Published online Aug 26, 2021. doi: 10.12998/wjcc.v9.i24.7169
Peer-review started: January 11, 2021
First decision: April 25, 2021
Revised: May 1, 2021
Accepted: July 5, 2021
Article in press: July 5, 2021
Published online: August 26, 2021
Processing time: 224 Days and 17.9 Hours
Idiopathic basal ganglia calcification (IBGC) is a neurodegenerative disease characterized by symmetrical calcification of basal ganglia and other brain region, also known as Fahr’s disease. It can be sporadic or familial, and there is no definite etiology at present. With the development of neuroimaging, the number of reports of IBGC has increased in recent years. However, due to its hidden onset, diverse clinical manifestations, and low incidence, it is likely to be misdiagnosed or ignored by potential patients and their family.
We report a case of a 61-year-old man who presented with symptoms of dysphagia and alalia. His computed tomography scan of the brain revealed bilateral symmetric calcifications of basal ganglia, cerebellum, thalamus, and periventricular area. The genetic test showed a new mutation sites of MYORG, c.1438T>G mutation and c.1271_1272 TGGTGCGC insertion mutation. He was finally diagnosed with IBGC.
It is important to detect MYORG mutation when IBGC is suspected, especially in those without an obvious family history, for better understanding of the underlying mechanism and identifying potential treatments.
Core Tip: Idiopathic basal ganglia calcification (IBGC) is characterized by calcification of basal ganglia and other regions of the brain. IBGC is clinically heterogeneous and usually exhibits an autosomal dominant pattern of inheritance. We report a case of a 61-year-old man who presented with symptoms of dysphagia and alalia. His computed tomography scan of the brain revealed bilateral symmetric calcifications of basal ganglia, cerebellum, thalamus, and periventricular area. The genetic test showed a new mutation site of MYORG.
- Citation: Fei BN, Su HZ, Yao XP, Ding J, Wang X. Idiopathic basal ganglia calcification associated with new MYORG mutation site: A case report. World J Clin Cases 2021; 9(24): 7169-7174
- URL: https://www.wjgnet.com/2307-8960/full/v9/i24/7169.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i24.7169
Idiopathic basal ganglia calcification (IBGC) is characterized by calcification of basal ganglia and other regions of the brain, commonly known as Fahr’s disease[1]. IBGC is clinically heterogeneous and usually exhibits an autosomal dominant pattern of inheritance. Wang et al[2] discovered the first pathogenic gene of IBGC: SLC20A2 in 2012. Three other IBGC pathogenic genes (PDGFRB[3], PDGFB[4], and XPR1[5]) were subsequently identified. In 2018, Yao et al[6] first reported that MYORG is a new pathogenic gene for IBGC with an autosomal-recessive trait. Here, we report a family with novel mutation sites of MYORG.
The patient is a 61-year-old man with the complaint of progressive severe dysphagia and alalia.
Patient’s symptoms started 4 years ago and were getting worse. He denied any headache, seizures, and psychiatric symptoms.
Patient has hypertension for more than 10 years.
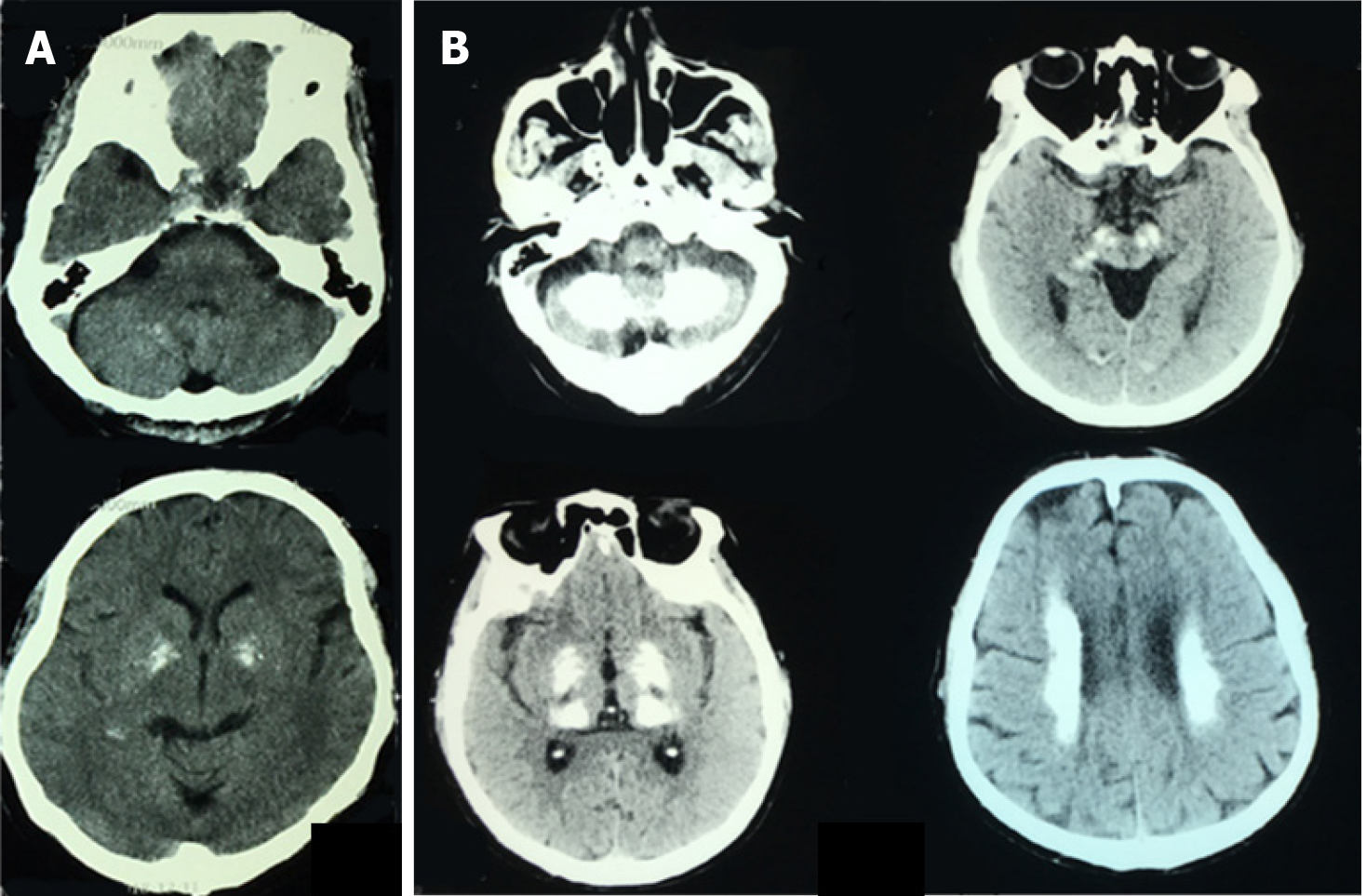
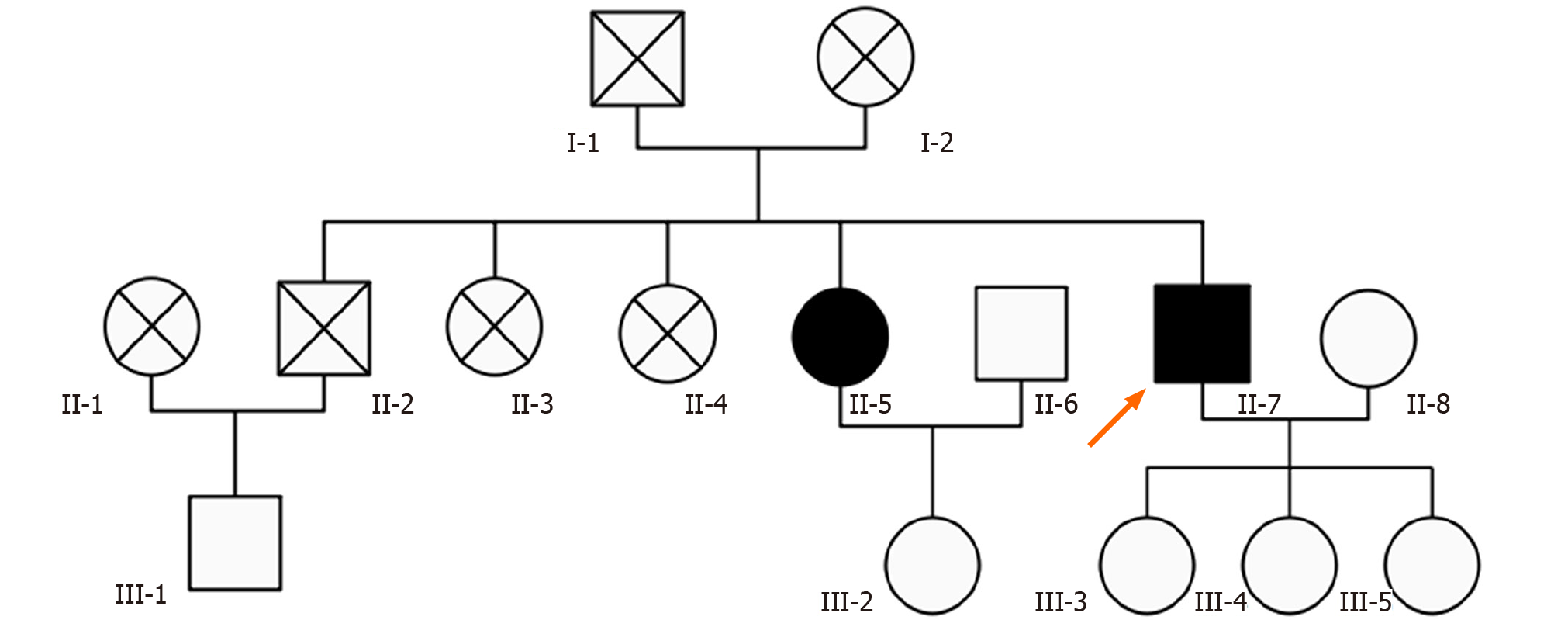
Patient’s parents passed away in their sixties with unknown reason. He had an old brother and three old sisters, three of them died of unknown reason. The surviving sister is clinically asymptomatic but has calcifications on computed tomography (CT) scan (Figure 1A), and total calcification[7] score was 10. His three daughters were also asymptomatic and with normal CT scan. The pedigree chart is shown in Figure 2.
The neurological examination was normal.
Laboratory tests, including serum calcium, phosphorus, and parathyroid hormone levels, were within normal limits (calcium 2.22 mmol/L, phosphorus 0.84 mmol/L, parathyroid hormone 37.5 ng/L).
Patient’s CT scan revealed multiple symmetric calcifications of bilateral basal ganglia, cerebellum, thalamus, and periventricular area (Figure 1B). Total calcification score was 54.
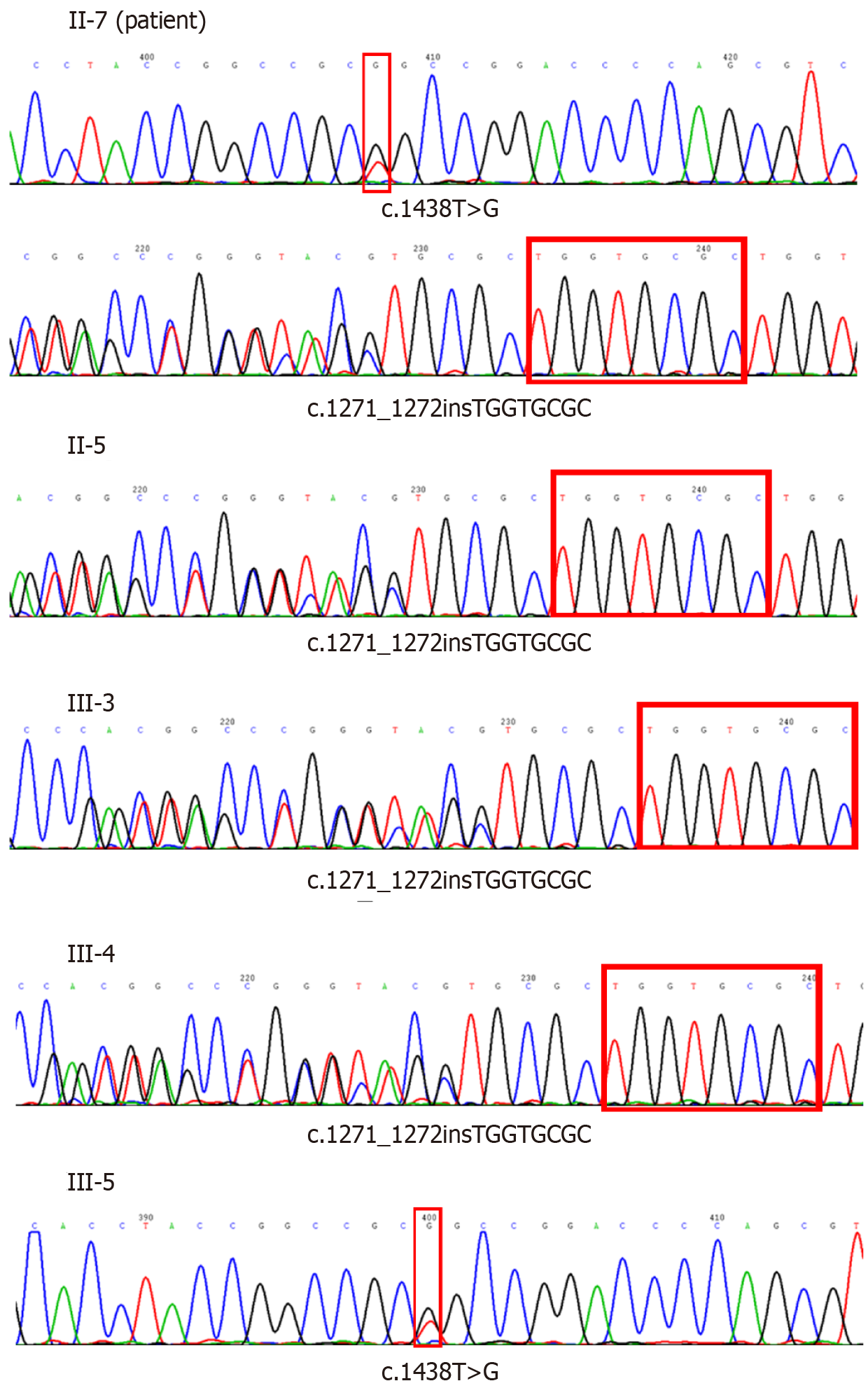
Genetic tests were done for the patient and his family. Excluding the dead and unreachable individuals, 7 people over two generations of the patient’s family were investigated. The patient’s genetic test showed c.1438T >G mutation and c.1271_1272 TGGTGCGC insertion mutation in exon 2 of MYORG gene. Besides the patient, his sister and two of his daughter have MYORG c.1438T>G mutation, while one daughter has MYORG c.1271_1272 TGGTGCGC insertion mutation (Table 1 and Figure 3).
| II-5 | II-7 | II-8 | III-1 | III-3 | III-4 | III-5 | |
| Age at evaluation | 66 | 61 | 61 | 51 | 36 | 34 | 32 |
| Education level | II | II | II | III | IV | IV | IV |
| MMSE (/30) | 27 | 26 | 28 | 29 | 30 | 30 | 30 |
| MoCA (/30) | 26 | 19 | 27 | 28 | 30 | 29 | 29 |
| Clinic symptom | - | + | - | - | - | - | - |
| Calcifications on CT | + | + | - | - | - | - | - |
| Total calcification score | 54 | 10 | 0 | 0 | 0 | 0 | 0 |
| MYORG mutation | + | + | - | - | + | + | + |
| c.1438T>G mutation | - | + | - | - | - | - | + |
| c.1271_1272 TGGTGCGC insertion mutation | + | + | - | - | + | + | - |
According to these laboratory tests and neuroimaging, he was diagnosed with IBGC.
Oxiracetam and nicergoline were given for neurotrophic treatments and improving cerebrovascular circulation.
The symptoms were not approved with these agents.
IBGC is a hereditary disease of the nervous system. With the wide application of CT, the detection rate of IBGC becomes higher and higher. Most patients were healthy in the adolescent period. Even if characteristic calcification lesions had appeared in the head CT examination, there might not be obvious abnormal symptoms of the nervous system. Neuropsychiatric and motor disorders would gradually present in the middle-aged and elderly patients with progressive parkinsonism and spasticity.
In this pedigree, the patient had compound heterozygous mutations, one point mutation and one insertion mutation. His sister and daughters only had one mutation site, and they did not present with any symptoms. Some research found that homozygotes have more serious brain calcification, whereas with the heterozygous mutation, only punctated calcification was found in the globus pallidus. The clinical symptoms in homozygotes are various neurological symptoms, while heterozygotes are usually clinically asymptomatic[8]. Although the patient’s sister and daughters are asymptomatic, they had the MYORG mutation. We suggested to them annual cranial CT scans, because with age the disease may progress and clinical symptoms may appear.
Previous studies reported that IBGC is inherited as an autosomal dominant disease[9,10] and identified SLC20A2 coding for the phosphate transporter PiT2, PDGFRB3 coding for the platelet derived growth factor receptor, as a cause of IBGC. However, SLC20A2, PDGFRB, PDGFB, and XPR1 mutations were only detected in 16.8% of familial brain calcification. The underlying reason of IBGC remains unclear, especially in those without a prominent family history. In 2018, Yao et al[6] identified MYORG as the new recessive causal gene for IBGC, and reported 10 different mutation sites. Our patient and his family have MYORG novel mutation sites, c.1438T>G mutation and c.1271_1272 TGGTGCGC insertion mutation, which have not been reported previously.
MYORG, also known as KIAA1161 or NET37, is located on chromosome 9p13.3. It encodes a protein containing 714 amino acids, which is a member of the glycosylase 31 family. MYORG gene was specifically expressed in S100b positive astrocytes. Bilateral symmetrical nodules of cerebral calcification were observed in MYORG knockout mice. The main components of calcium nodules were calcium and phosphorus, which were highly similar to the elements of brain calcification in clinical patients, which proved that MYORG autosomal recessive function deletion mutation was an important pathogenic gene of IBGC. The mutation of this gene causes dysfunction of astrocytes, which may affect the combination of astrocytes and pericytes, interfere with the normal function of neurovascular unit, and finally may lead to the calcification of the brain. According to the clinical manifestations, Chen et al[11] found that 76.5% patients with MYORG mutation were symptomatic, and the most recurrent symptom was movement disorder. Our patient’s manifestation was progressive dysphagia and alalia.
Since many patients will exhibit a progressive neurological disorder with no known treatment, it is important to detect MYORG mutation, especially in those without obvious family history, for better understanding the underlying mechanism and identifying potential treatments.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tamune H S-Editor: Fan JR L-Editor: Filipodia P-Editor: Wang LYT
| 1. | Manyam BV. What is and what is not 'Fahr's disease'. Parkinsonism Relat Disord. 2005;11:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 224] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 2. | Wang C, Li Y, Shi L, Ren J, Patti M, Wang T, de Oliveira JR, Sobrido MJ, Quintáns B, Baquero M, Cui X, Zhang XY, Wang L, Xu H, Wang J, Yao J, Dai X, Liu J, Zhang L, Ma H, Gao Y, Ma X, Feng S, Liu M, Wang QK, Forster IC, Zhang X, Liu JY. Mutations in SLC20A2 link familial idiopathic basal ganglia calcification with phosphate homeostasis. Nat Genet. 2012;44:254-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 309] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 3. | Nicolas G, Pottier C, Maltête D, Coutant S, Rovelet-Lecrux A, Legallic S, Rousseau S, Vaschalde Y, Guyant-Maréchal L, Augustin J, Martinaud O, Defebvre L, Krystkowiak P, Pariente J, Clanet M, Labauge P, Ayrignac X, Lefaucheur R, Le Ber I, Frébourg T, Hannequin D, Campion D. Mutation of the PDGFRB gene as a cause of idiopathic basal ganglia calcification. Neurology. 2013;80:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 220] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 4. | Keller A, Westenberger A, Sobrido MJ, García-Murias M, Domingo A, Sears RL, Lemos RR, Ordoñez-Ugalde A, Nicolas G, da Cunha JE, Rushing EJ, Hugelshofer M, Wurnig MC, Kaech A, Reimann R, Lohmann K, Dobričić V, Carracedo A, Petrović I, Miyasaki JM, Abakumova I, Mäe MA, Raschperger E, Zatz M, Zschiedrich K, Klepper J, Spiteri E, Prieto JM, Navas I, Preuss M, Dering C, Janković M, Paucar M, Svenningsson P, Saliminejad K, Khorshid HR, Novaković I, Aguzzi A, Boss A, Le Ber I, Defer G, Hannequin D, Kostić VS, Campion D, Geschwind DH, Coppola G, Betsholtz C, Klein C, Oliveira JR. Mutations in the gene encoding PDGF-B cause brain calcifications in humans and mice. Nat Genet. 2013;45:1077-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 258] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 5. | Legati A, Giovannini D, Nicolas G, López-Sánchez U, Quintáns B, Oliveira JR, Sears RL, Ramos EM, Spiteri E, Sobrido MJ, Carracedo Á, Castro-Fernández C, Cubizolle S, Fogel BL, Goizet C, Jen JC, Kirdlarp S, Lang AE, Miedzybrodzka Z, Mitarnun W, Paucar M, Paulson H, Pariente J, Richard AC, Salins NS, Simpson SA, Striano P, Svenningsson P, Tison F, Unni VK, Vanakker O, Wessels MW, Wetchaphanphesat S, Yang M, Boller F, Campion D, Hannequin D, Sitbon M, Geschwind DH, Battini JL, Coppola G. Mutations in XPR1 cause primary familial brain calcification associated with altered phosphate export. Nat Genet. 2015;47:579-581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 237] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 6. | Yao XP, Cheng X, Wang C, Zhao M, Guo XX, Su HZ, Lai LL, Zou XH, Chen XJ, Zhao Y, Dong EL, Lu YQ, Wu S, Li X, Fan G, Yu H, Xu J, Wang N, Xiong ZQ, Chen WJ. Biallelic Mutations in MYORG Cause Autosomal Recessive Primary Familial Brain Calcification. Neuron. 2018;98:1116-1123.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 124] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 7. | Nicolas G, Pottier C, Charbonnier C, Guyant-Maréchal L, Le Ber I, Pariente J, Labauge P, Ayrignac X, Defebvre L, Maltête D, Martinaud O, Lefaucheur R, Guillin O, Wallon D, Chaumette B, Rondepierre P, Derache N, Fromager G, Schaeffer S, Krystkowiak P, Verny C, Jurici S, Sauvée M, Vérin M, Lebouvier T, Rouaud O, Thauvin-Robinet C, Rousseau S, Rovelet-Lecrux A, Frebourg T, Campion D, Hannequin D; French IBGC Study Group. Phenotypic spectrum of probable and genetically-confirmed idiopathic basal ganglia calcification. Brain. 2013;136:3395-3407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 171] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 8. | Bauer M, Rahat D, Zisman E, Tabach Y, Lossos A, Meiner V, Arkadir D. MYORG Mutations: a Major Cause of Recessive Primary Familial Brain Calcification. Curr Neurol Neurosci Rep. 2019;19:70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Volpato CB, De Grandi A, Buffone E, Facheris M, Gebert U, Schifferle G, Schönhuber R, Hicks A, Pramstaller PP. 2q37 as a susceptibility locus for idiopathic basal ganglia calcification (IBGC) in a large South Tyrolean family. J Mol Neurosci. 2009;39:346-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Dai X, Gao Y, Xu Z, Cui X, Liu J, Li Y, Xu H, Liu M, Wang QK, Liu JY. Identification of a novel genetic locus on chromosome 8p21.1-q11.23 for idiopathic basal ganglia calcification. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:1305-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Chen Y, Fu F, Chen S, Cen Z, Tang H, Huang J, Xie F, Zheng X, Yang D, Wang H, Huang X, Zhang Y, Zhou Y, Liu JY, Luo W. Evaluation of MYORG mutations as a novel cause of primary familial brain calcification. Mov Disord. 2019;34:291-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |