Published online Aug 26, 2021. doi: 10.12998/wjcc.v9.i24.7092
Peer-review started: February 19, 2021
First decision: March 25, 2021
Revised: April 6, 2021
Accepted: July 14, 2021
Article in press: July 14, 2021
Published online: August 26, 2021
Processing time: 185 Days and 14.4 Hours
Cancer of unknown primary (CUP) is a histological proven malignant tumor whose origin cannot be detected despite careful examination. Most cervical lymph node metastases in CUP (80%) will originate from head and neck sites, and 15% show infiltration of squamous carcinoma cells. The survival rates of CUP are poor: The 5-year-survival rate ranges from 10% to 15%. First-line treatment recom
Here, we report a case of a 42-year-old female patient with cervical and abdominal lymph node and distant bone metastases of an occult primary of the head and neck (squamous cell carcinoma, human papillomavirus positive). The cancer was diagnosed during pregnancy 10 years ago, and after giving birth, the patient was treated with cetuximab plus platinum-fluorouracil chemotherapy achieving complete remission (CR). CR lasted 26 mo when new metastases (abdominal lymph node, lumbar vertebral body) emerged. Both manifestations were irradiated. From then on, the patient has not received any further treatment, and her disease has remained controlled. Ten years after the initial cancer diagnosis, the patient is still alive and in good health, representing an exceptional case of HNSCC.
This case illustrates the exceptional clinical course and benefits of combined therapy approaches in advanced metastatic HNSCC with occult primary.
Core Tip: A 42-year-old female patient suffered from metastatic squamous-cell head and neck cancer with occult primary with lymph node and bone metastases. She received palliative immune-chemotherapy with cetuximab, 5-fluorouracil, and platinum as first-line treatment, and subsequent irradiation of further abdominal lymph node and bone metastases. Although survival rates are very poor in this setting, the patient is still alive 10 years after treatment and in complete remission. Here, we present a rare case of long-term survival despite poor prognosis after palliative immunotherapy even before the era of immune checkpoint inhibitors.
- Citation: Große-Thie C, Maletzki C, Junghanss C, Schmidt K. Long-term survivor of metastatic squamous-cell head and neck carcinoma with occult primary after cetuximab-based chemotherapy: A case report. World J Clin Cases 2021; 9(24): 7092-7098
- URL: https://www.wjgnet.com/2307-8960/full/v9/i24/7092.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i24.7092
Cervical lymph node metastases originate from a head and neck site in 80% of cases, and 15% show infiltration of squamous carcinoma cells. Human papillomavirus (HPV) infection is thought to be related to approximately 25% of all head and neck squamous cell carcinoma (HNSCC)[1]. Despite extensive physical examination and medical imaging, the primary lesion remains undetected. Approximately 50% of untreated advanced head and neck cancer patients will die within 4 mo[2]. In patients with metastatic HNSCC of unknown primary, the 5-year-survival rate is also poor, and ranges from 5% to 15%[3,4]. Therefore, treatments are aimed at symptom control and enhancement of quality of life. According to the EXTREME study, first-line treatment for metastatic HNSCC used to be platinum-based chemotherapy combined with fluorouracil and an epidermal growth factor receptor monoclonal antibody (cetuximab)[3] until recently, when checkpoint inhibitors demonstrated clinical benefit. However, the treatment goal for patients with this type of cancer remains palliative. Following EXTREME, response rate is 36%, and even fewer patients became long-term survivors (2.7%)[5]. Patients who did become long-term survivors were defined as at least partial response to therapy after 5 years. The specific reasons why a patient may have an exceptional response to therapy are still obscure. Therefore, clinical and imaging features need to be collected from individual case reports. Here, we report an exceptional case of long-term survival of a woman, who continues to live 10 years since the time of diagnosis.
