Published online Aug 26, 2021. doi: 10.12998/wjcc.v9.i24.7154
Peer-review started: January 6, 2021
First decision: July 5, 2021
Revised: July 7, 2021
Accepted: July 14, 2021
Article in press: July 14, 2021
Published online: August 26, 2021
Processing time: 229 Days and 19.4 Hours
Hepatic hemangioma is the most common benign tumor of the liver. However, patients with large hemangiomas that cause compression symptoms or that are at risk of rupture may need further intervention. It is necessary to explore additional minimally invasive and personalized treatment options for hemangiomas.
A 47-year-old woman was diagnosed with a right hepatic hemangioma for more than 10 years. Abdominal contrast-enhanced computed tomography (CT) and contrast-enhanced ultrasound revealed that there was a large hemangioma in the right liver, with a size of approximately 95 mm × 97 mm × 117 mm. Due to the patient's refusal of surgical treatment, hepatic artery embolization was performed in the first stage. After 25 d of liver protection treatment, the liver function indexes decreased to normal levels. Then, ultrasound-guided microwave ablation of the giant hepatic hemangioma was performed. Ten days after the treatment, hepatobiliary ultrasonography showed that the hemangioma of the right liver was smaller than the previous size (the volume was reduced by approximately 30%). Then the patient was discharged from the hospital. One year after discharge, CT showed that the hepatic hemangioma had shrunk by about 80%
Transcatheter arterial embolization combined with microwave ablation is a safe and effective minimally invasive treatment for hepatic hemangioma.
Core Tip: Hepatic hemangioma is the most common benign tumor of the liver. However, patients with large hemangiomas that cause compression symptoms or that are at risk of rupture may need further intervention. It is necessary to explore additional minimally invasive and personalized treatment options for hemangiomas. Here, we present the case of a 47-year-old woman who was diagnosed with a right hepatic hemangioma for more than 10 years. Abdominal contrast-enhanced computed tomography and contrast-enhanced ultrasound revealed that there was a large hemangioma in the right liver, with a size of approximately 95 mm × 97 mm × 117 mm. Hepatic artery embolization was performed in the first stage. Then, ultrasound-guided microwave ablation of the giant hepatic hemangioma was performed.
- Citation: Wang LZ, Wang KP, Mo JG, Wang GY, Jin C, Jiang H, Feng YF. Minimally invasive treatment of hepatic hemangioma by transcatheter arterial embolization combined with microwave ablation: A case report. World J Clin Cases 2021; 9(24): 7154-7162
- URL: https://www.wjgnet.com/2307-8960/full/v9/i24/7154.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i24.7154
Hepatic hemangioma is the most common benign tumor of the liver[1], which usually only needs regular reexamination, but surgical intervention is usually required when it becomes larger or causes symptoms[2]. With the deepening of the concept of minimally invasive and rapid rehabilitation, exploring the minimally invasive treatment of hepatic hemangioma is a hot topic. In recent years, there have been many studies on interventional therapy[3] and ablation of hepatic hemangioma[4]. However, the safety and efficacy of interventional therapy and ablation on hepatic hemangioma need to be confirmed by further study[5]. Previous studies mostly focused on comparing the safety and effectiveness of simple interventional therapy or simple ablation and surgical treatment, and they are limited to hepatic hemangiomas with a diameter less than 5 cm[6]. In fact, interventional therapy combined with ablation may be more effective than either interventional therapy or ablation therapy alone[7].
However, there are no reports of transcatheter arterial embolization (TAE) combined with microwave ablation in the treatment of hepatic hemangiomas larger than 10 cm in diameter. Here, we report a case of hepatic hemangioma with a diameter of 10 cm treated successfully by TAE combined with microwave ablation. We further summarize the literature and our successful experience in order to provide new ideas for minimally invasive and personalized treatment options for hepatic hemangioma.
A 47-year-old woman complained that the physical examination found a hemangioma for more than 10 years.
The patient was diagnosed with a right hepatic hemangioma for more than 10 years and had failed interventional hepatic artery embolization at a local hospital 1 year ago. The hemangioma showed an increase in size during 10 years (from 3 cm to near 10 cm in diameter).
The patient had a history of caesarean section and unilateral salpingectomy and had failed interventional hepatic artery embolization at a local hospital.
The patient’s temperature was 36.5 °C, heart rate was 75 bpm, respiratory rate was 19 breaths per minute, blood pressure was 120/72 mmHg, and oxygen saturation in room-temperature air was 99%. She had no tenderness or rebound pain in the abdomen. The frequency of bowel sounds was four per minute, and there were no other pathological signs.
Blood, biochemistry, and urine analyses were normal. Electrocardiogram, chest X-ray, and arterial blood gas results were also normal.
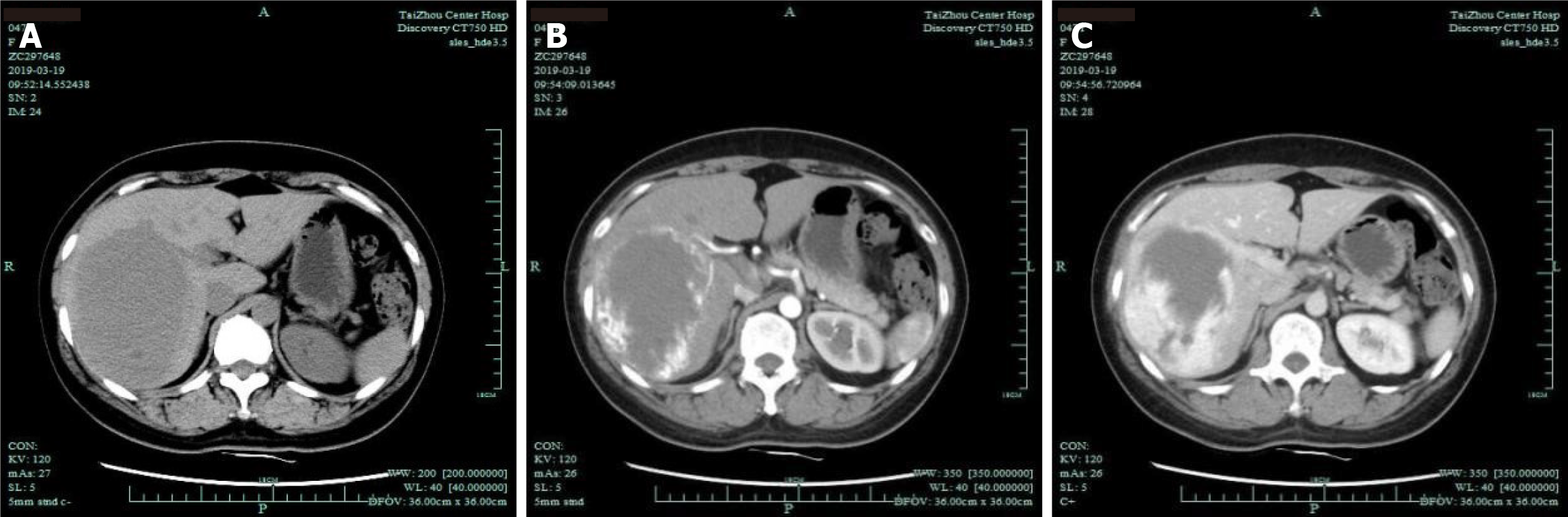
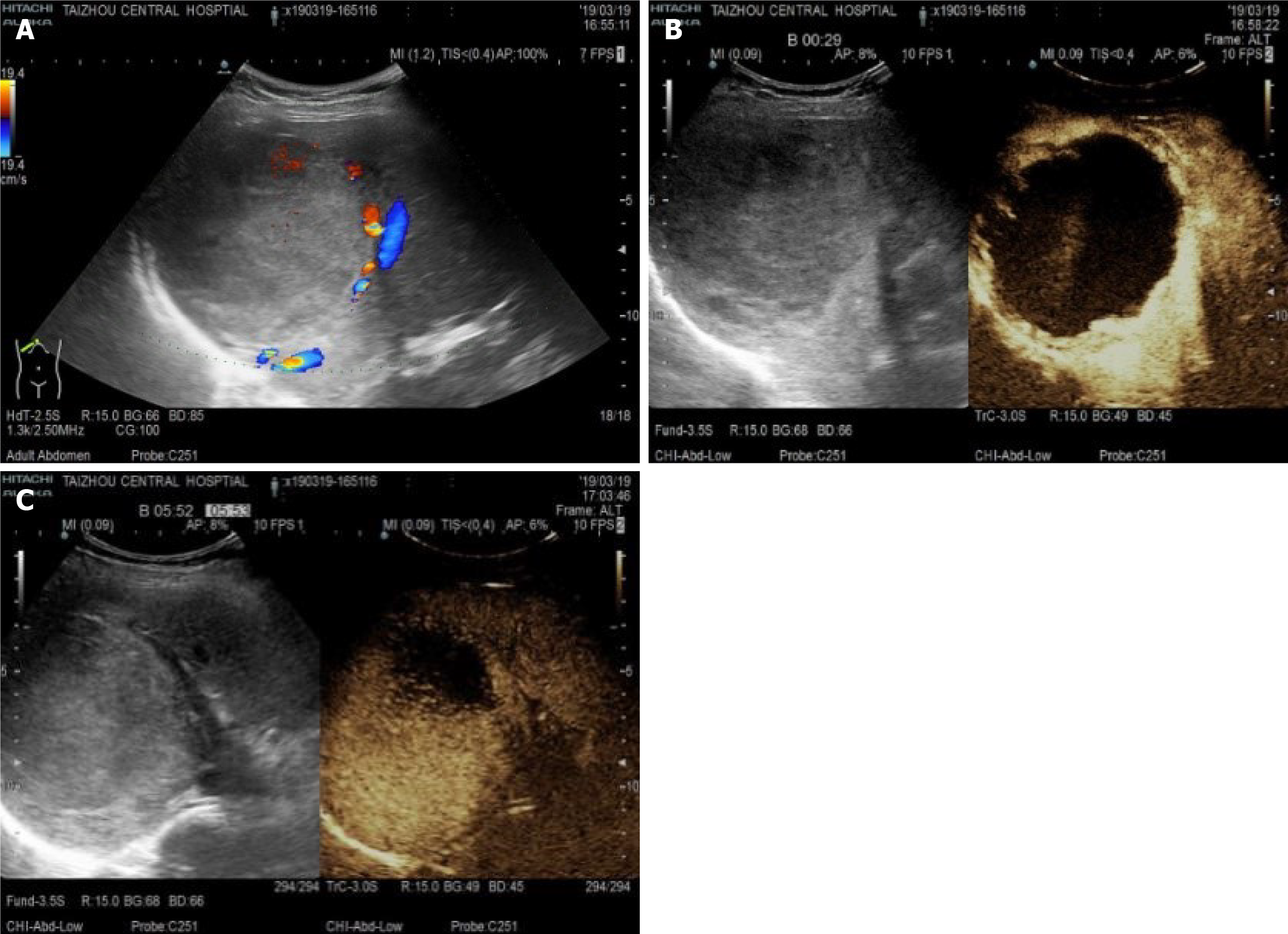
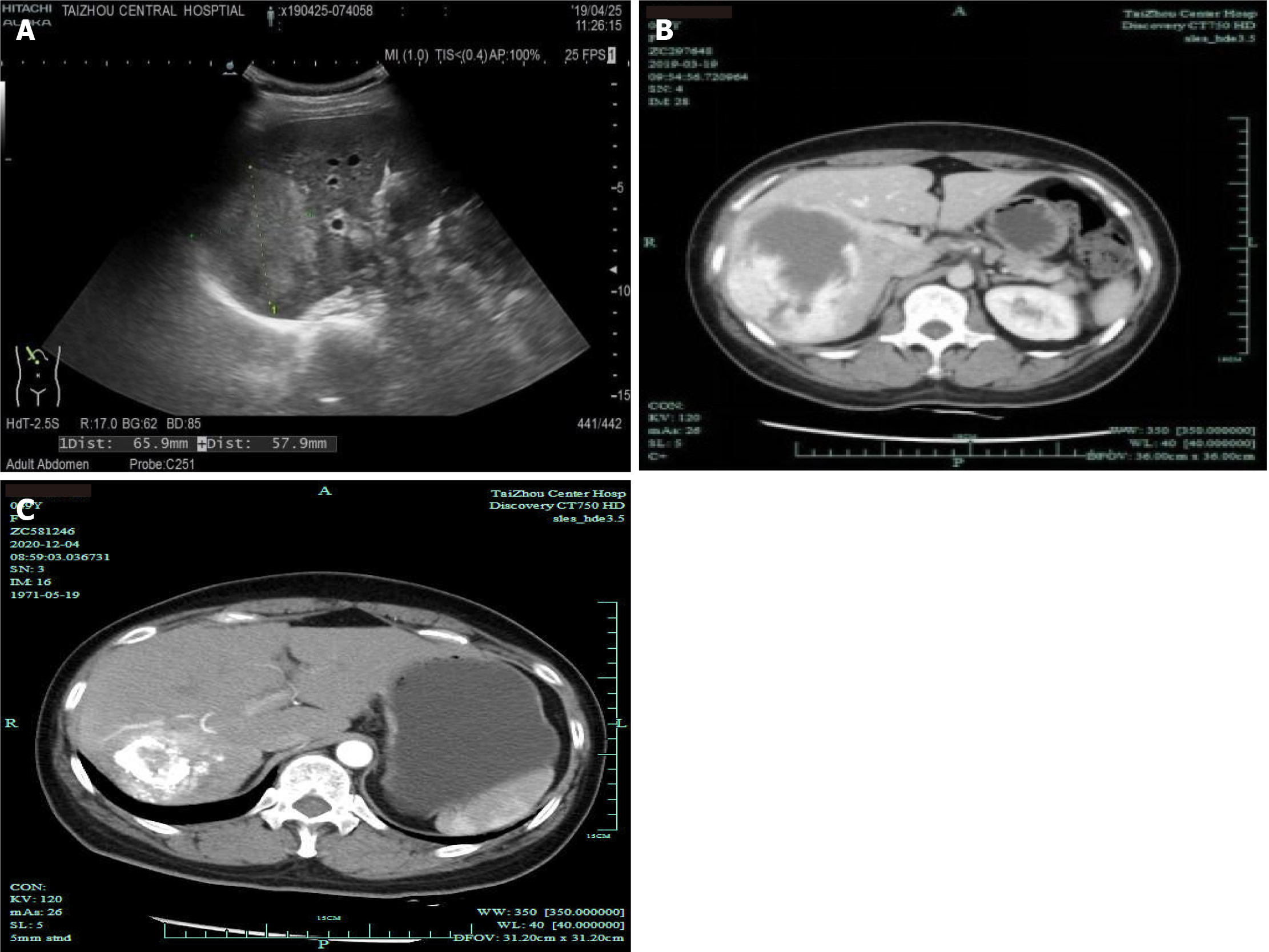
Abdominal contrast-enhanced computed tomography (CT) showed the following: The right lobe of the liver had a low-density mass with a size of approximately 95 mm × 97 mm × 117 mm, the boundary was clear, and the density was uniform, which suggested a right hepatic hemangioma (Figure 1). Ultrasound angiography showed that the right posterior hepatic lobe displayed a strong echogenic area with a size of 95 mm × 97 mm × 117 mm, and the boundary was clear. After a total of 2.4 mL of sulfur hexafluoride microbubble solution was injected through the anterior elbow vein, a small amount of enhancement was observed around the arterial phase in the strong echo zone. During this period, the contrast agent was significantly filled. Contrast-enhanced ultrasound showed a right hepatic posterior hemangioma (Figure 2).
The final diagnosis of the presented case was hepatic hemangioma.
Since the patient refused surgical treatment, she was strongly encouraged to undergo microwave ablation due to the consideration of a large local lesion as well as to protect the surrounding important blood vessels, to ensure the scope of ablation, and to reduce intraoperative bleeding caused by puncture and ablation. The detailed plan according to the location and size of the lesion was as follows: One-stage hepatic artery interventional embolization, followed by short-term ultrasound-guided microwave ablation of hepatic giant hemangioma.
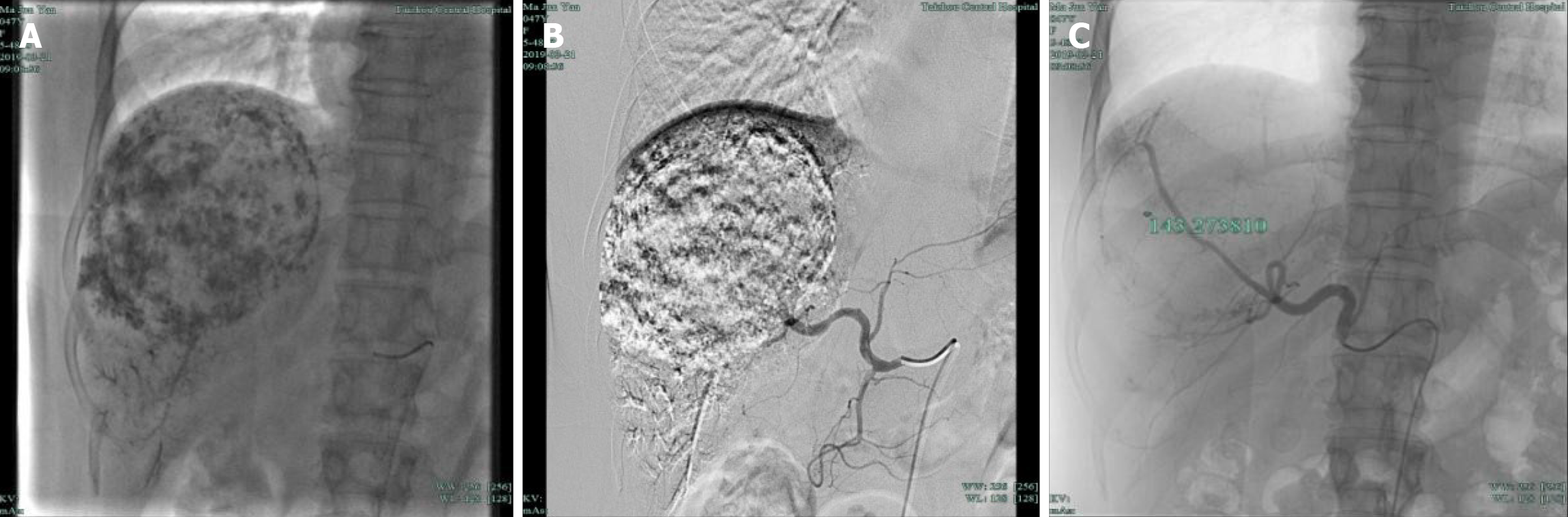
Intraoperatively, a catheter was inserted for celiac artery angiography, which showed that the hepatic artery was significantly thickened and tortuous, and a large mass-like abnormal staining area was seen in the right liver (Figure 3A and B). A microcatheter and micro-guidewire were introduced into the right hepatic artery from the contrast catheter, and the micro-guidewire was withdrawn. Then, 10 mg dexamethasone and 8 mg ondansetron were perfused, and the microcatheter and micro-guidewire were superselected to the right lobe of the liver. In the small branch, angiography was performed to determine the supply of the blood vessels. Under fluoroscopy, 20 mL of iodized oil, 15000 units of bleomycin, and a small amount of contrast medium mixture were injected slowly via the microcatheter, and then a small amount of gelatin sponge was injected into the main supply vessels. There was mild reflux, and angiography showed that the abnormal staining area of the liver had disappeared (Figure 3C). The patient had no obvious discomfort, and the catheter was withdrawn. The sheath was removed after treatment, the puncture site was pressure-bandaged, the sandbag was pressed to stop the bleeding, and the patient was placed in a supine position for 24 h. The side was continuously pressed for 8 h. In terms of postoperative monitoring of vital signs, there were no complaints of discomfort, no transient fever, and a maximum body temperature of 38 °C; routine blood tests showed a white blood cell count of 13.3 × 109/L, 87.4% central granulocytes, and increased liver function indicators [alanine aminotransferase (ALT) 105 U/L, aspartate aminotransferase (AST) 74 U/L, and gamma-glutamyltransferase (GGT) 74 U/L]. Postoperative prophylactic antibiotics and liver protection treatment were administered. Two days later, routine blood examination showed that the white blood cell levels decreased to normal, and the liver function indicators were normal (ALT 105 U/L, AST 74 U/L, and GGT 76 U/L), so liver protection treatment was continued.
After 25 d, the liver function indexes decreased (ALT 61 U/L, AST 24 U/L, and GGT 164 U/L), and ultrasound-guided microwave ablation of the hepatic hemangioma was carried out. The patient was placed in a supine position, general anesthesia was administered, and routine surgery and disinfection of the surgical field were carried out; for ultrasonic positioning, an 11-point blade with a 2 mm small mouth was positioned on the skin, avoiding color Doppler blood flow, and two 15 G needles were punctured into the right posterior hepatic lobe for real-time ultrasound-guided microwave ablation. The following procedures were used for the lesions: N1: Right lower hepatic lobe lesion in the lower front, energy: 55 W × 600 s; N2: Right posterior hepatic lobe lesion in the posterior segment, energy: 55 W × 600 s (Figure 4A); N3: Right hepatic posterior lobe lesion in the middle front, energy: 55 W × 600 s; N4: Right posterior lobe lesion in the middle of the posterior region, energy: 55 W × 600 s (Figure 4B); and N5: In the upper segment of the right posterior hepatic lobe, energy: 55 W × 600 s. The double needle (N1N2, N3N4) had a total length of 1200 s, and the single needle (N5) had a total length of 600 s. The dynamic observation of the mass area was covered by a strong echo (Figure 4C), and the double needle was resected into a single needle. Postoperative monitoring of vital signs was normal. Routine blood tests showed a white blood cell count of 12.1 × 109/L, 84.5% central granulocytes, and increased liver function indicators (ALT 165 U/L, AST 92 U/L, and GGT 86 U/L). Postoperative prophylactic antibiotics and liver protection treatment were administered. Five days later, routine blood examination showed that the white blood cell levels decreased to normal, and the liver function indicators were normal (ALT 75 U/L, AST 42 U/L, and GGT62 U/L). The patient had no serious complication after ablation
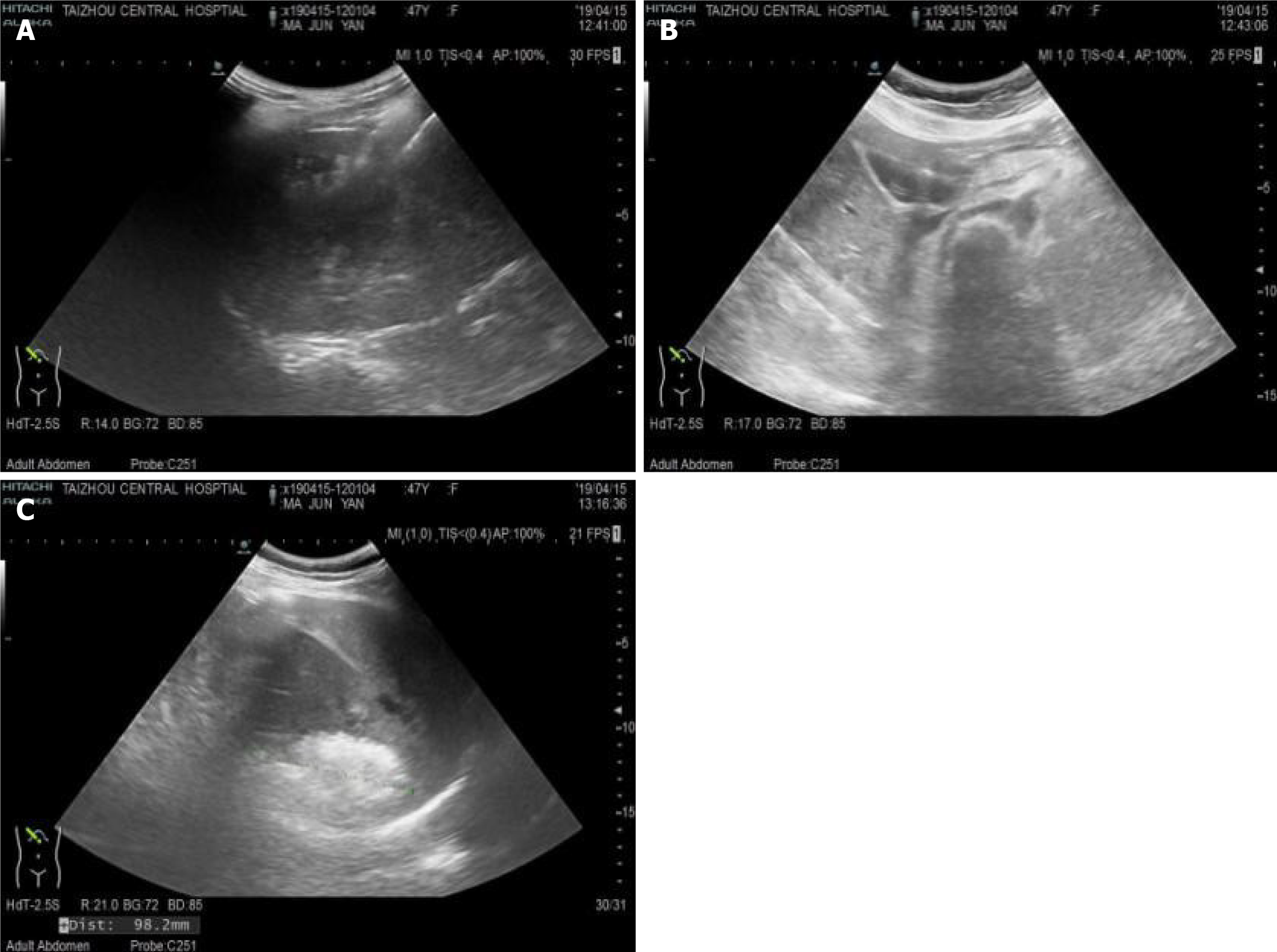
Hepatobiliary ultrasonography 10 d after the treatment showed that the posterior lobe of the right liver had a strong echo region with a size of 71 mm × 66 mm × 58 mm and a relatively clear boundary, and the hemangioma in the right liver decreased in size compared with the previous size (approximately 30% smaller in volume) (Figure 5A), Then, the patient was discharged from the hospital. CT 2 mo after discharge showed a right lamellar hepatic mass with a high-density shadow after microwave ablation of the hepatic hemangioma (Figure 5B). One year after discharge, CT showed that the hepatic hemangioma had shrunk by about 80% (Figure 5C).
The incidence of hepatic hemangioma is 0.4%-20.0%[8], and the autopsy discovery rate is 0.4%-7.3%[9]. Hepatic hemangioma mostly grows slowly and has no tendency to become malignant; additionally, spontaneous rupture is rare. Some scholars abroad have classified large hemangiomas as those > 5 cm in diameter. Most hepatic hemangiomas do not require treatment or only need regular follow-up. However, giant hemangiomas larger than 5 cm in diameter may cause compression symptoms, such as obstructive jaundice, gastric outlet obstruction, and Budd-Chiari syndrome, or coagulopathy (Kasabach-Merritt syndrome) requiring further intervention[10]. The current treatments include surgery, interventional therapy[3], radiofrequency, and microwave ablation[4].
TAE is a common method used for the treatment of hepatic hemangioma. Most of large hepatic hemangiomas requiring clinical treatment have a multi-arterial blood supply. Some arteries that are a part of this blood supply are not easily found during angiography, and collateral circulation easily forms after embolization of these arteries, resulting in recurrence of hepatic hemangioma. If the tumor is over-embedded, it may cause serious complications such as abnormal liver function, intrahepatic bile duct injury, or ectopic embolism. Therefore, TAE is often used as an adjunct therapy or alternative[3]. For large (5-10 cm) symptomatic hepatic hemangiomas, the safety and efficacy of microwave ablation have not been fully confirmed[5]. The patient had previously undergone TAE treatment for hepatic hemangioma, but the effect was dissatisfying. The hepatic hemangioma was more than 10 cm in size, and the surgical trauma was substantial. Simple TAE or microwave and radiofrequency treatments may not meet the treatment needs.
Microwaves have a higher thermal efficiency than radiofrequency ablation[11]. This is mainly due to the different ways in which microwaves and radiofrequencies interact with tissues. The heat-generating component of radiofrequency ablation is mainly located within a few millimeters around the electrode and is susceptible to tissue carbonization. The expansion of the solidification range mainly depends on conduction heat dissipation, while microwaves have a certain penetrating power in the tissue, their heating range is large, and they are subject to the impact of carbonization, which is small. Since microwaves have a higher tissue solidification temperature than radiofrequencies, they have a strong coagulation ability in blood vessels, and their coagulation volume and shape are less affected by blood vessels; thus, microwave ablation has a larger ablation range, more easily resulting in intratumor ablation. The sufficiently high temperature for ensuring a large ablation range through heat transfer reflects the higher thermal efficiency of microwave ablation. The volume of microwave ablation is larger than that of radiofrequency ablation, which can reduce the number of punctures and the incidence of complications. For tumors with a diameter ≥ 5 cm, microwave ablation can be combined with multiple needles to significantly expand the ablation volume. Therefore, for large tumors, microwave ablation is more advantageous than radiofrequency ablation[12]. Microwave ablation is also less affected by blood perfusion. It is more suitable for treating tumors adjacent to large blood vessels. Multiple needles do not interfere with each other at the same time during ablation, and they have a synergistic effect, thereby making the ablation range larger and the ablation time shorter[13].
We decided to adopt microwave ablation combined with TAE treatment for our patient since the safety and efficacy of microwave ablation combined with TAE treatment are better than those of ablation alone[14]. The reasons are as follows: (1) TAE can effectively reduce the blood supply in the tumor by embolizing the blood vessels of the hemangioma, which can reduce the heat taken away by the "heat sink effect" caused by the rich blood flow during the ablation process, resulting in better microwave ablation efficiency; (2) After TAE blocks the artery supplying the hemangioma, it can effectively reduce the risk of intraoperative bleeding; and (3) The application of iodized oil to the tumor in the TAE procedure can further define the hemangioma boundary and accurately locate it to avoid damage to the surrounding organs[15]. Of course, the optimal interval and the choice of vascular sealing materials during TAE treatment require further research.
The main complications of ablation are hemorrhage, important organ puncture injuries, and organ thermal injuries, such as pleura, diaphragm, and lung injuries. Hemolysis-related complications, such as anemia, jaundice, hemoglobinuria, and temporary kidney injury, can lead to acute renal failure[16]. To reduce the risk of ablation and puncture, we believe that the puncture process should pass through more normal liver tissue as much as possible to reduce the risk of bleeding; furthermore, precise needle insertion, avoidance of repeated puncture, and utilizing the safety distance from “high-risk organs” when placing needles should be implemented. To avoid thermal damage, the needle should be ablated to prevent bleeding when withdrawing the needle; to avoid the excessive volume of single ablation, adequate rehydration should be administered during the perioperative period, especially during surgery, and if necessary, alkalinized urine should be implemented to avoid acute kidney injury or even renal failure. Complete ablation of the tumor should not be excessively pursued in order to avoid causing excessive side damage; if necessary, the tumor can be ablated once the puncture causes bleeding. Feasible local ablation of the tumor can be carried out while considering conservative treatment, including hepatic artery embolization and surgical hemostasis.
In summary, after slight treatment by TAE combined with ablation, the hemangioma was mostly necrotic, and the volume was significantly reduced; at the very least, the tumor will not continue to grow rapidly and affect liver function, in line with the therapeutic purposes and concepts of benign liver tumors. TAE combined with microwave ablation is a safe and effective minimally invasive treatment for hepatic hemangioma. For hepatic hemangioma that has no rupture and bleeding and has surgical indications, this kind of combined treatment can be adopted.
We are very grateful to our colleagues from the Department of Imaging for providing the computed tomography pictures.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sawada K S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Wang LYT
| 1. | Berlin-Wahlén A, Evers H, Haegerstam G, Vinnars E. Systemic absorption of ketocaine following epicutaneous administration. Acta Pharm Suec. 1981;18:113-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Slagel DE, DeSimone P, Dillon M, LePage DJ, Bogden AE. Subrenal capsule assay: feasibility of transporting tissues to a central facility for testing. Cancer Treat Rep. 1985;69:717-718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Tsuji Y, Tsubota N, Maeda K, Nishijima E, Yamashita C, Kozawa S, Okada M, Nakamura K, Okada S. [A case of thoracic empyema with broncho-pleural fistula existing for 57 years. Histological findings of squamous cell carcinoma in the resected lung]. Kyobu Geka. 1984;37:768-772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Wang Z, Tang X, Qi X, Shi Y, Chi J, Li P, Zhai B. Feasibility, safety, and efficacy of ultrasound-guided percutaneous microwave ablation for giant hepatic hemangioma. Int J Hyperthermia. 2018;35:246-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Tang X, Ding M, Lu B, Chi J, Wang T, Shi Y, Wang Z, Cui D, Li P, Zhai B. Outcomes of ultrasound-guided percutaneous microwave ablation vs surgical resection for symptomatic large hepatic hemangiomas. Int J Hyperthermia. 2019;36:632-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Takaki H, Yamakado K, Uraki J, Nakatsuka A, Fuke H, Yamamoto N, Shiraki K, Yamada T, Takeda K. Radiofrequency ablation combined with chemoembolization for the treatment of hepatocellular carcinomas larger than 5 cm. J Vasc Interv Radiol. 2009;20:217-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Kang SG, Yoon CJ, Jeong SH, Kim JW, Lee SH, Lee KH, Kim YH. Single-session combined therapy with chemoembolization and radiofrequency ablation in hepatocellular carcinoma less than or equal to 5 cm: a preliminary study. J Vasc Interv Radiol. 2009;20:1570-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | European Association for the Study of the Liver (EASL). EASL Clinical Practice Guidelines on the management of benign liver tumours. J Hepatol. 2016;65:386-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 325] [Article Influence: 36.1] [Reference Citation Analysis (2)] |
| 9. | Toro A, Mahfouz AE, Ardiri A, Malaguarnera M, Malaguarnera G, Loria F, Bertino G, Di Carlo I. What is changing in indications and treatment of hepatic hemangiomas. A review. Ann Hepatol. 2014;13:327-339. [PubMed] |
| 10. | Hasan HY, Hinshaw JL, Borman EJ, Gegios A, Leverson G, Winslow ER. Assessing normal growth of hepatic hemangiomas during long-term follow-up. JAMA Surg. 2014;149:1266-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 11. | Guo SF. [Measurement of the lumbar bony canal and spinal stenosis]. Zhonghua Wai Ke Za Zhi. 1984;22:623-626, 640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 12. | Natarajan AT, Ahnström G, Sharma RP. Heterochromatin and chromosomal aberration in microtus agrestis: role of chromosomal association. Mutat Res. 1974;22:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 13. | Vietti Violi N, Duran R, Guiu B, Cercueil JP, Aubé C, Digklia A, Pache I, Deltenre P, Knebel JF, Denys A. Efficacy of microwave ablation vs radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with chronic liver disease: a randomised controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2018;3:317-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 212] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 14. | Heggie AD. Pathogenesis of the rubella exanthem. Isolation of rubella virus from the skin. N Engl J Med. 1971;285:664-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Dormandy TL, Munro JG. Oxyhaemoglobin without oxygen. Nature. 1965;206:410-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Saint-Laurent J, Beaugrand J. Brain stimulation, reinforcement and behavior. Rev Can Biol. 1972;31 Suppl:193-Suppl:213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 0.4] [Reference Citation Analysis (0)] |