Published online Aug 26, 2021. doi: 10.12998/wjcc.v9.i24.7175
Peer-review started: February 23, 2021
First decision: May 11, 2021
Revised: May 19, 2021
Accepted: July 7, 2021
Article in press: July 7, 2021
Published online: August 26, 2021
Processing time: 181 Days and 12.9 Hours
Geleophysic dysplasia (GD) presents the characterized clinical manifestations of acromelic dysplasia, including extremely short stature, short limbs, small hands and feet, stubby fingers and toes, joint stiffness and others. It is clinically distinct from the other acromelic dysplasia in terms of symptoms such as cardiac valvular abnormalities, progressive hepatomegaly and tracheal stenosis.
We report on a Chinese 9-year-old girl with GD with the c.5243G>T (p.C1748F) mutation in FBN1 (fibrillin 1, OMIM 134797). She was born in Guangxi Zhuang Autonomous Region of China. The patient presented with typical clinical features of GD and recurrent respiratory tract infections over 6 years. Laboratory studies and chest computed tomography (CT) scan indicated bronchopneumonia. Her echocardiography revealed mild mitral valve thickening with regurgitation. La
GD is a rare genetic condition that can cause life-threatening cardiovascular and respiratory problems. This study also found that the identified genotype of GD could be related to different clinical phenotypes.
Core Tip: We aim to report a 9-year-old girl with geleophysic dysplasia (GD) with mutation c.5243G>T (p.C1748F) in FBN1. Other than the patient we reported, a total of 9 acromelic dysplasia cases due to mutations in c.5242T, c.5243G or c.5244T of FBN1 have been reported, which all are predicted to result in the substitution of cysteine at codon 1748. This study also found that the identified genotype of GD could be related to different clinical phenotypes.
- Citation: Tao Y, Wei Q, Chen X, Nong GM. Geleophysic dysplasia caused by a mutation in FBN1: A case report. World J Clin Cases 2021; 9(24): 7175-7180
- URL: https://www.wjgnet.com/2307-8960/full/v9/i24/7175.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i24.7175
Geleophysic dysplasia (GD) belongs to acromelic dysplasia and has unique features[1]. GD is clinically distinct from other acromelic dysplasia in its symptoms such as cardiac valvular abnormalities, progressive hepatomegaly and tracheal stenosis[1] . Three genes, ADAMTSL2 (OMIM 612277), FBN1 and LTBP2 (OMIM 602091), have been associated with GD[2-4]. Nearly all the mutations in FBN1 associated with GD are located in exons 41 and 42[5]. FBN1, located at 15q21, encodes an extracellular matrix protein that forms a major component of microfibrils of the extracellular matrix in connective tissues[6]. The most common inherited disease caused by FBN1 mu
A 9-year-old Chinese girl presented with labored breathing, cough with wheeze and nasal discharge.
The patient had no intellectual development disorder, hepatomegaly or hand joint stiffness.
The patient had a history of hypothyroidism, short stature, obstructive sleep apnea hypopnea syndrome and recurrent respiratory tract infections (RRTIs).
The patient was born in Guangxi Zhuang Autonomous Region of China and had no special personal or family history.
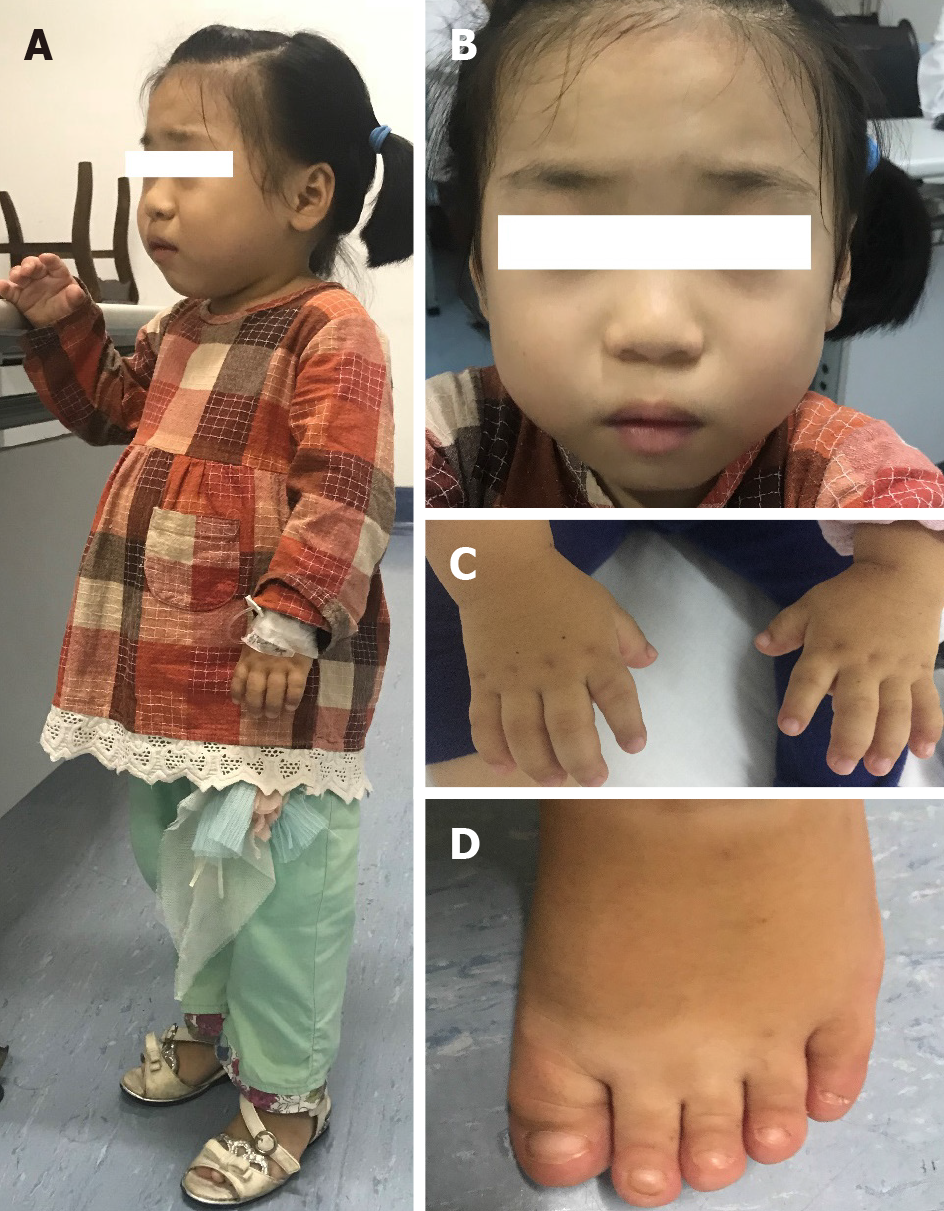
The patient had extremely short stature (94 cm, < -3 SD) (Figure 1A), low weight (17 kg, < -3 SD)[9], unique facial features (round face, small nose with anteverted nostrils, broad and depressed nasal bridge and thin upper lip) (Figure 1B), short limbs, and short hands and feet (Figure 1C and D).
Laboratory tests suggested inflammation.