A 42-year-old woman presented with a painfully swollen right leg and an indolent swelling of the supraclavicular lymph node up to 4 cm in diameter at week 36 of her otherwise unremarkable pregnancy. A deep vein thrombosis was excluded by Doppler sonography. A caesarean section was performed as complaints exacerbated in week 37 of pregnancy. At that time, a new urinary obstruction, grade III, on the right side caused by an extraluminal lymph node required a double J stent. Meanwhile, the supraclavicular lymph node increased further in size.
There was no history of weight loss, night sweats, or fever, but the general state of health declined recently.
The patient had no history of past illness.
The patient had no notable personal or family history. Classical risk factors, tobacco and alcohol, were denied.
On admission, the patient presented with a painfully swollen right leg in combination with indolent swollen right inguinal lymph nodes with diameters of up to 4 cm. Further indolent lymph nodes were found in the left supraclavicular fossa. There were no sensory or motor deficits in her limbs or abnormalities in the cardiovascular system detected on clinical examination. Ear, nose, and throat were inconspicuous by inspection.
Blood analysis showed deviations that could be explained by pregnancy rather than by lymphoma: anemia [hemoglobin 7.1 mmol/L (7.5-10.0 mmol/L)], elevated lactate dehydrogenase [298 U/L (< 247 U/L)]; faster sed rate [54 mm/h (< 20 mm/h)]. There were no signs of hemolysis, thrombopenia, or leucopenia. Initially, the only elevated tumor marker was CA 125 (46.1 U/mL; normal range < 35 U/mL). Elevation was interpreted as pregnancy related[6]. CEA, CA 19-9, CYFRA 21-1, and NSE were within the normal range. There was evidence of a previous Epstein-Barr virus infection. Immunofixation was unremarkable.
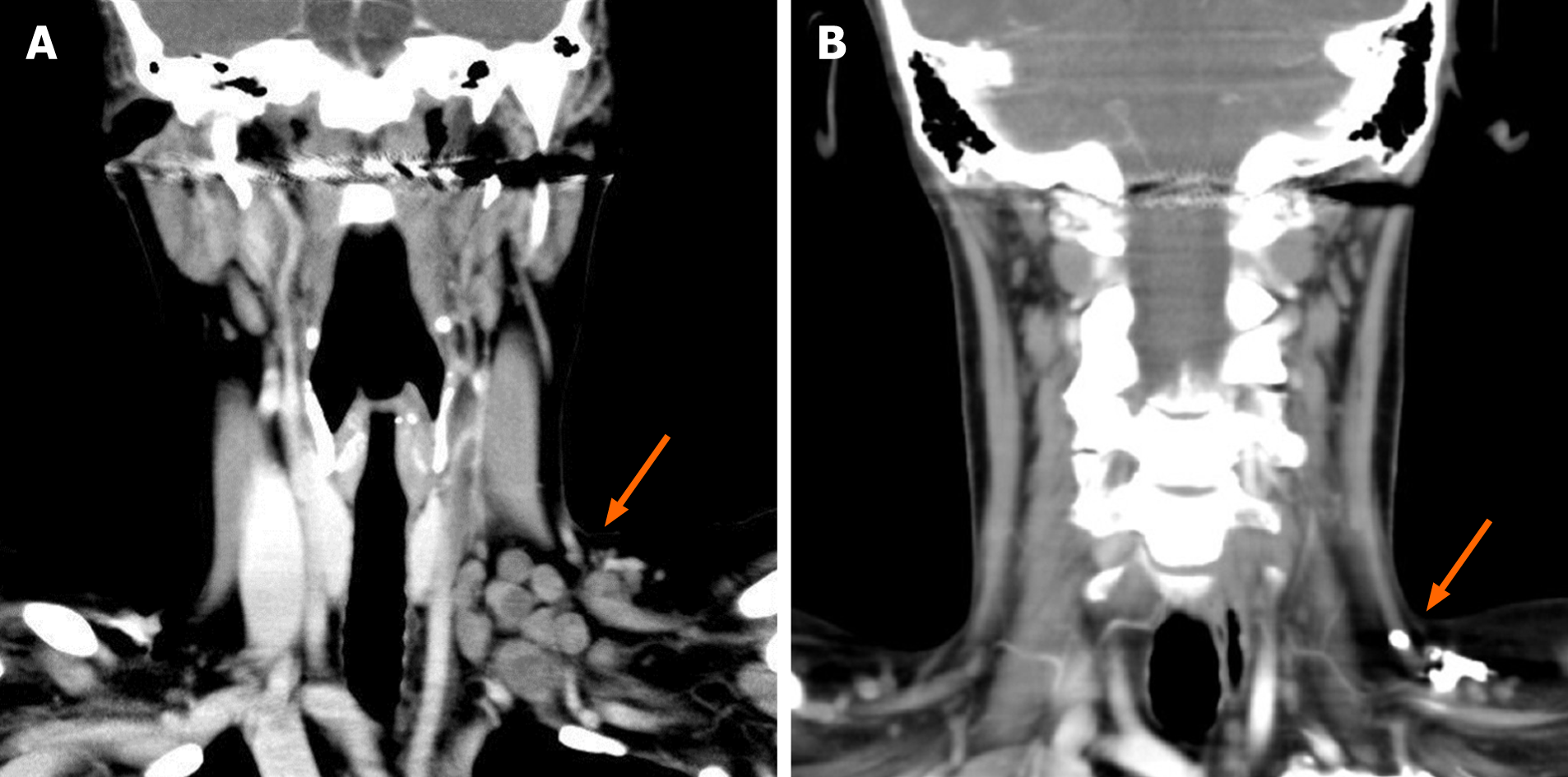
Swelling of the cervical, supraclavicular, abdominal, and inguinal lymph nodes up to 4 cm in diameter were detected using ultrasonography. The right renal pelvis was dilated due to an external compression of the ureter by lymph nodes. Computed tomography of the whole body confirmed the above-mentioned swollen lymph nodes and showed compression of the right renal artery with minor perfusion of the right kidney (Figure 1). There were osteolyses of the third and fifth lumbar vertebral bodies, as well as of lower cervical vertebral bodies.
Gynecologic, dermatologic, and urologic admissions could not detect a primary tumor. Gastroscopy, colonoscopy, and bronchoscopy remained inconspicuous. Histologic diagnosis was made by an excisional biopsy of a cervical lymph node on the left side.
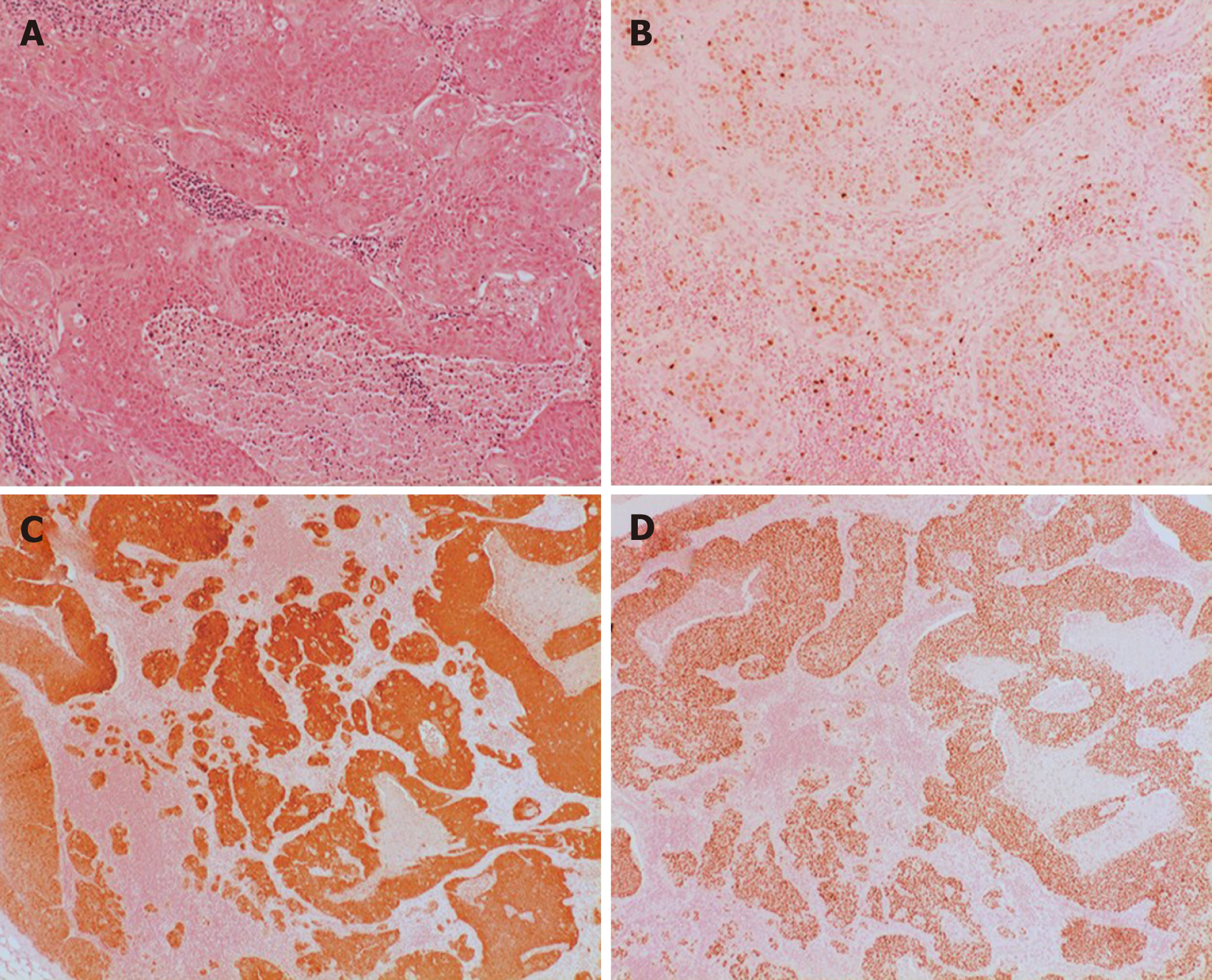
Hematoxylin and eosin stain described metastasis of moderate differentiation. The proliferation index, Ki67, was high. Tumor cell were immunoreactive for p40, confirming squamous cell differentiation. P16 immunohistochemistry showed diffuse cytoplasmatic and nuclear reactions in most of the tumor cells, which is a surrogate marker for HPV infection (Figure 2).
Metastatic, HPV positive HNSCC of unknown primary.
The patient received 6 courses of 5-fluorouracil, platinum, and cetuximab as described in the EXTREME study[3] (5-fluorouracil 1000 mg/m²; cisplatin 40 mg/m²; cetuximab first course 400 mg/m², following courses 250 mg/m²). After the fifth course, cisplatin was replaced by carboplatin (area under the curve 5) because of severe emesis. Docetaxel (70 mg/m²) was added to the first course. Acute toxicity during the therapy included severe nausea with emesis grade 4 and mucositis grade 3. Apart from this, the patient tolerated the treatment well.
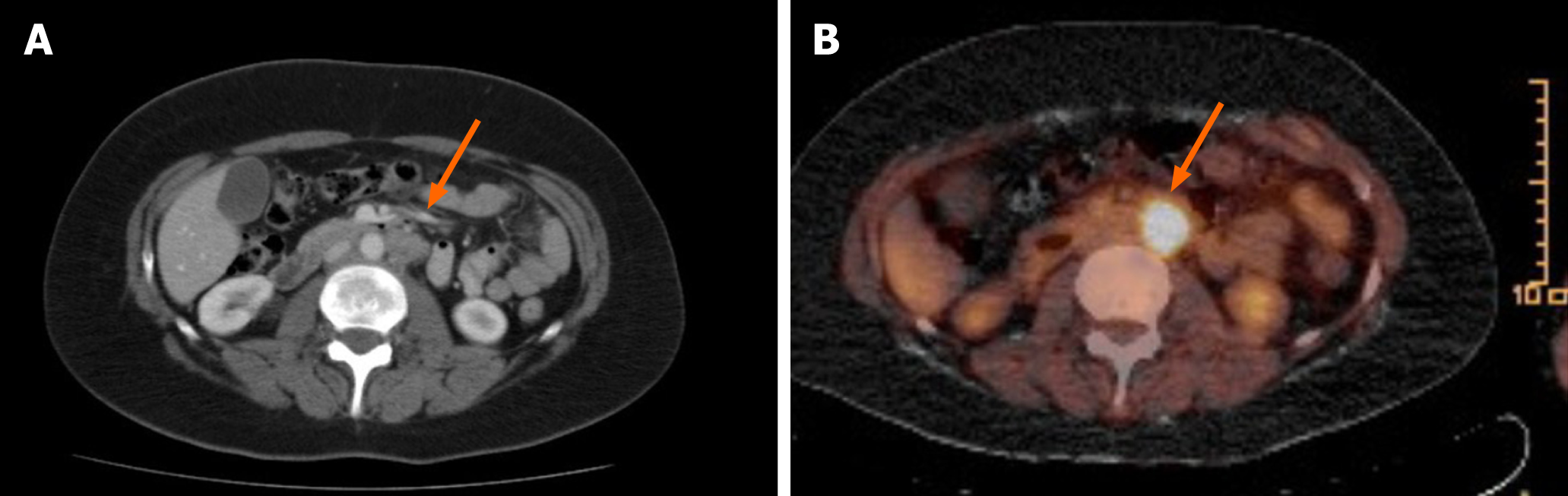
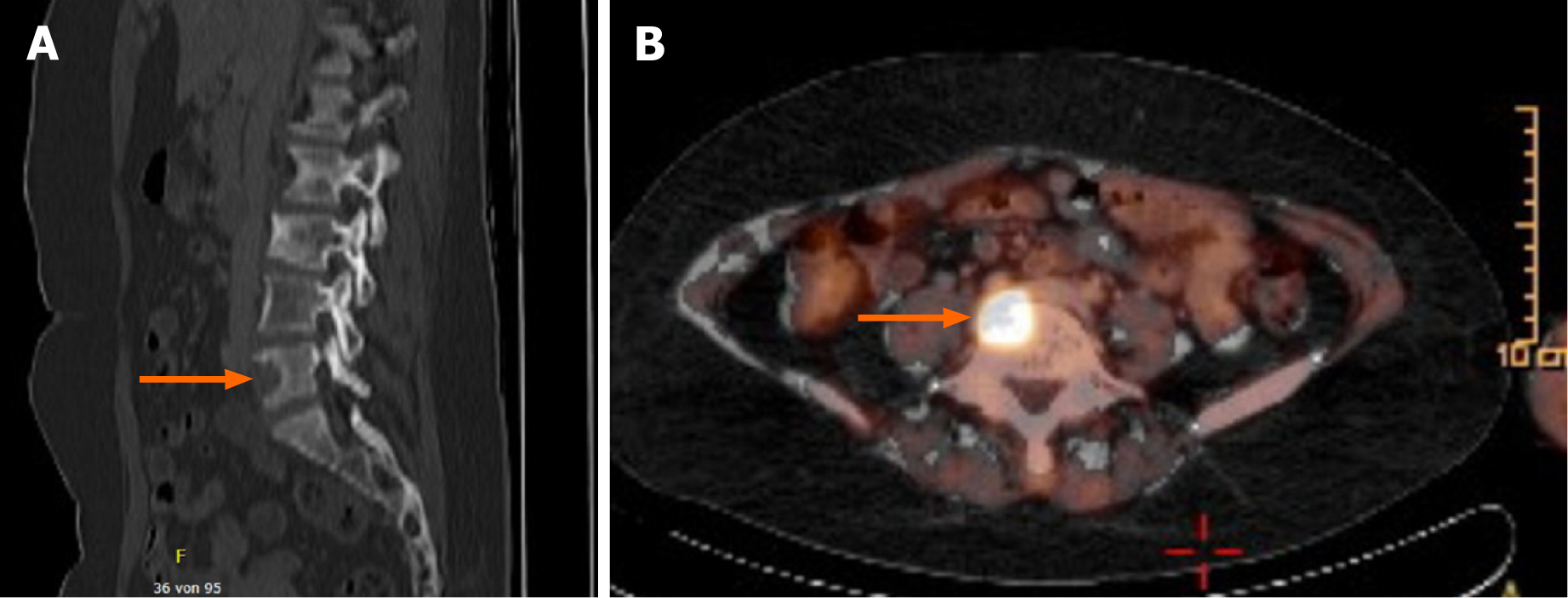
The patient achieved CR after four courses (Figure 1). During follow up (clinical examination and computer tomographic scan every 3 mo), the patient remained in CR until 26 mo, when in a fluorodeoxyglucose positron emission tomography (FDG-PET) signal positive lymph nodes in the para-aortic region were detected (Figure 3). Therapy was comprised of irradiation (36 gray). Only 4 mo later, a likewise FDG-PET positive bone metastasis in the fifth lumbar bone was detected and irradiated (35 gray) (Figure 4). No other metastasis, as surveilled by clinical examination and computed tomography scan, occurred from then on. The patient is living 10 years after her cancer diagnosis, remains in CR, and the general state of health is Eastern Cooperative Oncology Group (ECOG) performance status 0.
The prognosis of metastatic HNSCC with unknown primary is dismal. A placebo-controlled, randomized study performed by ECOG showed a better response rate to treatment with cetuximab and cisplatin (26%) than to cisplatin alone (10%)[7]. Overall survival (OS) and the rate of long-term survivors improved with the implementation of cetuximab-based therapies as well[8], but expected survival is still 10 mo according to the EXTREME study (open-label, randomized, phase III trial)[3,5]. The population of long-term survivors has been neglected due to its rarity. Few case reports have been published describing this clinically significant subgroup[9-11]. Linares et al[5] describe the prevalence of long-term surviving patients at 2.7% (7/225). Reliable biomarkers in advanced HNSCC are limited. Single case reports of long-term survival merit attention because they may yield information about predicting markers of good response. Argiris et al[12] described some clinical factors to be predictive for better OS in advanced HNSCC, such as limited weight loss, good performance status, good tumor cell differentiation, and no prior radiation. Our patient had ECOG 0 performance status, no prior radiation, and moderate cell differentiation consistent with her long-term survival. Weight loss was not a reliable parameter because of pregnancy.
In addition to traditional carcinogens like alcohol and tobacco, it became apparent that HPV infection is associated with HNSCC, and p16 status is used as a surrogate marker of HPV infection[13,14]. Rosenthal et al[15] demonstrated that p16-positive patients have a longer OS than p16-negative patients. These findings led to a revision of the staging system by the American Joint Committee on Cancer and the Union for International Cancer Control (UICC) in 2016. Alterations of the tumor staging from UICC7 to UICC8 included mainly UICC stage III or IVa according to the 7th edition. Our patient remained at the same stage UICC IVc, and had in both tumor, node and metastasis (TNM) classifications the lowest OS (OS UICC IVc TNM7 12 mo; OS UICC IVc TNM8 5 mo[16]). But regardless of p16 status, the addition of cetuximab to radiation therapy improved patient outcome. This is in accordance with the finding that 90% of HPV negative HNSCC show EGFR overexpression[17]. Nevertheless, overexpression of EGFR and the EGFR copy number alterations[18] are not predictive of the response to cetuximab[19,20]. Thus, p16 overexpression should be included in treatment planning, but cannot be the only predictive factor of long-term survival of our patient. Gene expression profiling, as a new diagnostic technique, has the potential to identify the origin of tumor tissue. It raises expectations that survival will improve by site-specific treatment. So far, there is no significant improvement in 1-year survival from site-specific treatment in comparison to empirical therapy[21] for patients with cancer of unknown primary. Meanwhile, in this case, cetuximab-based therapy as site-specific therapy was successful. This suggests that site-specific therapy has a prognostic value.
We believe that long-term survivors in the HNSCC CUP setting demand our attention, as it reveals unexpected treatment results and identifies biomarkers besides p16 status and EGFR overexpression.
The present case of long-term survival demonstrates that a cetuximab-based therapy has the potential to succeed. A key future question will be to identify biomarkers that predict an outstanding and long-lasting therapy response. To our knowledge, thus far, there is only limited data on long-term survivors in advanced HNSCC. Hopefully, immunotherapy will increase the prevalence and provide the unique chance to focus on this subgroup.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Deng JY, Mitani S, Uraguchi K, Yang TY S-Editor: Zhang H L-Editor: Filipodia P-Editor: Zhang YL
| 1. | D'Souza G, Dempsey A. The role of HPV in head and neck cancer and review of the HPV vaccine. Prev Med. 2011;53 Suppl 1:S5-S11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 183] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 2. | Kowalski LP, Carvalho AL. Natural history of untreated head and neck cancer. Eur J Cancer. 2000;36:1032-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 137] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 3. | Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol D, Peyrade F, Benasso M, Vynnychenko I, De Raucourt D, Bokemeyer C, Schueler A, Amellal N, Hitt R. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2546] [Cited by in RCA: 2544] [Article Influence: 149.6] [Reference Citation Analysis (0)] |
| 4. | Randén M, Rutqvist LE, Johansson H. Cancer patients without a known primary: incidence and survival trends in Sweden 1960-2007. Acta Oncol. 2009;48:915-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Linares J, Rullan A, Taberna M, Vazquez S, Mesia R. Emergence of long-term surviving patients with the introduction of Cetuximab in recurrent/metastatic disease of squamous cell carcinoma of head and neck. Oral Oncol. 2016;55:e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Ercan Ş, Kaymaz Ö, Yücel N, Orçun A. Serum concentrations of CA 125, CA 15-3, CA 19-9 and CEA in normal pregnancy: a longitudinal study. Arch Gynecol Obstet. 2012;285:579-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Burtness B, Goldwasser MA, Flood W, Mattar B, Forastiere AA; Eastern Cooperative Oncology Group. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol. 2005;23:8646-8654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 705] [Cited by in RCA: 628] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 8. | Sacco AG, Cohen EE. Current Treatment Options for Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma. J Clin Oncol. 2015;33:3305-3313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 255] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 9. | Verduzco-Rodríguez L, Aguirre-González EH, Verduzco-Aguirre HC. Durable complete response induced by paclitaxel-nimotuzumab-methotrexate chemotherapy in a patient with metastatic head and neck squamous cell carcinoma. Hematol Oncol Stem Cell Ther. 2011;4:182-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Guigay J, Even C, Mayache-Badis L, Debbah M, Saada-Bouzid E, Tao Y, Deschamps F, Janot F, Lezghed N, Michel C. Long-term response in patient with recurrent oropharyngeal carcinoma treated with cetuximab, docetaxel and cisplatin (TPEx) as first-line treatment followed by cetuximab maintenance. Oral Oncol. 2017;68:114-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Szturz P, Specenier P, Van Laer C, Van Den Weyngaert D, Corthouts B, Carp L, Van Marck E, Vanderveken O, Vermorken JB. Long-term remission of locally recurrent oropharyngeal cancer after docetaxel-based chemotherapy plus cetuximab. Eur Arch Otorhinolaryngol. 2016;273:1629-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Argiris A, Li Y, Forastiere A. Prognostic factors and long-term survivorship in patients with recurrent or metastatic carcinoma of the head and neck. Cancer. 2004;101:2222-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 146] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Salazar CR, Anayannis N, Smith RV, Wang Y, Haigentz M Jr, Garg M, Schiff BA, Kawachi N, Elman J, Belbin TJ, Prystowsky MB, Burk RD, Schlecht NF. Combined P16 and human papillomavirus testing predicts head and neck cancer survival. Int J Cancer. 2014;135:2404-2412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 14. | Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, Zahurak ML, Daniel RW, Viglione M, Symer DE, Shah KV, Sidransky D. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2225] [Cited by in RCA: 2190] [Article Influence: 87.6] [Reference Citation Analysis (0)] |
| 15. | Rosenthal DI, Harari PM, Giralt J, Bell D, Raben D, Liu J, Schulten J, Ang KK, Bonner JA. Association of Human Papillomavirus and p16 Status With Outcomes in the IMCL-9815 Phase III Registration Trial for Patients With Locoregionally Advanced Oropharyngeal Squamous Cell Carcinoma of the Head and Neck Treated With Radiotherapy With or Without Cetuximab. J Clin Oncol. 2016;34:1300-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 162] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 16. | Boeker R, Stromberger C, Heiland M, Beck-Broichsitter B, Hofmann VM, Neumann K, Ochsenreither S, Olze H, Dommerich S, Piwonski I, Coordes A. Carcinoma of Unknown Primary and the 8th Edition TNM Classification for Head and Neck Cancer. Laryngoscope. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Rubin Grandis J, Melhem MF, Gooding WE, Day R, Holst VA, Wagener MM, Drenning SD, Tweardy DJ. Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. J Natl Cancer Inst. 1998;90:824-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 813] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 18. | Temam S, Kawaguchi H, El-Naggar AK, Jelinek J, Tang H, Liu DD, Lang W, Issa JP, Lee JJ, Mao L. Epidermal growth factor receptor copy number alterations correlate with poor clinical outcome in patients with head and neck squamous cancer. J Clin Oncol. 2007;25:2164-2170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 291] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 19. | Licitra L, Störkel S, Kerr KM, Van Cutsem E, Pirker R, Hirsch FR, Vermorken JB, von Heydebreck A, Esser R, Celik I, Ciardiello F. Predictive value of epidermal growth factor receptor expression for first-line chemotherapy plus cetuximab in patients with head and neck and colorectal cancer: analysis of data from the EXTREME and CRYSTAL studies. Eur J Cancer. 2013;49:1161-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 121] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 20. | Licitra L, Mesia R, Rivera F, Remenár É, Hitt R, Erfán J, Rottey S, Kawecki A, Zabolotnyy D, Benasso M, Störkel S, Senger S, Stroh C, Vermorken JB. Evaluation of EGFR gene copy number as a predictive biomarker for the efficacy of cetuximab in combination with chemotherapy in the first-line treatment of recurrent and/or metastatic squamous cell carcinoma of the head and neck: EXTREME study. Ann Oncol. 2011;22:1078-1087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 183] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 21. | Hayashi H, Kurata T, Takiguchi Y, Arai M, Takeda K, Akiyoshi K, Matsumoto K, Onoe T, Mukai H, Matsubara N, Minami H, Toyoda M, Onozawa Y, Ono A, Fujita Y, Sakai K, Koh Y, Takeuchi A, Ohashi Y, Nishio K, Nakagawa K. Randomized Phase II Trial Comparing Site-Specific Treatment Based on Gene Expression Profiling With Carboplatin and Paclitaxel for Patients With Cancer of Unknown Primary Site. J Clin Oncol. 2019;37:570-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (0)] |