Published online Aug 26, 2021. doi: 10.12998/wjcc.v9.i24.7133
Peer-review started: December 31, 2020
First decision: June 15, 2021
Revised: June 25, 2021
Accepted: July 6, 2021
Article in press: July 6, 2021
Published online: August 26, 2021
Processing time: 235 Days and 23.4 Hours
Leigh syndrome (LS) is one of the most common mitochondrial diseases in infants and children. LS often manifests as early-onset with delayed phenotypic development. However, late-onset LS with normal development and white matter lesions in the brain is rarely reported, thereby highlighting the phenotypic variability of LS expression.
We report a 12-year-old boy who presented with an unusual late-onset and fulminant form of LS that is maternally inherited without developmental delay. The patient was admitted to the hospital with symptoms of ptosis and somnolence, and died within 2 mo. Analysis of peripheral blood leukocytes showed a homoplasmic m.9176T>C mutation in the patient. Magnetic resonance imaging also revealed lesions in bilateral white matter as well as symmetrical lesions in the basal ganglia and brain stem. The patient was diagnosed with LS. The patient was treated with vitamin C, vitamin D, and adenosine-triphosphate. The patient died within 2 mo of hospital admission.
LS can present in both infants and older children with different phenotypes.
Core Tip: Leigh syndrome (LS) often manifests as early-onset with delayed phenotypic development. However, late-onset LS with normal development and white matter lesions in the brain is rarely reported. Here, we present a late-onset LS case of a previously healthy12-year-old boy that suddenly and unexpectedly died within 2 mo after the onset of symptoms that included myasthenia oculi and subsequent energy failure. Gene analysis revealed that the boy had a T-to-C transition at nucleotide 9176 of the mitochondrial adenosine triphosphatase 6 gene. The current case is reported for the first time in China and highlights the variability of phenotypic expression of LS.
- Citation: Liang JM, Xin CJ, Wang GL, Wu XM. Late-onset Leigh syndrome without delayed development in China: A case report. World J Clin Cases 2021; 9(24): 7133-7138
- URL: https://www.wjgnet.com/2307-8960/full/v9/i24/7133.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i24.7133
Leigh syndrome (LS) is an early-onset neurodegenerative pediatric disorder and is characterized by bilateral symmetrical necrotic lesions of gray matter nuclei in the basal ganglia and brainstem, as well as in the diencephalon and cerebellum[1]. LS patients traditionally present in early infancy, resulting in regression or psychomotor delay in their early age[2]. Late-onset LS has also been reported, however, it is very rare. Here, we present a late-onset LS case of a previously healthy 12-year-old boy that suddenly and unexpectedly died within 2 mo after the onset of symptoms that included myasthenia oculi and subsequent energy failure. Gene analysis revealed that the boy had a T-to-C transition at nucleotide 9176 of the mitochondrial adenosine triphosphatase (ATPase) 6 gene. Biochemical evaluation and magnetic resonance imaging (MRI) revealed positive acetylcholine antibody in the blood, a positive oligoclonal band in the cerebral fluid, and abnormal signals in white matter. The current case is reported for the first time in China and highlights the variability of phenotypic expression of LS.
A previously healthy 12-year-old boy was admitted to the hospital on February 12, 2016 with complaints of blepharoptosis, voice hoarseness, fever, and the inability to walk and talk.
The patient exhibited persistent blepharoptosis, voice hoarseness, and fever for about 2 mo. He then became severely agitated with the main complaints of sleepiness, dysphagia, limb weakness, and respiratory and circulatory failure. The patient unfortunately died soon thereafter.
The patient was diagnosed with subacute ptosis at 12-years-old.
The patient was delivered vaginally at term with a birth weight of 3250 g and without developmental delay. His height was 156 cm and weight was 50 kg upon admission to the hospital. The patient's mother exhibited no abnormal symptoms after the birth. The family history of the patient regarding a similar condition was negative.
Clinical examination showed that the patient presented with pyramidal signs and very easily elicited reflexes and extensor toe signs on both feet.
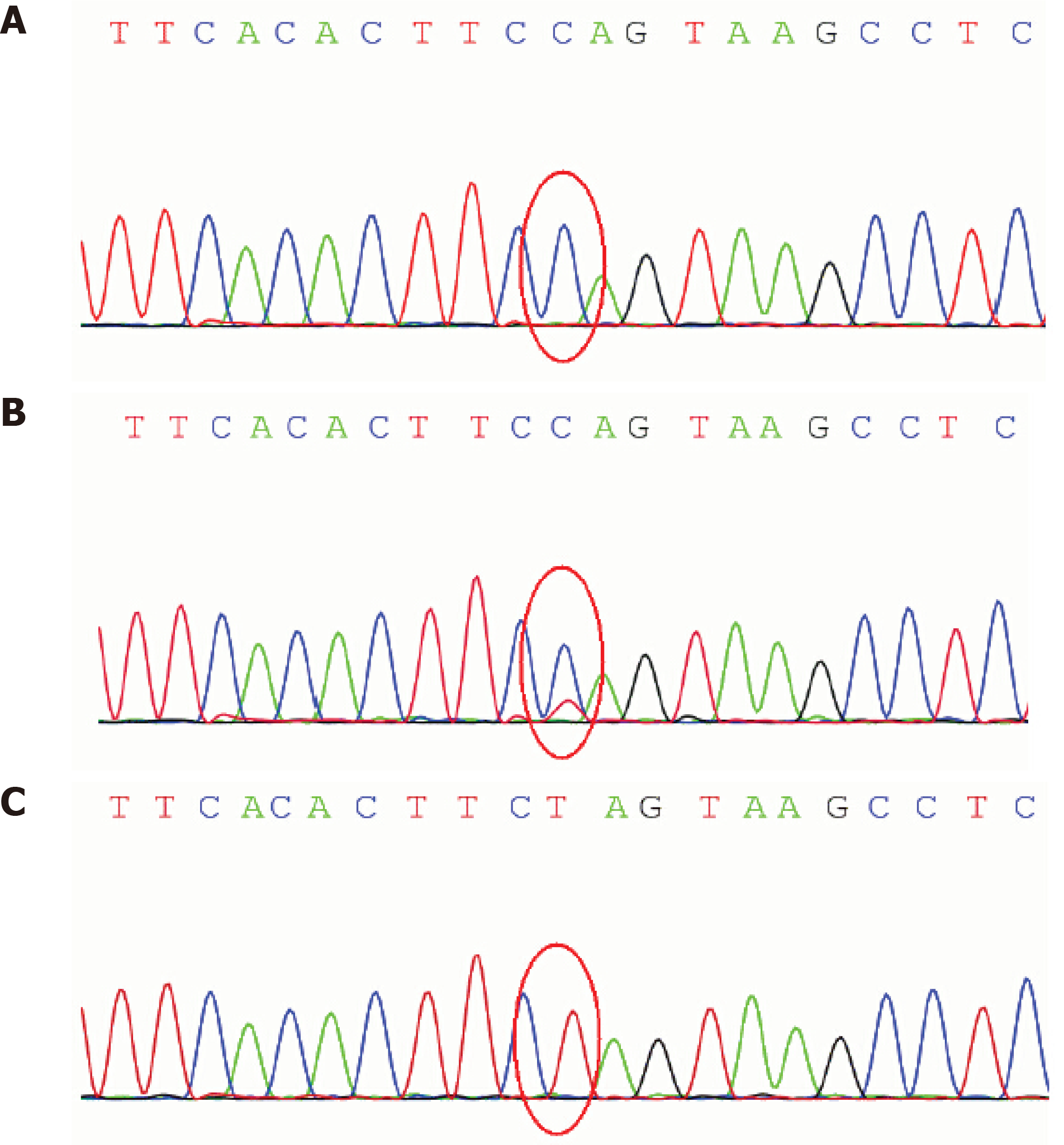
The routine blood and urine tests, urinary sediment examination, routine fecal tests, and occult blood test were performed, which all showed normal results. The arterial blood gas was tested and the pH value was 7.32. The oxygen pressure was 11.3 kPa, the carbon dioxide pressure was 4.9 kPa, and the oxygen saturation was 99%. The arterial plasma lactate level was slightly elevated to 3 mg/dL. Ammonia and blood sugar levels were normal. Other blood biochemistry indexes were also normal. The cytological and biochemical examination showed the normal presence of cerebrospinal fluid (CSF)-positive antibodies to acetylcholine, presynaptic membrane receptor, and thymoma in the blood, and positive oligoclonal bands in the CSF. The antibody tests to Mycoplasma pneumoniae, herpes simplex virus, and Epstein-Barr virus were negative. Gene sequencing analysis revealed a T-to-C transition at nucleotide 9176 of the mitochondrial ATPase 6 gene (Leu to Pro) in the patient and a heteroplasmic mutation in the patient’s mother (Figure 1).
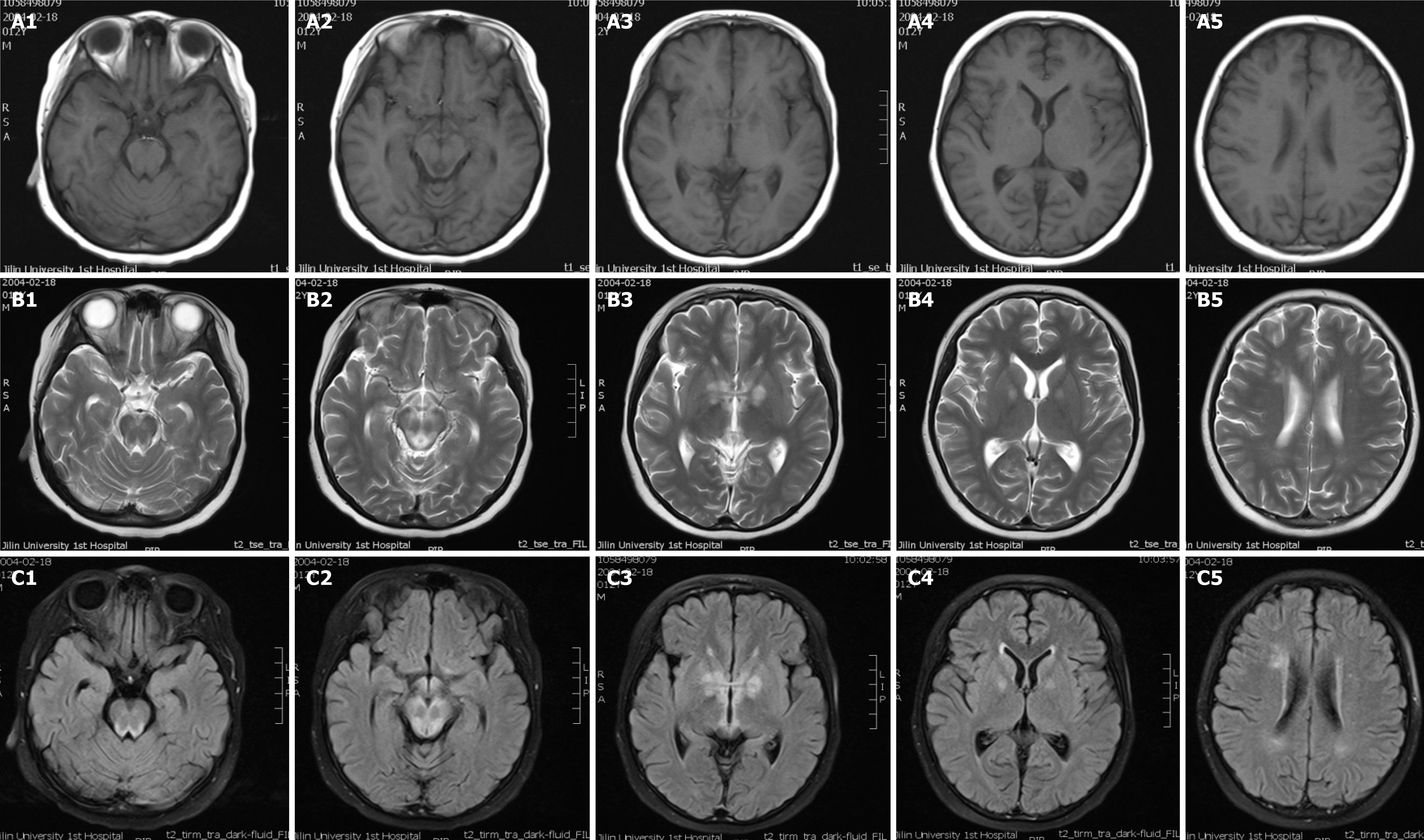
Electroencephalogram analysis revealed slow-waves of background activity. The brain MRI scan showed symmetrical abnormal signals in the basal ganglia, medial thalami, periaqueductal region of the midbrain and pons, and the bilateral white matter around the lateral ventricles (Figure 2).
The physician diagnosed mitochondrial encephalopathy or LS based on the clinical phenotypes, the observation of lactic acidosis and cerebral lesions, as well as the gene sequencing analysis. This case was an unusual late-onset and fulminate form of LS and was maternally inherited.
The patient was treated for 2 mo with antiviral and dehydration medication to reduce intracranial pressure. Otherwise, the physician prescribed therapy (daily 100 mg vitamin B complex, 300 mg vitamin C, 50 mg vitamin E, 90 mg coenzyme Q10, and 300 mg carnitine).
The clinical features of the patient rapidly intensified after admission to the hospital. He died from progressive brainstem dysfunction 2 mo later.
LS (OMIM 256000), also known as subacute necrotizing encephalomyelopathy, is a severe, fatal, early-onset progressive disorder that develops during infancy or childhood. LS is characterized by the presence of bilateral symmetrical necrotizing lesions in the brainstem, basal ganglia, thalamus, and spinal cord[3]. In this report, we described the clinical and molecular features of an unusual late-onset fulminant case in a previously healthy 12-year-old boy with a mitochondrial DNA T9176C mutation (which leads to a severe phenotype).
MRI showed that the patient presented with symmetrical lesions in the basal ganglia, consistent with onset symptoms typically observed in LS patients between 3 and 12 mo old[1]. McKelvie et al[4] reported a case of late-adult onset LS but failed to identify the specific underlying mutation with genetic studies. At the onset of LS, the main parental complaints describe the loss of motor milestones and cognitive deterioration. Nutritional troubles resulting from the decreased ability to feed may precede or exacerbate the underlying encephalopathy, leading to an apparent reduced body and head growth[2]. Rahman et al[3] proposed stringent criteria for defining LS in infants and children. In this study, a 12-year-old boy with late-onset LS was previously healthy with normal body and head growth. The motor and intellectual delay in early age is indispensable for LS diagnosis but was missed in this unusual case. The clinical presentations of LS are highly variable and lead to difficulty in defining diagnostic criteria. Survival is variable, with most patients dying between the ages of 1 mo to 21 years (the average is 2.4 years). Poor survival has been associated with age of disease onset, genetically identified markers, epileptic seizures, brainstem lesions as observed on neuroimaging data, failure to gain weight, and intensive care admission[5-7]. In this study, the onset of the unusual LS case was 12-years-old and led to patient death within 2 mo after hospital admission. This case highlights the variability of phenotypic expression of LS.
MRI of the brain was used to identify rare symmetrical lesions in white matter, in addition to typical symmetrical lesions of gray matter in the basal ganglia and brainstem. Characteristic neuropathologies includes spongiform lesions throughout the brain, including in the basal ganglia, brain stem, and cerebellum[2,8]. In this study, the bilateral white matter around the lateral ventricles was detected in this unusual case, which might be associated with demyelination or gliosis. White matter lesions on MRI are so prominent and can often mimic leukodystrophy. The typical lesions are observed bilaterally and symmetrically throughout the brain but have a particular predilection for the brainstem and are often accompanied by demyelination[1,9,10]. Laboratory test results of this case revealed a positive antibody presence to acetylcholine, presynaptic membrane receptor, and thymoma in blood, suggesting that LS was accompanied by an immune-disorder.
