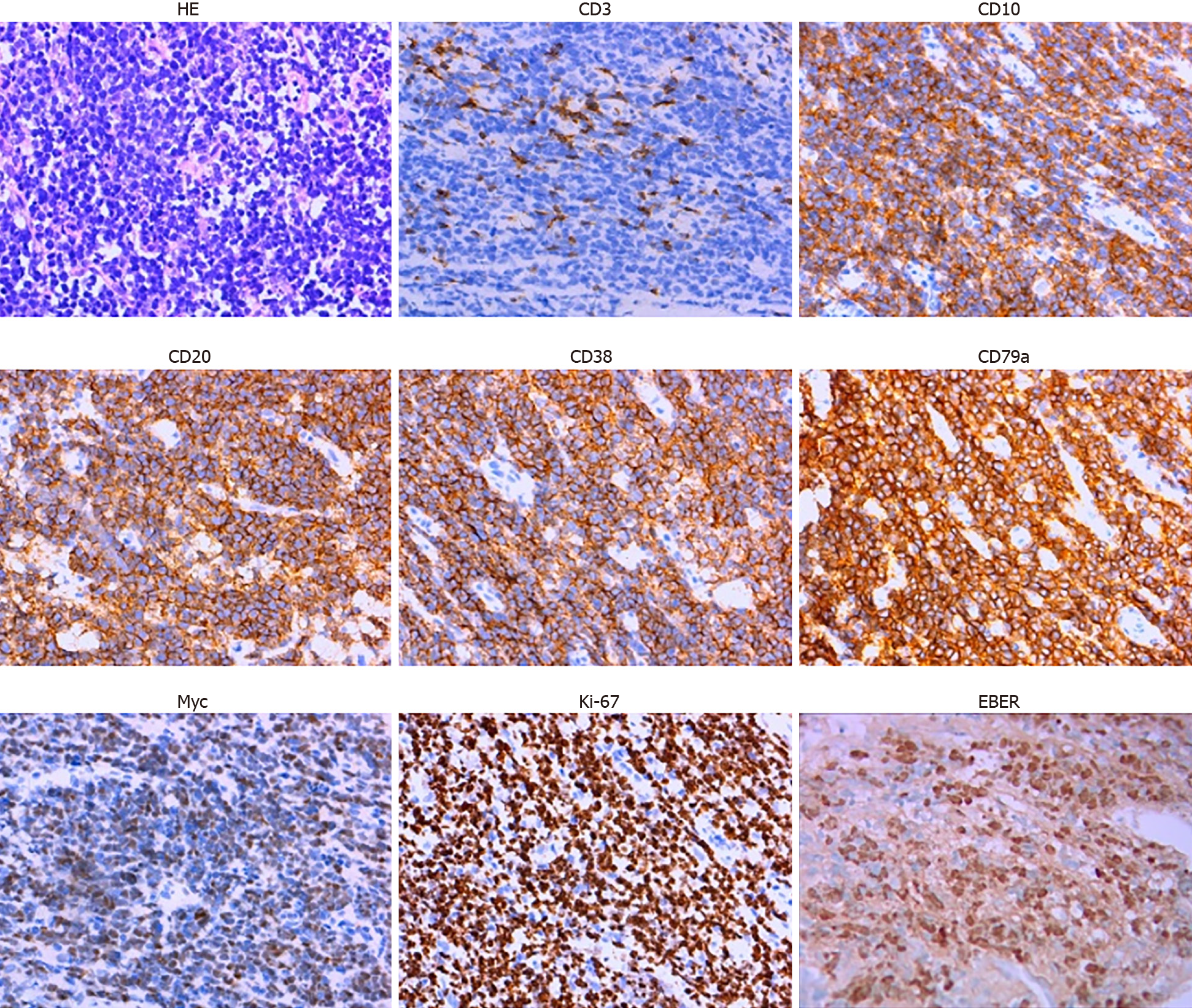
Ultrasound-guided pathological biopsy of the mass on the lower left abdomen showed local necrosis. The immunohistochemistry results were as follows: cluster of differentiation 20 (CD20)+, CD79a+, CD3-, CD43-, CD21+, Ki-67 (90%+), CD10+, B-cell lymphoma 6 (Bcl-6)+, CD38+, terminal deoxynucleotidyl transferase-, CD5-, CD30-, CD23-, Cyclin D1-, Bcl-2-, melanoma ubiquitous mutated protein 1+/-, myeloperoxidase-, CD117-, creatine kinase-, vimentin+, CD56-, CD99-, S-100-, c-Myc (70%+) and EBV encoded small RNAs (EBER)+ (Figure 1).
Quantitative serum EBV was 2.35 × 104 copies/mL. The morphology of bone marrow cells indicated active bone marrow hyperplasia and a lymphocyte ratio of 18.0%, with a ratio of 3.0% for lymphoblasts and prolymphocytes. Immunotyping revealed that lymphocytes accounted for approximately 15% of the karyocytes, with a normal distribution of lymphocyte subsets. The protoregional cells accounted for approximately 1% of the karyocytes, with a scattered distribution. Monocytes accounted for approximately 5.5% of the karyocytes, with a mature phenotype. Granulocytes accounted for about 67.5% of the karyocytes, without abnormal development. Positron emission tomography-computed tomography (PET/CT) showed fluorodeoxyglucose uptake in the nasopharyngeal superior/posterior wall with multiple lymph nodes in the bilateral neck, multiple nodules and masses in the abdominal and pelvic cavities and local thickening of the ascending colon, partial small intestine and rectum. Burkitt lymphoma Stage IV in group A with high risk was diagnosed, complicated with uterine leiomyoma and chronic hepatitis B.
Burkitt lymphoma.
The patient received entecavir to treat the HBV infection. On May 6, 2017, she received a low-dose dexamethasone (10 mg, days 1-5) + cyclophosphamide (0.2 g, days 1-3) regimen for cytoreduction. Two cycles of chemotherapy with the hyper-CVAD (A + B) regimen were performed on May 12, 2017 and July 19, 2017. PET/CT on October 9, 2017 showed complete remission. Next, another cycle of chemotherapy with the hyper-CVAD (A + B) regimen was performed on October 13, 2017.
The PET/CT in January 2018 showed complete remission.
During the following 2.5 years, the patient was admitted to our hospital three times because of interstitial pneumonia, and antifungal treatment was performed. The follow-up PET/CT on April 18, 2019 showed increased fluorodeoxyglucose metabolism in the bilateral nasopharynx walls, bilateral submaxillary, bilateral neck, bilateral axilla and bilateral groin multiple lymphadenectasis, thickened intercostal pleura between the 11th and 12th right ribs and multiple nodules in peritoneal, retroperitoneal and pelvic cavities. Recurrence of lymphoma was suspected. However, the patient refused to be hospitalized for re-examination and treatment.
On July 16, 2020, the patient was readmitted to our hospital due to severe diarrhea episodes for 5 d. The diarrhea was brought under control after oral administration of levofloxacin, but the stools were still sparse (once per day). Physical examination revealed cervical lymphadenectasis. Routine blood examination results were as follows: white blood cells: 8.43 × 109/L, neutrophils: 4.12 × 109/L, hemoglobin: 112 g/L, platelets: 49 × 109/L, EBV polymerase chain reaction: 5.63 × 104 copies/mL, erythrocyte sedimentation rate: 80.0 mm/h and lactate dehydrogenase: 322.4 U/L.
Ultrasound-guided biopsy of the lower left cervical lymph nodes showed CD3(+), granzyme B (+), CD8 (+), CD4 (-), CD20 (-), CD19 (-), CD79a (-), Bcl-2 (-), Bcl-6 (-), CD10 (-), CD21 (-), Cyclin D1 (-), anaplastic lymphoma receptor tyrosine kinase (-), CD30 (-), creatine kinase (-), CD56 (-), creatine kinase 5/6 (-), SRY-related HMG-box-11 (-), c-Myc (+, approximately 20%), melanoma ubiquitous mutated protein 1 (+, approximately 50%), Ki-67 (+, approximately 70%), CD5 (partially +) and EBER (+). T-cell lymphoma was suspected.
Colonoscopy results showed a submucosal eminence of approximately 1.0 cm × 1.0 cm with a smooth surface in the cecum. Further biopsy results showed this submucosal eminence was consistent with lymphoma in structure and consistent with the cervical lymph nodes in terms of classification.
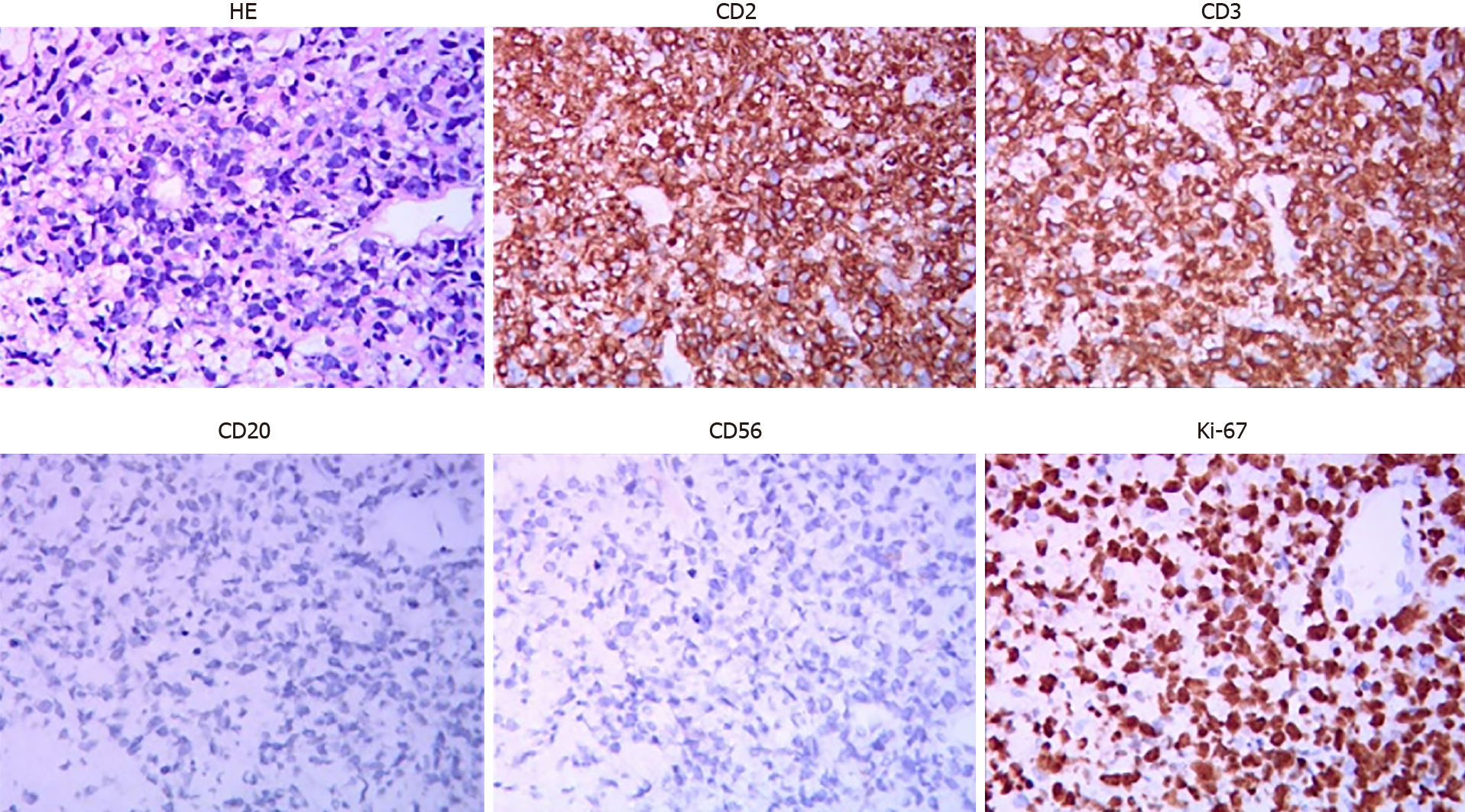
Another ultrasound-guided biopsy of the left cervical lymph nodes on July 31, 2020 showed CD2 (+), CD3 (+), CD8 (-), CD56 (-), Ki67 (70%), T-cell intracytoplasmic antigen-1 (+), granzyme B (+), P53 (+, wild type), c-Myc (10%+), CD20 (-), CD7 (+), programmed cell death protein 1 (40%+), programmed death-ligand 1 (interstitial cell +) and EBER (-) (Figure 2). PTCL-NOS was diagnosed. Moreover, TCR β rearrangement was positive in PTCL-NOS.
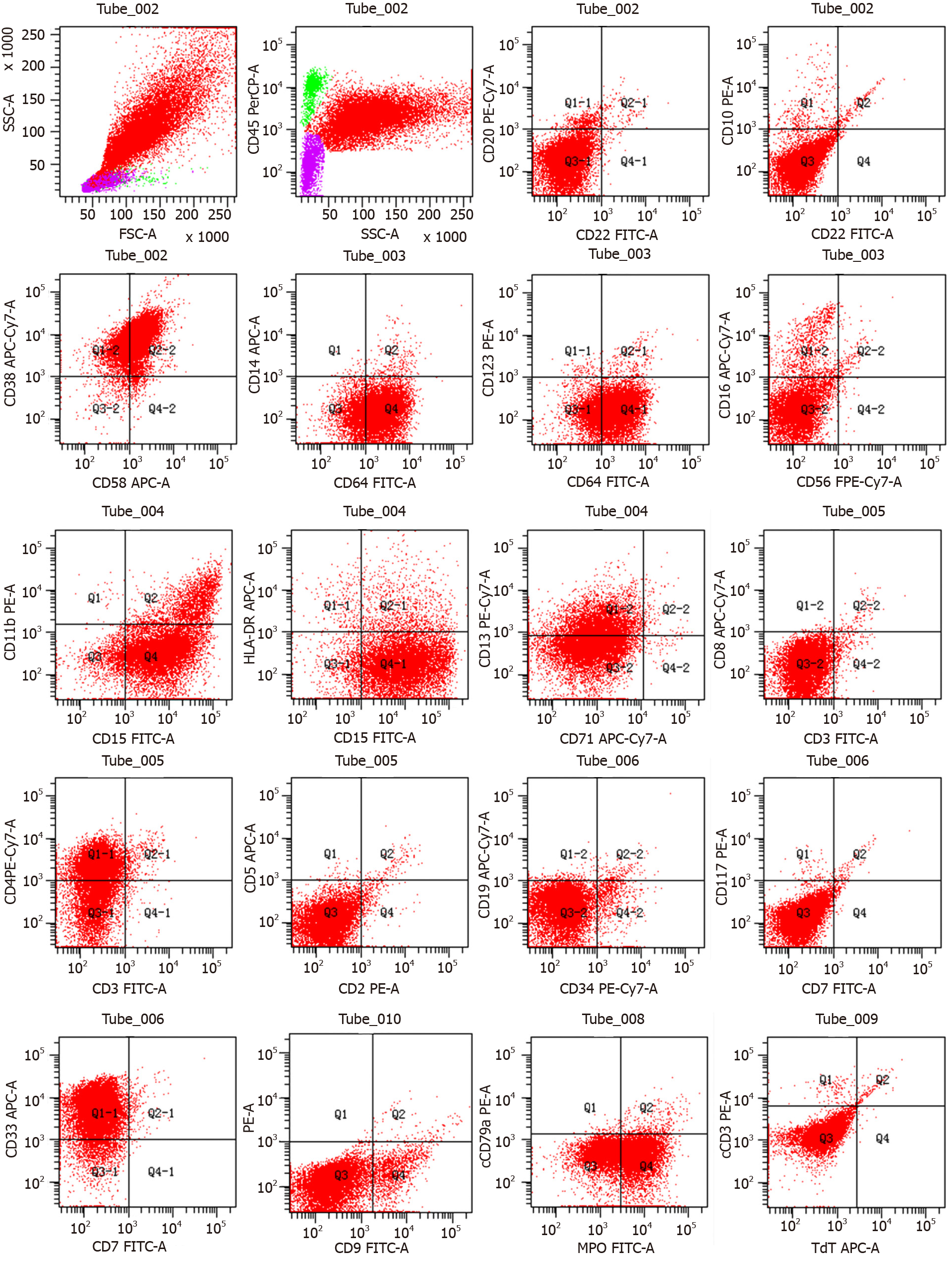
Considering the patient’s poor condition and gastrointestinal tumor invasion, COP (cyclophosphamide, vincristine and methylprednisolone) was administered on August 17, 2020 for cytoreduction. Bone marrow morphology examination on September 4, 2020 showed an abnormal cell colony in the region where myeloid cells extended to the protocells. The colony accounted for approximately 80% of the karyocytes, expressing CD4, CD13, CD15, CD33, CD38, CD58, CD64 and myeloperoxidase and partially expressing CD11b (Figure 3). Lymphoid proliferation was inhibited. AML was diagnosed.
Considering her poor health condition, the patient was scheduled to receive decitabine (25 mg, days 1-5) and venetoclax (100 mg, once daily on days 1-7 and 200 mg on days 8-28) treatment supplemented with symptomatic and supportive treatments starting from October 2, 2020. However, she developed gastrointestinal bleeding, peptic ulcers and hemorrhagic shock on the second day of treatment, and the chemotherapy was suspended. Although many symptomatic and supportive treatments were given, the patient developed diarrhea in the afternoon of October 18, and her blood pressure dropped. She was critically ill, but her family members refused any further appropriate treatments, such as tracheal intubation. She was then discharged from the hospital.
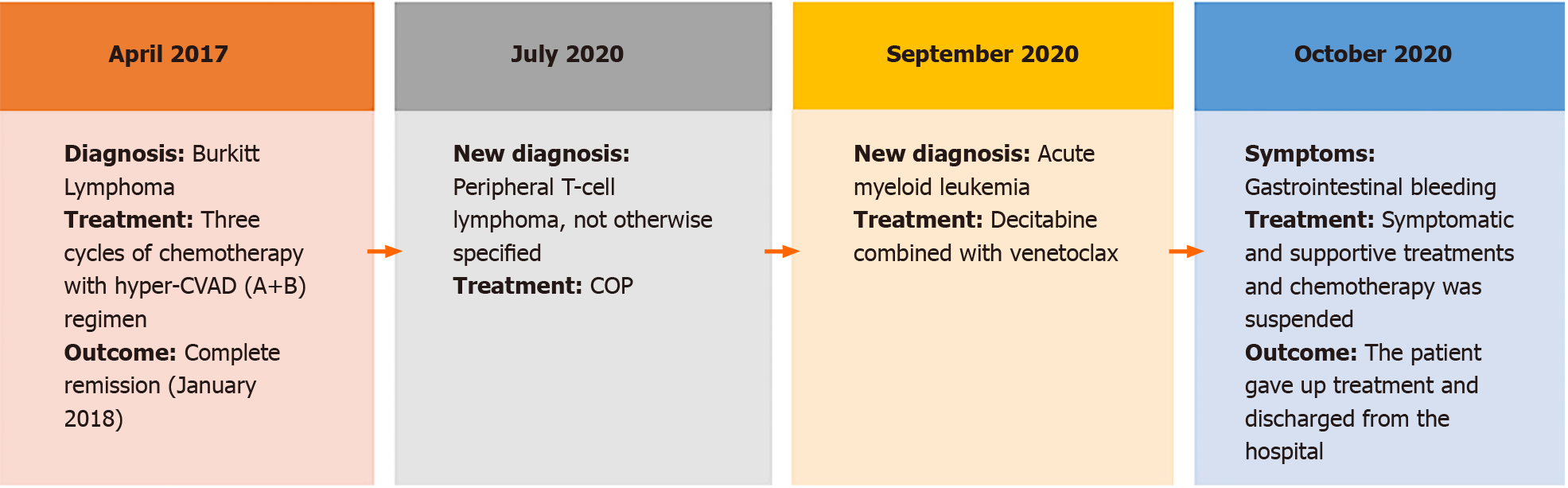
The clinical course of the present case was summarized in Figure 4.
MPC was first recognized and reported by Owen[5] in 1921 and later by other researchers[6,7]. The frequency of MPC is reported to be in the range of 2%-17%[1], and the reported frequency in China is only 0.84% to 1.31%[8-10]. With the progress in early cancer diagnosis and treatment, patient survival time with malignant tumors has been prolonged, leading to an increased frequency of MPC. MPCs always occur as solid cancers. Although MPC associated with lymphoma and leukemia has been reported[11], the present case is the first report on secondary peripheral T-cell lymphoma and AML after the treatment of Burkitt lymphoma. Two years after treatment for Burkitt lymphoma, lymphadenectasis and gastrointestinal neoplasm occurred, which could have been easily misdiagnosed as metastatic or recurrent primary cancer. Currently, pathological biopsy is still the formal diagnosis method for tumors. Therefore, MPC diagnosis should be based on repeated multisite biopsies and should be conformed after multicenter consultation.
The mechanism for MPC remains unclear. Relevant factors may be roughly divided into endogenous factors (low immune function of patients with tumors), exogenous factors (such as environmental reasons and exposure to radiation and chemicals), genetic factors (such as family genetic defects and continuous action of carcinogenic factors) and factors related to appropriate therapy (such as the carcinogenic effects of radiation and chemotherapy)[1,12,13]. In this case, the patient developed PTCL-NOS together with AML approximately 2 years after being treated for Burkitt lymphoma. Although they were all hematological malignancies, the initial B-cell lymphoma and subsequent PTCL-NOS and AML had different sources of malignant cells. In this case, both heterochronous and synchronal MPCs occurred. The former is related to DNA strand breaks, gene mutations and chromosome aberrations caused by multiple chemotherapies, while the latter is scarce. The patient’s constitution may have contributed to this. Next-generation sequencing can help understand if tumors originate from different clones, thus guiding appropriate treatment. However, the patient refused further diagnosis and treatment due to economic reasons. Moreover, both Burkitt lymphoma and PTCL-NOS in the presented case had EBER, indicating common B/T progenitors may have EBV in this case. However, we could not perform Southern blotting analysis at our center to confirm this hypothesis.
The prognosis for MPC is poor, and there is still no consensus on the appropriate treatment. Currently, a patient-centered multidisciplinary comprehensive treatment model is recommended[14]. For patients with solid tumors, all lesions should be completely removed either simultaneously or successively. If a lesion cannot be excised, comprehensive therapies such as immunotherapy, targeted therapy and chemotherapy can be employed. However, the accumulation of drug toxicity or radiation doses in the same area should be monitored[15]. Burkitt lymphoma in this patient was in complete remission after the initial treatment until January 2018 when lymphadenectasis reoccurred, and PTCL-NOS complicated with AML newly occurred. For further diagnosis and treatment, chemotherapy is considered the primary choice. The patient was unable to withstand intense chemotherapy owing to her weak physical condition. Consequently, optimal supportive treatment was considered. However, she did not continue the treatment due to economic limitations.
In conclusion, we report a case of secondary PTCL-NOS complicated with AML and Burkitt lymphoma, with both heterochronous and synchronal MPCs. When a new tumor is diagnosed, its relationship to the old tumor treatment should be determined. If the economic condition allows, second-generation sequencing should be performed to identify the origin of tumors.
We thank Pro. Lin L from the Department of Hematology, Hainan Provincial People’s Hospital Hainan Affiliated Hospital of Hainan Medical University for guiding the organization and revising medical records, Pro. Fu XJ from the Department of Hematology, Hainan Provincial People’s Hospital Hainan Affiliated Hospital of Hainan Medical University for providing relevant medical information for the case summary and Dr. Liu D, Dr. Meng C and Dr. Lin XY for their help in medical writing.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Intagliata E, Yoshida N S-Editor: Gao CC L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Vogt A, Schmid S, Heinimann K, Frick H, Herrmann C, Cerny T, Omlin A. Multiple primary tumours: challenges and approaches, a review. ESMO Open. 2017;2:e000172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 369] [Article Influence: 46.1] [Reference Citation Analysis (1)] |
| 2. | Zhang ZG, Chen Y, Ji R, Zhao YJ, Wang J, Robinson L, Chen XP, Zhang L. Synchronous cancers of gallbladder carcinoma and combined hepatocellular cholangiocarcinoma: an unusual case and literature review. BMC Cancer. 2018;18:1046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Nezu K, Okubo T, Shiraiwa M, Nozawa Y, Kyan A. Systemic treatment for coexisting mucinous urethral adenocarcinoma and prostate adenocarcinoma. IJU Case Rep. 2020;3:261-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Xu Y, Chen QN, Wang H, Liu NB, Shi BM. Synchronous hepatocellular carcinoma and gallbladder adenocarcinoma with neuroendocrine differentiation: a case report and literature review. BMC Surg. 2020;20:246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Hanlon FR. Multiple Primary Carcinomas. Am J Cancer. 1931;15:2001. |
| 7. | Warren S. Multiple primary malignant tumors. A survey of the literature and a statistical study. Am J Cancer. 1932;16:1358-1414. |
| 8. | Liu Z, Liu C, Guo W, Li S, Bai O. Clinical analysis of 152 cases of multiple primary malignant tumors in 15,398 patients with malignant tumors. PLoS One. 2015;10:e0125754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Lv M, Zhang X, Shen Y, Wang F, Yang J, Wang B, Chen Z, Li P, Li S. Clinical analysis and prognosis of synchronous and metachronous multiple primary malignant tumors. Medicine (Baltimore). 2017;96:e6799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 10. | Jiao F, Yao LJ, Zhou J, Hu H, Wang LW. Clinical features of multiple primary malignancies: a retrospective analysis of 72 Chinese patients. Asian Pac J Cancer Prev. 2014;15:331-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Wu RJ, Zheng RJ, Huang YQ, Ma XD. [Chidamide combined with chemotherapy for treatment of therapy-related acute myeloid leukemia secondary to peripheral T-cell lymphoma: a case report and literatures review]. Zhonghua Xueye Xue Zazhi. 2019;40:685-687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Wood ME, Vogel V, Ng A, Foxhall L, Goodwin P, Travis LB. Second malignant neoplasms: assessment and strategies for risk reduction. J Clin Oncol. 2012;30:3734-3745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 232] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 13. | Copur MS, Manapuram S. Multiple Primary Tumors Over a Lifetime. Oncology (Williston Park). 2019;33. [PubMed] |
| 14. | Digestive tract Oncology Professional Committee of Chinese Association of Research Hospitals; Multidisciplinary Comprehensive Treatment Committee SB; Chinese Medical Doctors Association. Expert consensus on the diagnosis and treatment model of the multidisciplinary treatment collaboration group for gastric cancer. Zhongguo Shiyong Waike Zazhi. 2017;37:37-38. |
| 15. | Kumar S. Second malignant neoplasms following radiotherapy. Int J Environ Res Public Health. 2012;9:4744-4759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |