Published online Aug 26, 2021. doi: 10.12998/wjcc.v9.i24.7189
Peer-review started: February 1, 2021
First decision: March 7, 2021
Revised: April 6, 2021
Accepted: May 18, 2021
Article in press: May 18, 2021
Published online: August 26, 2021
Processing time: 203 Days and 11 Hours
Targeted therapy based on pathway analysis of hepatitis B-related hepatocellular carcinoma (HCC) may be a promising remedy.
The present case involved an advanced hepatocellular carcinoma (HCC) patient who did not receive local regional therapy and was intolerant to sorafenib. Total RNA extracted from the patient’s tumor tissue was used to obtain the gene mutation profile. The c.3676A>T and c.4402A>T stop-gain mutations in adeno
Exclusive mutations of APC of all the Wnt pathway elements could be a therapeutic target in HCC, with aspirin as an effective treatment option.
Core Tip: Hepatocellular carcinoma (HCC) is a highly heterogeneous disease. Due to the differences in etiology and ethnicities, the driving genes in HCC are likely to be different globally. Adenomatous polyposis coli (APC) mutations are critical in a fraction of HCC patients, as APC mutations might trigger HCC by activating the Wnt pathway. The effects of this mutation could be consistently suppressed by aspirin. Thus, APC mutation-triggered HCC might be a new subgroup of chronic hepatitis B virus infection-related HCC. Wnt pathway inhibition could be an effective remedy for this subgroup of patients.
- Citation: Lin Q, Bai MJ, Wang HF, Wu XY, Huang MS, Li X. Aspirin-induced long-term tumor remission in hepatocellular carcinoma with adenomatous polyposis coli stop-gain mutation: A case report. World J Clin Cases 2021; 9(24): 7189-7195
- URL: https://www.wjgnet.com/2307-8960/full/v9/i24/7189.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i24.7189
Some small molecular agents with multiple targets, including sorafenib, lenvatinib, and regorafenib, are reported to be effective against hepatocellular carcinoma (HCC)[1]. However, the efficacy of these agents is unpredictable. As the average overall survival of patients with advanced HCC is only approximately three months[2], primary resistance to first-line treatment may prevent the possibility of second-line remedies. Thus, precise therapy is needed for the treatment of advanced HCC.
In this era of precise therapy, treatments can be administered with maximum efficacy if the target is clearly identified. For the multi-targeted agents used for HCC, unsatisfactory safety profiles and limited improvement in overall survival have hindered their use in the treatment of advanced HCC. RNA sequencing can be used to identify the causative gene mutations in tumor tissues to provide more accurate targets for the treatment of HCC.
In this case of advanced HCC, we screened for mutations in an HCC patient using the Illumina Hiseq2000 platform and identified mutated adenomatous polyposis coli (APC) as the major driver gene. Aspirin was chosen as the targeted agent to treat this patient, which achieved disease control for at least four years.
Recurrent HCC with APC stop-gain mutation.
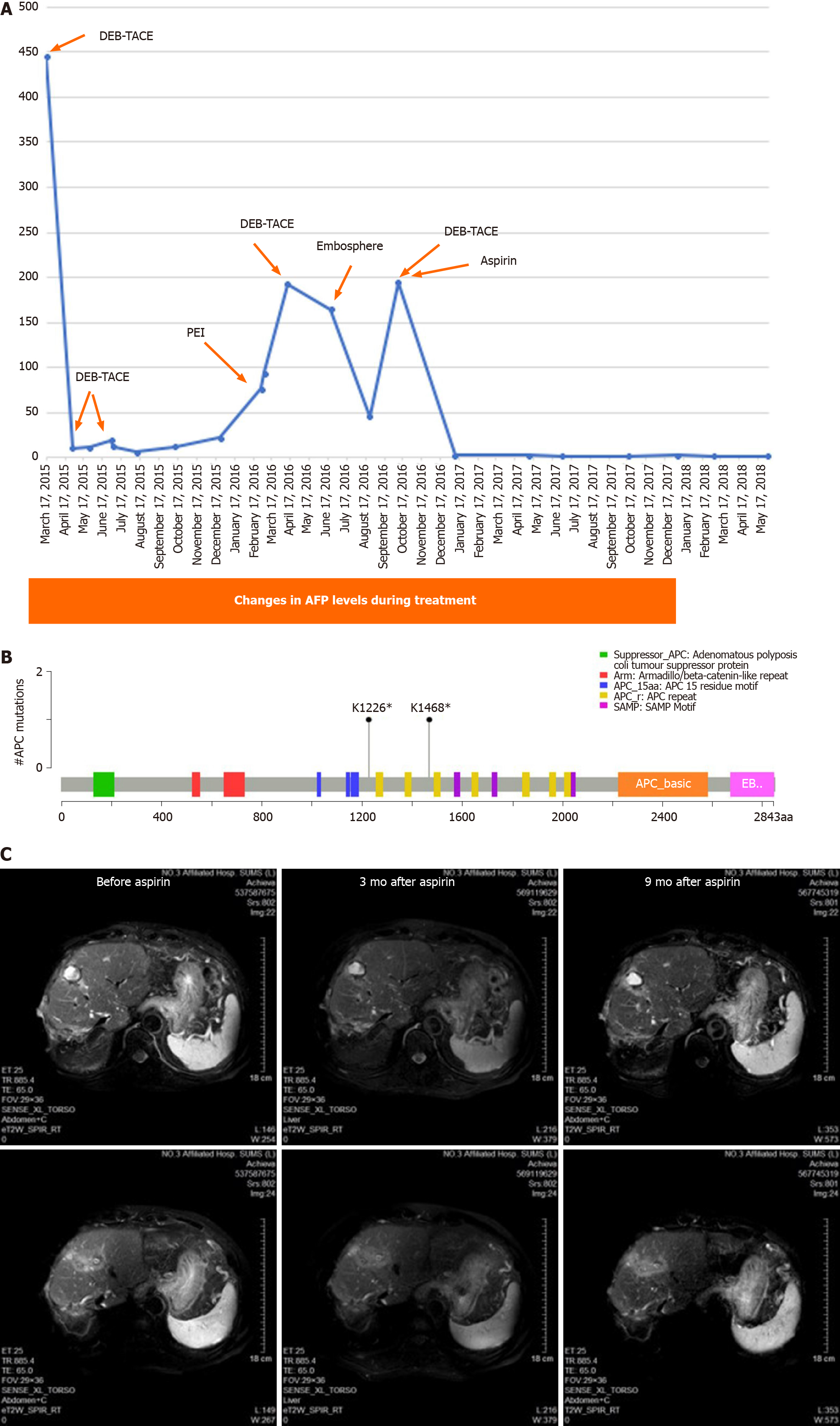
A Chinese male patient with chronic hepatitis B infection was admitted to the Cancer Center of Sun Yat-sen University for the treatment of HCC identified in the S6 segment of the liver by contrast computed tomography (CT) scan in June 2012. Radical resection was performed. Tumor recurrence was observed in March 2014. Transcatheter arterial chemoembolization (TACE) was performed followed by radiofrequency ablation with radical intent. However, the tumor recurred at the juncture of the S2 and S3 sections in September 2014. The tumor was resected. The tumor recurred in February 2015. A series of TACE procedures were performed as palliative local regional therapy from February 2015 to October 2016 at the Third Affiliated Hospital of Sun Yat-sen University. Sorafenib was administered after the first cycle of palliative TACE, but the treatment was suspended one month later due to grade III diarrhea. In that period, tumor progression was noted three times, which made local regional therapy an inappropriate choice for disease control. Alteration of alpha-fetoprotein indicated the efficacy of each therapy (Figure 1A).
Untreated chronic hepatitis B.
None.
Normal.
RNA sequence-based precise therapy was also considered. RNA sequencing was conducted on recurrent tumor tissue from a needle core biopsy in April 2016 to identify the gene mutation(s). Sequencing revealed that APC mutations are the major driver mutations. Stop-gain c.3676A>T and c.4402A>T APC mutations were the most prevalent (42.2% and 35.1%, respectively). ABL1 missense mutations, TP53 stop-gain, and TNFAIP3 missense mutations were less frequent (Table 1).
| Gene | Mutation type | Mutation ratio (%) | Nucleotide change | Amino acid change | Chromosome | Genbank transcript ID |
| ABL 1 | Missense | 30.2 | c.1363G>C | p.Asp455His | 9 | NM_005157 |
| APC | Stop-gain | 42.2 | c.3676A>T | p.Lys1226X | 5 | NM_000038 |
| APC | Stop-gain | 35.1 | c.4402A>T | p.Lys1468X | 5 | NM_000038 |
| TP53 | Stop-gain | 27.7 | c.193A>T | p.Arg65X | 17 | NM_000546 |
| TNFAIP3 | Missense | 5.5 | c.1787G>A | p.Arg596Gln | 6 | NM_001270507 |
| Gene | Mutation type | Folds | Starting position | Ending position | Chromosome | Genbank transcript ID |
| MDM4 | Amplification | 1.9 | 204485510 | 204527248 | 1 | N/A |
The amino acid sequences of APC mutations indicated a strongly truncated APC protein (Supplementary data). MutationMapper analysis indicated that the functional domain of APC was lost in both mutants in HCC tissue (Figure 1B).
Recurrent HCC was revealed by CT.
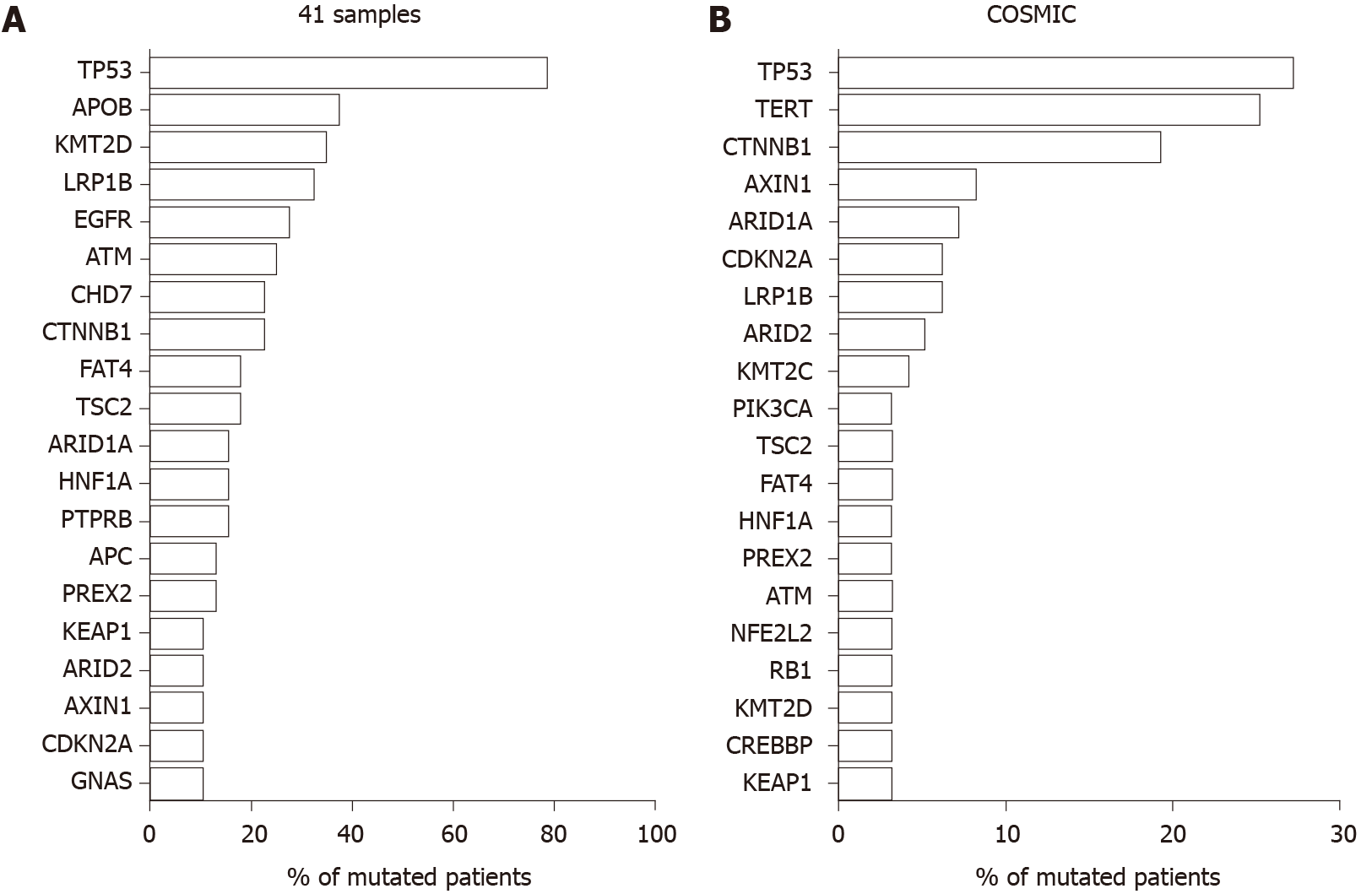
Gene mutations were screened from HCC tissue samples from four patients and circulating tumor DNA samples from 41 HCC patients at The Third Affiliated Hospital of Sun Yat-sen University between February 2016 to January 2018 using the Illumina Hiseq2000 platform. Their gene mutation profiles were compared with those of the global HCC population from the Catalog of Somatic Mutations in Cancer (COSMIC) database. For patients with APC mutations, the amino acid sequences of APC mutations were evaluated using the Mutation Taster (http://www.mutation taster.org/) program. Using amino acid sequences generated by Mutation Taster, MutationMapper[3] was used to analyze the functional defects of the APC mutants.
The gene mutation profile of Chinese patients with HCC was different to that of the global HCC population from the COSMIC database. According to our RNA sequence analysis of tumor tissues (n = 4) and circulating tumor DNA samples (n = 41) from hepatitis B-related HCC patients, the most prevalent mutated genes were TP53, APOB, KMT2D, LRP1B, EGFR, ATM, CHD7, CTNNB1, ARID1A, TSC2, FAT4, HNF1A, PTPRB, and APC (Figure 2A). This profile differed from the COSMIC global data (Figure 2B). Among the top 20 most frequent mutation genes, APOB, EGFR, ATM, CHD7, PTPRB, APC, and GNAS mutations were much more frequent among Chinese patients with HCC. Thus, these mutations may be potential targets for precise therapy.
APC is a major suppressive regulator of the Wnt pathway[4]. In this pathway, APC and glycogen synthase kinase 3 beta (GSK3β) combined with Wnt pathway key regulator β-catenin induces β-catenin phosphorylation and degradation to avoid Wnt pathway over-activation. The Wnt pathway can be activated by multiple causes in HCC, including mutations in Axin1, Axin2, and β-catenin[5]. However, mutations in APC and GSK3β are rare[6]. In the present study, APC was the only mutated gene in the Wnt pathway, which was presumed to be the exclusive cause of Wnt pathway over-activation. Due to the potentially normal function of other elements of the Wnt pathway, decreased β-catenin phosphorylation was the only trigger for this pathway. Thus, acceleration of β-catenin phosphorylation might abrogate the Wnt pathway, implicating GSK3β as a potential target.
Recurrent HCC with APC stop-gain mutation.
Aspirin is reported to be effective in inducing β-catenin phosphorylation by activating GSK3β due to inhibition of the cyclooxygenase 2 (COX2) pathway[7]. As the COX2 pathway was assumed to act normally, high-dose aspirin (0.3 g/day) was chosen as a remedy from April 2016.
This strategy achieved disease control for almost 5 years until February 2021, as confirmed by magnetic resonance imaging and monitoring of alpha fetoprotein. The treatment was well tolerated (Figure 1A and C).
HCC is a highly heterogeneous disease[8]. The etiology of HCC in Chinese individuals is mainly chronic hepatitis B virus infection, which is quite different to that in European patients, for whom HCC is caused mainly by alcohol and chronic hepatitis C virus infection[2]. Due to the differences in etiology and ethnicities, the driving genes in HCC must be different globally. According to our data, APC mutations were among the top 20 most frequent mutations. However, in the COSMIC database, APC mutations are very rare, and few APC mutations related to HCC have been reported[6]. In the present case, we found an APC mutation that triggered HCC. The effects of this mutation could be consistently suppressed by aspirin. Thus, APC mutation-triggered HCC might be a new subgroup of chronic hepatitis B virus infection-related HCC. Wnt pathway inhibition could be an effective remedy for this subgroup of patients.
The Wnt pathway has been reported to be very important in the development of malignancies[9]. Approximately 20% of HCC patients reportedly display Wnt pathway activation[5]. The causes of Wnt pathway over-activation are complex. The importance of Wnt over-activation in the development of HCC could differ from one patient to another, which might not be evident in a large cohort study. Thus, the Wnt inhibitor failed to show reliable efficacy among non-selected HCC patients. In the present case, an APC mutation triggered HCC and the Wnt pathway was exclusively activated by APC dysfunction, as other elements of the Wnt pathway were not mutated. Knowledge of the Wnt pathway and its association with the COX2 pathway has led to the use of COX2 inhibitors[7], including the COX inhibitor aspirin and the selective COX2 inhibitors celecoxib and meloxicam, which are clinically available. In the present case, we chose aspirin and achieved long-term disease control.
The diagnosis of HCC does not rely on a pathologic test[2], which decreases the availability of RNA sequences in tumor tissues. The lack of tumor tissue gene mutation information, especially for advanced and recurrent diseases, has limited the extensive application of precisely designed targeted therapy based on dominant driver genes. Thus, needle biopsy might be of potential benefit for advanced and recurrent HCC. The present case demonstrates that RNA sequencing of HCC tissues might be a valuable approach and that the current HCC diagnostic procedure without histological tests might be insufficient for precise targeted therapy.
In the present case, the success of aspirin treatment was based on the dominant genetically driven Wnt pathway and exclusive mutation of APC among all the Wnt pathway elements, as discovered by RNA sequencing.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Di Pasqua LG, lee Y S-Editor: Wang JL L-Editor: Webster JR P-Editor: Liu JH
| 1. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3017] [Article Influence: 431.0] [Reference Citation Analysis (3)] |
| 2. | Li X, Dong M, Lin Q, Chen ZH, Ma XK, Xing YF, Wan XB, Wen JY, Wei L, Chen J, Wu XY. Comparison of current staging systems for advanced hepatocellular carcinoma not amendable to locoregional therapy as inclusion criteria for clinical trials. Asia Pac J Clin Oncol. 2013;9:86-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Vohra S, Biggin PC. Mutationmapper: a tool to aid the mapping of protein mutation data. PLoS One. 2013;8:e71711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653:1-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 639] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 5. | Takigawa Y, Brown AM. Wnt signaling in liver cancer. Curr Drug Targets. 2008;9:1013-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 6. | Chen TC, Hsieh LL, Ng KF, Jeng LB, Chen MF. Absence of APC gene mutation in the mutation cluster region in hepatocellular carcinoma. Cancer Lett. 1998;134:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Gala MK, Chan AT. Molecular pathways: aspirin and Wnt signaling-a molecularly targeted approach to cancer prevention and treatment. Clin Cancer Res. 2015;21:1543-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Hammoud GM, Ibdah JA. Are we getting closer to understanding intratumor heterogeneity in hepatocellular carcinoma? Hepatobiliary Surg Nutr. 2016;5:188-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 9. | Barker N, Clevers H. Mining the Wnt pathway for cancer therapeutics. Nat Rev Drug Discov. 2006;5:997-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 572] [Article Influence: 31.8] [Reference Citation Analysis (0)] |