The inheritance pattern of LS can be different from the autosomal recessive, X-linked dominant or maternal pattern. LS can be caused by mutations in a number of both nuclear and mitochondrial genes[11]. Here, we reported a T9176C mutation in the mitochondrial ATPase 6 gene (OMIM 516060) in the late-onset LS patient. A heteroplasmic (T and C) 9176 mutation was also detected in a sample from the patient’s mother. The T9176C mutation was first identified in two brothers who were diagnosed with a milder variant of LS[12] and results in the most common maternally inherited form of LS. Nevertheless, a nucleotide change (T-to-G and T-to-C) at the same location (8993) of the mitochondrial DNA, which affects the ATP 6 subunit of ATPase, is more commonly associated with LS[11]. The degree of heteroplasmy producing LS is likely a key influencing factor that drives different phenotypes.
In conclusion, here we present the case of a 12-year-old boy with late-onset fulminant LS without developmental delay. The T9176C mutation was detected to confirm the severe phenotype of LS. The neuroradiological features were also typical for LS. The bilateral white matter around the lateral ventricles indicated that typical lesions were accompanied by demyelination. Overall, LS is highly variable regarding clinical genotypes and create a persistent challenge for clinicians to diagnosis LS.
Manuscript source: Unsolicited manuscript
Specialty type: Neurosciences
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Al-Haggar M S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Li JH
| 1. | Lake NJ, Bird MJ, Isohanni P, Paetau A. Leigh syndrome: neuropathology and pathogenesis. J Neuropathol Exp Neurol. 2015;74:482-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 2. | Baertling F, Rodenburg RJ, Schaper J, Smeitink JA, Koopman WJ, Mayatepek E, Morava E, Distelmaier F. A guide to diagnosis and treatment of Leigh syndrome. J Neurol Neurosurg Psychiatry. 2014;85:257-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 172] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 3. | Rahman S, Blok RB, Dahl HH, Danks DM, Kirby DM, Chow CW, Christodoulou J, Thorburn DR. Leigh syndrome: clinical features and biochemical and DNA abnormalities. Ann Neurol. 1996;39:343-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 550] [Cited by in RCA: 536] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 4. | McKelvie P, Infeld B, Marotta R, Chin J, Thorburn D, Collins S. Late-adult onset Leigh syndrome. J Clin Neurosci. 2012;19:195-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Sofou K, De Coo IF, Isohanni P, Ostergaard E, Naess K, De Meirleir L, Tzoulis C, Uusimaa J, De Angst IB, Lönnqvist T, Pihko H, Mankinen K, Bindoff LA, Tulinius M, Darin N. A multicenter study on Leigh syndrome: disease course and predictors of survival. Orphanet J Rare Dis. 2014;9:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 167] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 6. | Ruhoy IS, Saneto RP. The genetics of Leigh syndrome and its implications for clinical practice and risk management. Appl Clin Genet. 2014;7:221-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Aulbert W, Weigt-Usinger K, Thiels C, Köhler C, Vorgerd M, Schreiner A, Hoffjan S, Rothoeft T, Wortmann SB, Heyer CM, Podskarbi T, Lücke T. Long survival in Leigh syndrome: new cases and review of literature. Neuropediatrics. 2014;45:346-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Bonfante E, Koenig MK, Adejumo RB, Perinjelil V, Riascos RF. The neuroimaging of Leigh syndrome: case series and review of the literature. Pediatr Radiol. 2016;46:443-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Gerards M, Sallevelt SC, Smeets HJ. Leigh syndrome: Resolving the clinical and genetic heterogeneity paves the way for treatment options. Mol Genet Metab. 2016;117:300-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 10. | Hung PC, Wang HS. A previously undescribed leukodystrophy in Leigh syndrome associated with T9176C mutation of the mitochondrial ATPase 6 gene. Dev Med Child Neurol. 2007;49:65-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Lake NJ, Compton AG, Rahman S, Thorburn DR. Leigh syndrome: One disorder, more than 75 monogenic causes. Ann Neurol. 2016;79:190-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 354] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 12. | Thyagarajan D, Shanske S, Vazquez-Memije M, De Vivo D, DiMauro S. A novel mitochondrial ATPase 6 point mutation in familial bilateral striatal necrosis. Ann Neurol. 1995;38:468-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 126] [Article Influence: 4.2] [Reference Citation Analysis (0)] |