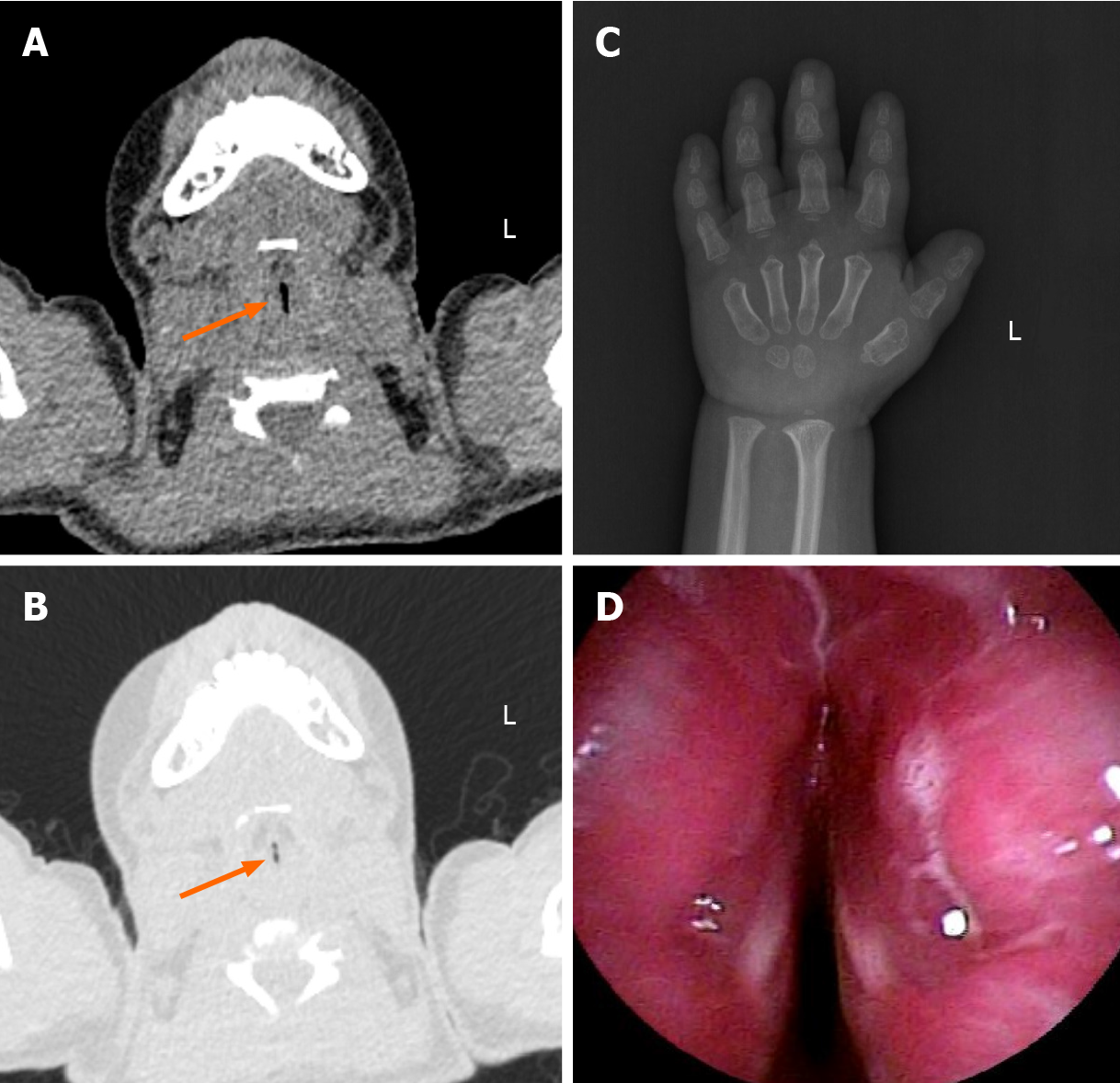
Her chest computed tomography (CT) scan indicated bronchopneumonia. Laryngopharyngeal CT revealed tracheal stenosis (Figure 2A and B).
GD.
The patient presented an oxygen saturation of 96% with a nasal catheter giving 0.5-1 L/min oxygen inhalation. She received human immunoglobulin (50 mL: 2.5 g [5%]) intravenously on the second and the last day of admission. Isopropyl compound ipratropium bromide solution (inhalation of Combivent aerosol budesonide) 1.25 mL + (Pulmicort) 200 μg + normal saline 1 mL atomization inhalation were continued daily during the hospital stay. The patient received 500 mg/d ceftriaxone sodium for injection until day 11, when the symptoms of the infection were resolved. For an
When discharged, patient’s symptoms of RRTIs had improved by using methylprednisolone 20 mg/d orally. Patient returned to the outpatient department regularly.
GD, acromicric dysplasia (AD) and Weill-Marchesani syndrome (WMS) are kinds of acromelic dysplasia[1], but these three disorders have their own unique features. GD is clinically distinct from AD and WMS in its symptoms such as cardiac valvular abnormalities, progressive hepatomegaly and tracheal stenosis. Ordinarily, conditions that resemble GD but without cardiac valvular abnormality are diagnosed as AD, and those accompanied by distinguishing eye anomalies, including lenticular myopia, ectopia lentis, glaucoma and spherophakia, are diagnosed as WMS[1].
Like the patients who have been reported to have FBN1-related diseases, the present patient also displayed an autosomal dominant inheritance pattern and had a mutation in the TB5 domain of FBN1. The inheritance method of FBN1 shows autosomal domi
We reviewed the literature of reported acromelic dysplasia cases, including GD, AD and WMS, due to mutations at c.5242T, c.5243G and c.5244T of FBN1, which all are predicted to result in the substitution of cysteine at codon 1748. Other than the patient we reported, a total of 9 patients were found, including one family with 7 patients. Patient 1[2] had the heterozygous mutation c.5243G>C (p.Cys1748Ser). Patient 2[1], like the girl we reported, had the same heterozygous mutation c.5243G>T (p.C1748F). Patient 3[11], the proband of the family, had the heterozygous mutation c.5242T>C (p.C1748R). There was no mutation reported at position c.5244T. All the patients had progressive growth delays from an early age and presented dysmorphic features such as short stature, short limbs and stubby fingers and toes. No severe abnormities were mentioned in either the mitral or tricuspid valve. However, Patient 3[11] developed a life-threatening subacute aortic dissection extending from the aortic root to the left subclavian artery of the thoracic aortic arch. Severe tracheal stenosis developed in our patient and Patient 1[2]. Thyroid hypofunction was found in both our patient and Patient 2[1]. It is worth noting that Patient 2, who had the same mutation as our patient, was diagnosed with WWS and had small, round lenses and moderate myopia but did not develop tracheal stenosis (Table 1).
| Our patient | Patient 1[2] | Patient 2[1] | Family[11] | ||||
| Patient 3 (proband) | Proband’s father | Proband’s sister | Proband’s children | ||||
| Mutation | c.5243G>T (p.C1748F) | c.5242T>C (p.Cys1748Ser) | c.5243G>T (p.C1748F) | c.5242T>C (p.C1748R) | |||
| Disorder | GD | GD | WMS | WMS | |||
| Short stature | + | + | + | + | + | + | + |
| Short limbs | + | + | + | + | + | + | + |
| Stubby fingers and toes | + | + | + | + | + | + | + |
| Mitral valve thickening and regurgitation | Mild | - | NA | - | NA | NA | NA |
| Aortic dissection | - | - | - | + | - | - | - |
| Tracheal stenosis | + | + | - | - | - | - | - |
| Myopia | - | + | + | + | + | + | NA |
| Ectopia lentis | - | + | + | + | + | + | NA |
| Thyroid hypofunction | + | - | + | NA | NA | NA | NA |
| Hepatosplenomegaly | - | - | - | NA | NA | NA | NA |
The present patient was diagnosed with GD due to the presence of mitral valve abnormalities and tracheal stenosis. However, Patient 2[1], with the same mutation as our patient, was diagnosed with WMS and lacked either mitral valve abnormalities or tracheal stenosis. These findings demonstrate that an identified genotype can be related to different clinical phenotypes. In addition to genotypes, environmental factors also play an important role in phenotype development. In this study, both the patient we reported and Patient 1[2] developed persistent tracheal stenosis with age. It is noteworthy that both of them had a history of RRTIs at an early age before having developed persistent tracheal stenosis. However, none of the same conditions were mentioned in Patient 2[1]. RRTIs might play an important role in tracheal stenosis development at an early age.
Progressive cardiac valvular abnormality is a common cause of death in patients with GD[12,13]. In this study, no severe abnormality was found in the mitral or tricuspid valve. However, Patient 3 developed a life-threatening subacute aortic dissection extending from the aortic root to the left subclavian artery of the thoracic aortic arch, which might be caused by aortic valve abnormalities. Moreover, severe tracheal stenosis developed in our patient and Patient 1, the latter of whom needed tracheostomy permanently. This demonstrated that respiratory problems, especially tracheal stenosis, might also be the cause of death in patients with GD. We should pay attention to cardiovascular and respiratory problems in patients with GD to prevent a severe, even life-threatening, event from occurring and to treat complications as early as possible.
Other than Patient 2[1], none of the patients with acromelic dysplasia were reported to present thyroid hypofunction. Our patient had mild thyroid hypofunction without impaired intelligence. The patient took Euthyrox orally and regularly, but her growth delay showed no improvement. This result indicates that thyroid hypofunction is one of the accompanying manifestations, rather than a causative factor, of GD. Intere
GD is a rare genetic condition that can cause life-threatening cardiovascular and respiratory problems. This study also found that the identified genotype of GD could be related to different clinical phenotypes.
Manuscript source: Unsolicited manuscript
Specialty type: Pediatrics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Malik S, Zavras N S-Editor: Liu M L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Wang Y, Zhang H, Ye J, Han L, Gu X. Three novel mutations of the FBN1 gene in Chinese children with acromelic dysplasia. J Hum Genet. 2014;59:563-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (2)] |
| 2. | Globa E, Zelinska N, Dauber A. The Clinical Cases of Geleophysic Dysplasia: One Gene, Different Phenotypes. Case Rep Endocrinol. 2018;2018:8212417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Li D, Dong H, Zheng H, Song J, Li X, Jin Y, Liu Y, Yang Y. A chinese boy with geleophysic dysplasia caused by compound heterozygous mutations in ADAMTSL2. Eur J Med Genet. 2017;60:685-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | McInerney-Leo AM, Le Goff C, Leo PJ, Kenna TJ, Keith P, Harris JE, Steer R, Bole-Feysot C, Nitschke P, Kielty C, Brown MA, Zankl A, Duncan EL, Cormier-Daire V. Mutations in LTBP3 cause acromicric dysplasia and geleophysic dysplasia. J Med Genet. 2016;53:457-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Sakai LY, Keene DR, Renard M, De Backer J. FBN1: The disease-causing gene for Marfan syndrome and other genetic disorders. Gene. 2016;591:279-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 236] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 6. | Le Goff C, Mahaut C, Wang LW, Allali S, Abhyankar A, Jensen S, Zylberberg L, Collod-Beroud G, Bonnet D, Alanay Y, Brady AF, Cordier MP, Devriendt K, Genevieve D, Kiper PÖ, Kitoh H, Krakow D, Lynch SA, Le Merrer M, Mégarbane A, Mortier G, Odent S, Polak M, Rohrbach M, Sillence D, Stolte-Dijkstra I, Superti-Furga A, Rimoin DL, Topouchian V, Unger S, Zabel B, Bole-Feysot C, Nitschke P, Handford P, Casanova JL, Boileau C, Apte SS, Munnich A, Cormier-Daire V. Mutations in the TGFβ binding-protein-like domain 5 of FBN1 are responsible for acromicric and geleophysic dysplasias. Am J Hum Genet. 2011;89:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 172] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 7. | Sakai LY, Keene DR. Fibrillin protein pleiotropy: Acromelic dysplasias. Matrix Biol. 2019;80:6-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Yang H, Ma Y, Luo M, Zhao K, Zhang Y, Zhu G, Sun X, Luo F, Wang L, Shu C, Zhou Z. Identification of gross deletions in FBN1 gene by MLPA. Hum Genomics. 2018;12:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Li H, Ji CY, Zong XN, Zhang YQ. [Height and weight standardized growth charts for Chinese children and adolescents aged 0 to 18 years]. Zhonghua Er Ke Za Zhi. 2009;47:487-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 55] [Reference Citation Analysis (0)] |
| 10. | Cheng SW, Luk HM, Chu YWY, Tung YL, Kwan EY, Lo IF, Chung BH. A report of three families with FBN1-related acromelic dysplasias and review of literature for genotype-phenotype correlation in geleophysic dysplasia. Eur J Med Genet. 2018;61:219-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Cecchi A, Ogawa N, Martinez HR, Carlson A, Fan Y, Penny DJ, Guo DC, Eisenberg S, Safi H, Estrera A, Lewis RA, Meyers D, Milewicz DM. Missense mutations in FBN1 exons 41 and 42 cause Weill-Marchesani syndrome with thoracic aortic disease and Marfan syndrome. Am J Med Genet A. 2013;161A:2305-2310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Legare JM, Modaff P, Strom SP, Pauli RM, Bartlett HL. Geleophysic dysplasia: 48 year clinical update with emphasis on cardiac care. Am J Med Genet A. 2018;176:2237-2242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Lee T, Takeshima Y, Okizuka Y, Hamahira K, Kusunoki N, Awano H, Yagi M, Sakai N, Matsuo M, Iijima K. A Japanese child with geleophysic dysplasia caused by a novel mutation of FBN1. Gene. 2013;512:456-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |