Published online Aug 26, 2021. doi: 10.12998/wjcc.v9.i24.7009
Peer-review started: March 13, 2021
First decision: June 3, 2021
Revised: June 12, 2021
Accepted: July 5, 2021
Article in press: July 5, 2021
Published online: August 26, 2021
Processing time: 163 Days and 12.6 Hours
Surgery is the primary curative option in patients with hepatocellular carcinoma (HCC). However, recurrence within 2 years is observed in 30%–50% of patients, being a major cause of mortality.
To construct and verify a non-invasive prediction model combining contrast-enhanced ultrasound (CEUS) with serology biomarkers to predict the early recurrence of HCC.
Records of 744 consecutive patients undergoing first-line curative surgery for HCC in one institution from 2016–2018 were reviewed, and 292 local patients were selected for analysis. General characteristics including gender and age, CEUS liver imaging reporting and data system (LIRADS) parameters including wash-in time, wash-in type, wash-out time, and wash-out type, and serology biomarkers including alanine aminotransferase, aspartate aminotransferase, platelets, and alpha-fetoprotein (AFP) were collected. Univariate analysis and multivariate Cox proportional hazards regression model were used to evaluate the independent prognostic factors for tumor recurrence. Then a nomogram called CEUS model was constructed. The CEUS model was then used to predict recurrence at 6 mo, 12 mo, and 24 mo, the cut-off value was calculate by X-tile, and each C-index was calculated. Then Kaplan-Meier curve was compared by log-rank test. The calibration curves of each time were depicted.
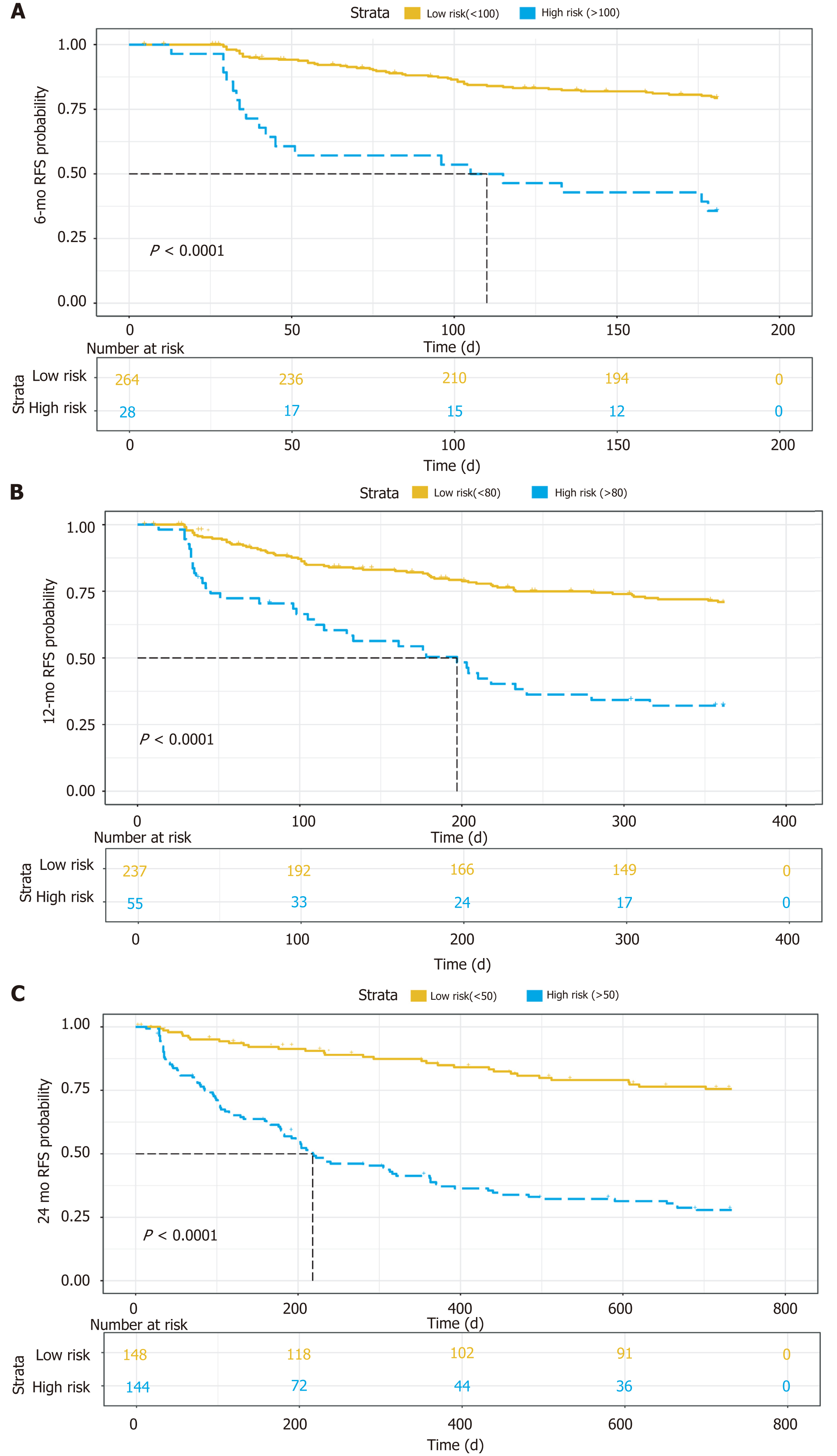
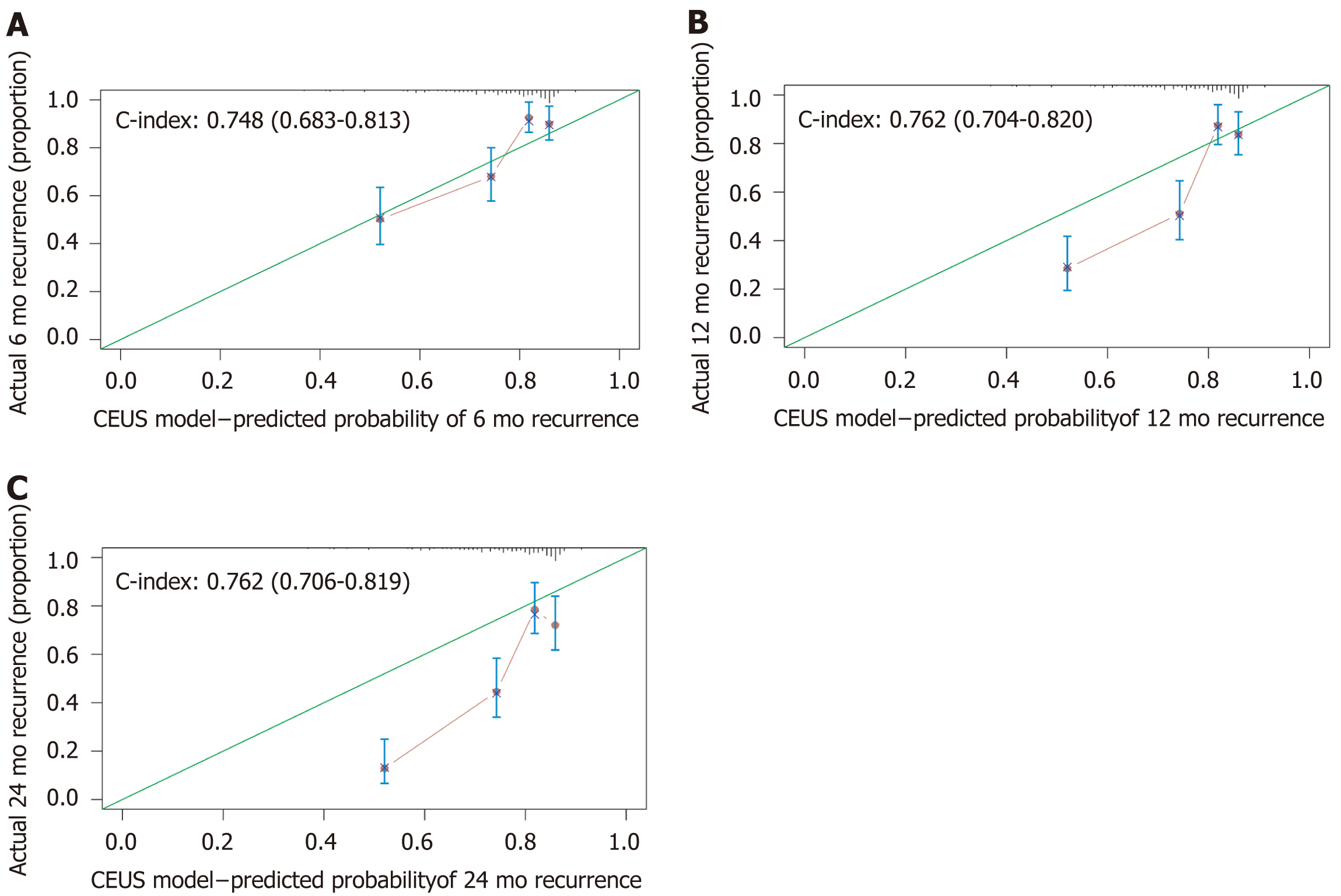
A nomogram predicting early recurrence (ER), named CEUS model, was formulated based on the results of the multivariate Cox regression analysis. This nomogram incorporated tumor diameter, preoperative AFP level, and LIRADS, and the hazard ratio was 1.123 (95% confidence interval [CI]: 1.041-1.211), 1.547 (95%CI: 1.245-1.922), and 1.428 (95%CI: 1.059-1.925), respectively. The cut-off value at 6 mo, 12 mo, and 24 mo was 100, 80, and 50, and the C-index was 0.748 (95%CI: 0.683-0.813), 0.762 (95%CI: 0.704-0.820), and 0.762 (95%CI: 0.706-0.819), respectively. The model showed satisfactory results, and the calibration at 6 mo was desirable; however, the calibration at 12 and 24 mo should be improved.
The CEUS model enables the well-calibrated individualized prediction of ER before surgery and may represent a novel tool for biomarker research and individual counseling.
Core Tip: This study aimed to construct and verify a non-invasive prediction model combining contrast-enhanced ultrasound with serology biomarkers to predict the early recurrence of hepatocellular carcinoma. Records of 292 local patients of hepatocellular carcinoma (HCC) were selected for analysis. A nomogram predicting early recurrence (ER) named contrasted-enhanced ultrasound (CEUS) model, incorporating tumor diameter, preoperative alpha-fetoprotein level, and LIRADS, was developed. The model showed satisfactory results, and the C-index was 0.762 (95%CI: 0.706–0.819). The calibration at 6 mo was desirable. The CEUS model enables the well-calibrated individualized prediction of ER before surgery and may represent a novel tool for biomarker research and individual counseling.
- Citation: Tu HB, Chen LH, Huang YJ, Feng SY, Lin JL, Zeng YY. Novel model combining contrast-enhanced ultrasound with serology predicts hepatocellular carcinoma recurrence after hepatectomy. World J Clin Cases 2021; 9(24): 7009-7021
- URL: https://www.wjgnet.com/2307-8960/full/v9/i24/7009.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i24.7009
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the third leading cause of cancer-related deaths worldwide[1]. Surgery is the primary curative option in patients with HCC. However, recurrence within 2 years occurs in 30%–50% of patients and is the major cause of mortality[2,3]. Identifying patients with a high recurrence risk after surgery is important so that clinicians can provide appropriate monitoring to detect the earliest stage of recurrent HCC in time and treatment may still be feasible.
The widely used tumor staging system, such as tumor node metastasis (TNM) and Barcelona clinic liver cancer (BCLC) staging, is useful for guiding surgery; however, their ability to predict recurrence has not been fully evaluated. Some models, such as the Singapore Liver Cancer Recurrence score[4], the Korean model[5], and the Surgery-Specific Cancer of the Liver Italian Program[6], have been specially developed to detect tumor recurrence after surgical resection; however, the most important parameter, that is, microvascular invasion, can only be evaluated pathologically on the resected specimens. A prognostic model that only requires preoperative available parameters may assist surgeons in planning a reasonable treatment strategy.
With the development of radiology technology and serology detection ability, predicting postoperative recurrence noninvasively is possible. Many scholars have used radiology technology or serological biomarkers to achieve this goal[7,8], but only a few have combined the two technologies; the combination of ultrasound and serological biomarkers has not been used for preoperative prediction. Ultrasound examination is favored by clinicians and patients for its lack of radiation, repeatability, and real-time monitoring. Contrast-enhanced ultrasound (CEUS) can observe tumor hemodynamics in real time, which plays an important role in evaluating the nature of a tumor. In this study, a nomogram model was constructed by fusing CEUS and a serological biomarker. The predictive ability of the model was evaluated and validated by internal resampling.
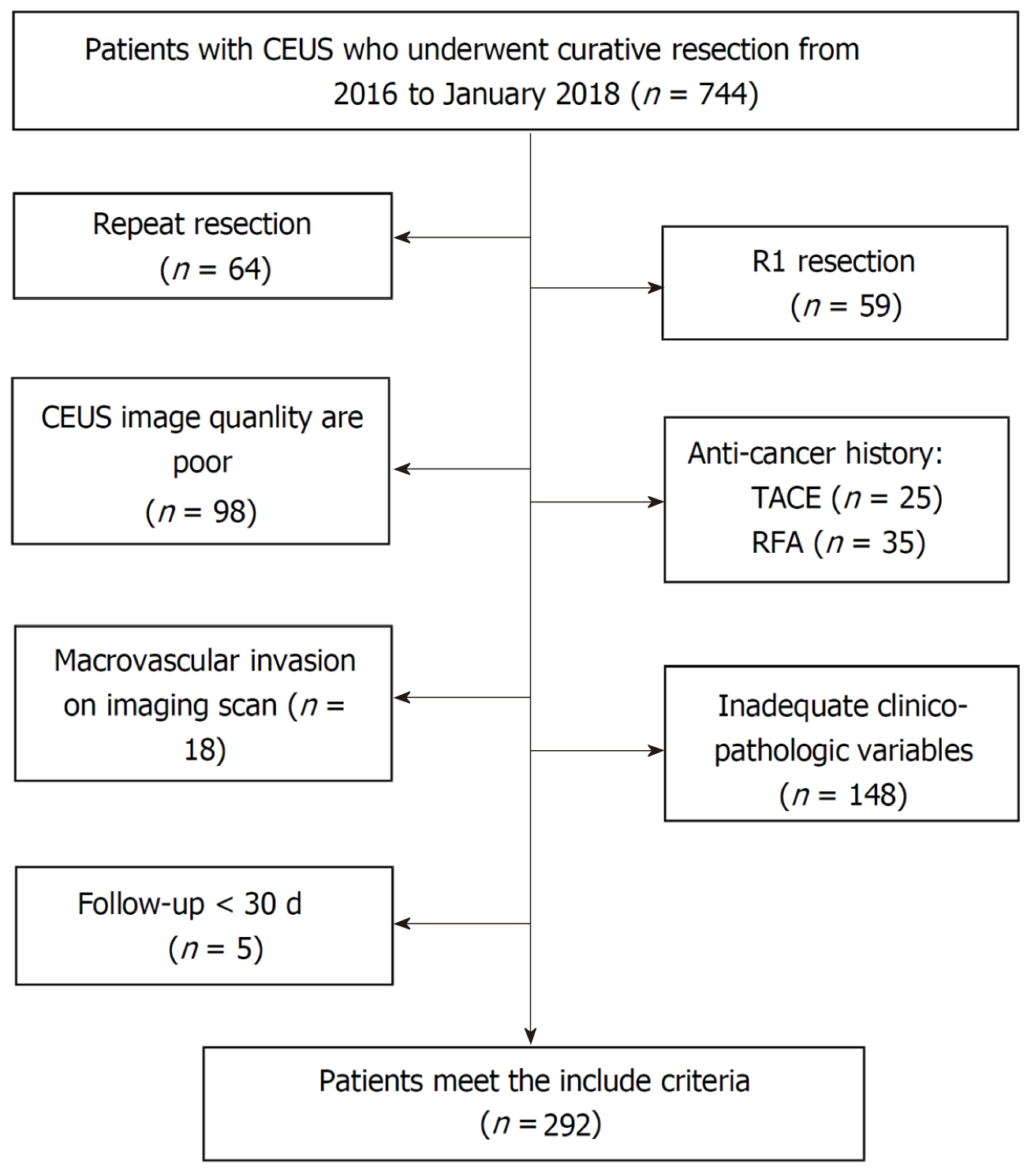
This study was approved by the hospital’s ethics committee (No. 2020-010-01), and all participating patients provided written informed consent. Between January 2016 and January 2018, 744 consecutive patients meeting the study’s criteria who underwent curative resection at our institution were retrospectively recruited. The exclusion criteria were as follows: (1) CEUS of the liver was unavailable; (2) CEUS was per
Ultrasound scanning was performed by a radiologist (LJL) who had 30 years of conventional ultrasound experience and 15 years of CEUS experience. She used a multi-frequency (5–2 MHz) convex array probe (C5-2) (Esaote) on a Mylab 90 system, and digital storage was recorded at the same time. Before CEUS, the whole liver must be thoroughly examined using a gray-scale ultrasound. Baseline scan included evaluation of lesions on B-mode imaging and color Doppler ultrasound in com
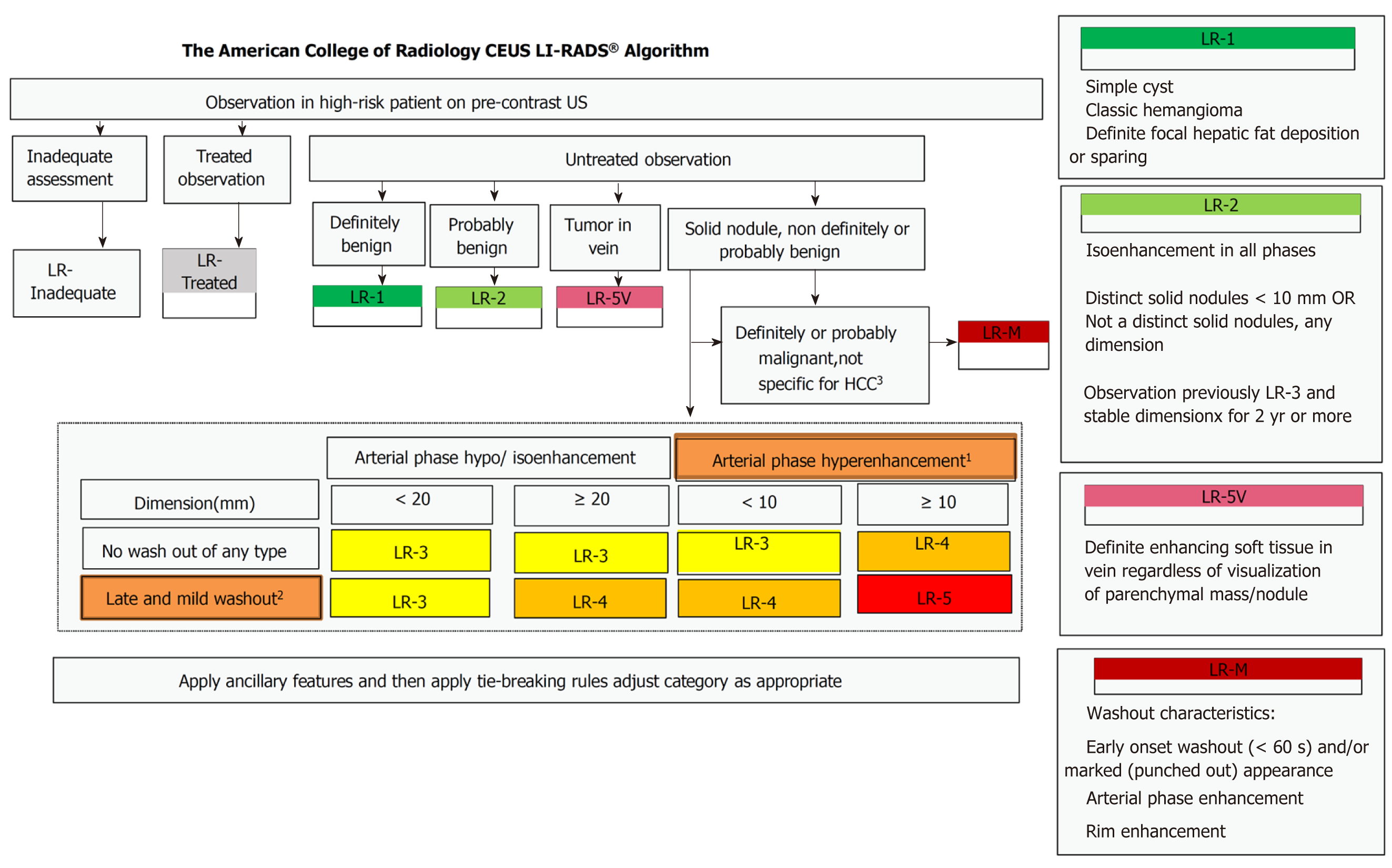
Before CEUS was performed, each patient rested for 10 min and received breathing training to obtain the best image. In CEUS, 2.4 mL of a microbubble contrast agent (Sonovue, Bracco, Milan, Italy) was rapidly injected via an antecubital vein followed by a 10 mL saline flush. A sufficiently large needle (20-gauge minimum diameter) was used to avoid bubble rupture. A low mechanical index (< 0.1) was used for CEUS examination. Different dynamic phases of contrast enhancement were identified in the liver study after a microbubble-based contrast agent was injected. The contrast side-by-side mode was used via a live dual-image display. The CEUS terminology follows the ACR CEUS LI-RADS working group standard[9]. Continuous scanning was used to observe the tumor. The duration of the study included the arterial phase (0–30 s), portal phase (31–120 s), and late phase (121–300 s). Three radiologists with 5 years of working experience with CEUS classified the tumor according to the LI-RADS (Figure 2)[10], and 5v was defined as 6. If more than one tumor was detected, the biggest one was reviewed. The following parameters were also collected: (1) The maximum diameter of the lesion; (2) Starting time (the time from injection to entering the tumor); (3) Peak time (the time from the injection to the maximum intensity of the tumor); (4) Iso-time (the time from the injection to tumor enhancement was equal to peripheral); (5) Washout time (the time from the injection to the tumor enhancement lower than peripheral); (6) Enhancement type (fast-in fast-out and non-fast-in fast-out); (7) Wash-in type (fast-in and non-fast-in); (8) Washout type (fast-out, < 30 s, and non-fast-out, ≥ 30 s); (9) Enhancement echogenicity (homogeneous and inhomogeneous); and (10) Tumor location (half liver and non-half-liver, half-liver means that the tumor was located only in the left or right liver).
Laboratory data within 3 d of morning fasting blood before curative treatment were obtained from medical records, which included alanine aminotransferase, aspartate aminotransferase, platelet, albumin, total bilirubin, serum creatinine, prothrombin time, prothrombin time activity, and international normalized ratio. Alpha-fetoprotein (AFP) was divided into 0–20 μg/L, 20–200 μg/L, and > 200 μg/L.
After resection, follow-up examination was performed every 3 mo during the study period, and data on serum AFP, liver function tests, contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) of the chest and abdomen, and ultrasound of the abdomen were obtained. The data were censored on January 3, 2020. Recurrence-free survival was defined as the time from the date of surgery to the date of first recurrence, metastasis, or last follow-up.
Continuous variables are expressed as the mean ± SD and were compared using an unpaired, 2-tailed t-test or Mann–Whitney test. Categorical variables were compared using the χ2 test or Fisher exact test. Survival curves were calculated using the Kaplan–Meier method and compared using the log-rank test. Multivariate Cox proportional hazards regression model was used to evaluate the independent prognostic factors for tumor recurrence. All variables associated with recurrence at a significant level were candidates for stepwise multivariate analysis. A nomogram was formulated based on the results of multivariate Cox regression analysis and by using the rms package of R, version 3.6.3 (http://www.r-project.org/). The nomogram is based on proportionally converting each regression coefficient in Cox regression to a 0-to-100-point scale. The effect of the variable with the highest β coefficient (absolute value) is assigned 100 points. The points are added across independent variables to derive the total points, which are converted to predict recurrence probabilities. The predictive performance of the nomogram was measured using C-index and calibration with 1000 bootstrap samples to decrease the over fit bias. For clinical use of the model, we tested the model’s prediction ability at 6, 12, and 24 mo and used X-tile, version 3.6.1 to calculate the optimal cut-off values. Then, Kaplan–Meier curve was analyzed[11]. The prediction error of the CEUS model and variables was assessed using the “Boot632plus” split method with 1000 iterations to calculate estimates of prediction error curves and is summarized as the integrated Brier score, which reflects a weighted average of the squared distances between observed recurrence status and predicted recurrence probability of a model; the integrated Brier score represents a valid measure of overall model performance and can range from 0, for a perfect model, to 0.25, for a noninformative model with a 50% incidence of the outcome[12]. In all analyses, P < 0.05 was considered statistically significant. All analyses were performed using R, version 3.6.3.
The basic characteristics are shown in Table 1. We collected 292 patients, including 238 males and 54 females, with a mean age of 55.7 ± 11.2 years.
| Total | Non-recurrence | Recurrence | P value | |
| Diameter (mean ± SD, cm) | 3.9 ± 2.3 | 3.2 ± 1.5 | 4.8 ± 2.8 | < 0.001 |
| Age (mean ± SD, yr) | 55.7 ± 11.2 | 54.9 ± 11.3 | 56.7 ± 11.1 | 0.17 |
| ALT (mean ± SD, U/L) | 35.5 ± 30.1 | 31.5 ± 21.4 | 40.7 ± 38.1 | 0.016 |
| AST (mean ± SD, U/L) | 36.1 ± 34.6 | 30.4 ± 15.3 | 43.5 ± 48.9 | 0.11 |
| PLT (mean ± SD, /L) | 150.2 ± 66.4 | 145.0 ± 62.9 | 157.2 ± 70.4 | 0.16 |
| ALB (mean ± SD, g/L) | 38.8 ± 4.6 | 38.7 ± 4.8 | 38.9 ± 4.3 | 0.94 |
| TBIL (mean ± SD, μmol/L) | 17.6 ± 8.9 | 16.9 ± 8.3 | 18.6 ± 9.6 | 0.16 |
| Cr (mean ± SD, μmol/L) | 74.0 ± 17.5 | 74.3 ± 19.8 | 73.6 ± 14.0 | 0.42 |
| PT (mean ± SD, s) | 13.8 ± 1.2 | 13.9 ± 1.2 | 13.8 ± 1.3 | 0.49 |
| PTA(mean ± SD, ) | 92.6 ± 14.9 | 92.3 ± 13.7 | 92.9 ± 16.3 | 0.91 |
| INR (mean ± SD) | 1.1 ± 0.1 | 1.1 ± 0.1 | 1.1 ± 0.1 | 0.97 |
| Start time (mean ± SD, s) | 16.8 ± 3.7 | 17.1 ± 3.9 | 16.5 ± 3.5 | 0.17 |
| Peak time (mean ± SD, s) | 24.1 ± 5.6 | 24.1 ± 5.3 | 24.0 ± 6.0 | 0.45 |
| Iso-time (mean ± SD, s) | 39.1 ± 16.9 | 40.9 ± 19.8 | 36.8 ± 11.7 | 0.079 |
| Washout time (mean ± SD, s) | 94.8 ± 63.3 | 98.5 ± 63.3 | 89.8 ± 63.3 | 0.11 |
| Sex, n (%) | ||||
| Male | 238 (81.5) | 129 (77.7) | 109 (86.5) | 0.068 |
| Female | 54 (18.5) | 37 (22.3) | 17 (13.5) | |
| LIRADS, n (%) | ||||
| 3 | 10 (3.4) | 8 (4.8) | 2 (1.6) | < 0.001 |
| 4 | 176 (60.3) | 118 (71.1) | 58 (46.0) | |
| 5 | 95 (32.5) | 37 (22.3) | 58 (46.0) | |
| 6 | 11 (3.8) | 3 (1.8) | 8 (6.3) | |
| AFP [n (%), μg/L] | ||||
| < 20 | 193 (66.1) | 133 (80.1) | 60 (47.6) | < 0.001 |
| 20-200 | 32 (11.0) | 13 (7.8) | 19 (15.1) | |
| > 200 | 67 (22.9) | 20 (12.0) | 47 (37.3) | |
| Enhancement type, n (%) | ||||
| Non-fast-in fast-out | 42 (14.4) | 24 (14.5) | 18 (14.3) | 1.00 |
| Fast-in fast-out | 250 (85.6) | 142 (85.5) | 108 (85.7) | |
| Wash-in type, n (%) | ||||
| Non-fast-in | 4 (1.4) | 2 (1.2) | 2 (1.6) | 1.00 |
| Fast-in | 288 (98.6) | 164 (98.8) | 124 (98.4) | |
| Washout type, n (%) | ||||
| Non-fast-out | 80 (27.4) | 41 (24.7) | 39 (31.0) | 0.29 |
| Fast-out | 212 (72.6) | 125 (75.3) | 87 (69.0) | |
| Enhancement type, n (%) | ||||
| Homogeneous enhancement | 223 (76.4) | 127 (76.5) | 96 (76.2) | 1.00 |
| Inhomogeneous enhancement | 69 (23.6) | 39 (23.5) | 30 (23.8) | |
| Location, n (%) | ||||
| Half liver | 261 (89.4) | 148 (89.2) | 113 (89.7) | 1.00 |
| Non-half liver | 31 (10.6) | 18 (10.8) | 13 (10.3) | |
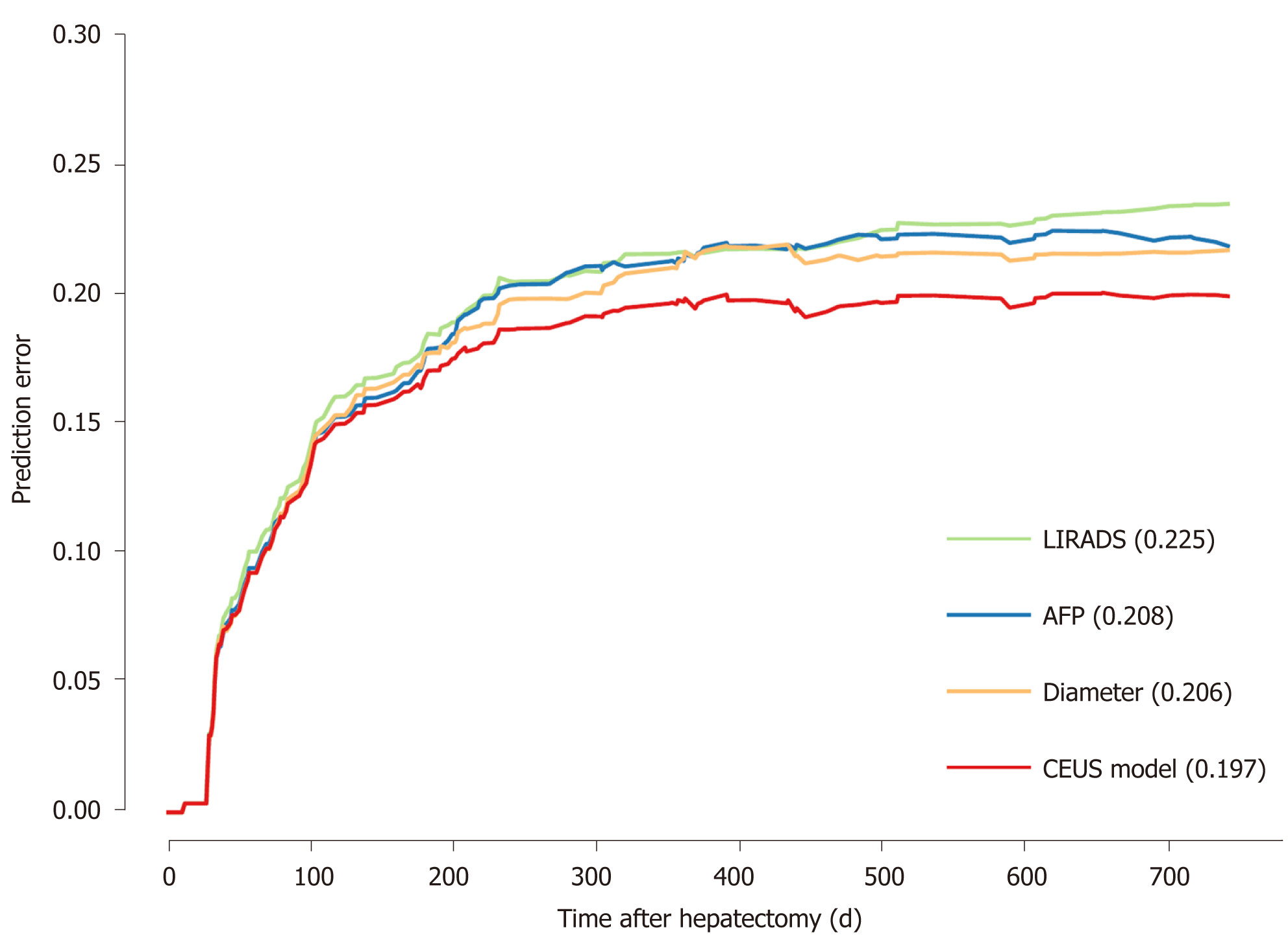
Cox regression results are shown in Table 2. LI-RADS, AFP level, and tumor diameter were entered into the final model. The hazard ratio (HR) was 1.428, 1.547, and 1.123, respectively. A prediction model, named CEUS model, was constructed. The CEUS model’s integrated Brier score of prediction error was the lowest (Figure 3).
| β | P value | HR | 95.0%CI | ||
| Lower | Upper | ||||
| LIRADS | 0.356 | 0.019 | 1.428 | 1.059 | 1.925 |
| AFP | 0.436 | 0.000 | 1.547 | 1.245 | 1.922 |
| Diameter | 0.116 | 0.003 | 1.123 | 1.041 | 1.211 |
| Age | 0.009 | 0.319 | 1.009 | 0.991 | 1.027 |
| ALT | 0.007 | 0.075 | 1.007 | 0.999 | 1.014 |
| AST | 0.003 | 0.322 | 1.003 | 0.997 | 1.008 |
| PLT | 0.002 | 0.225 | 1.002 | 0.999 | 1.005 |
| TBIL | 0.019 | 0.052 | 1.019 | 1.000 | 1.039 |
| Start time | -0.032 | 0.229 | 0.968 | 0.918 | 1.021 |
| Out time | -0.001 | 0.595 | 0.999 | 0.996 | 1.002 |
| Sex | -0.270 | 0.312 | 0.763 | 0.453 | 1.288 |
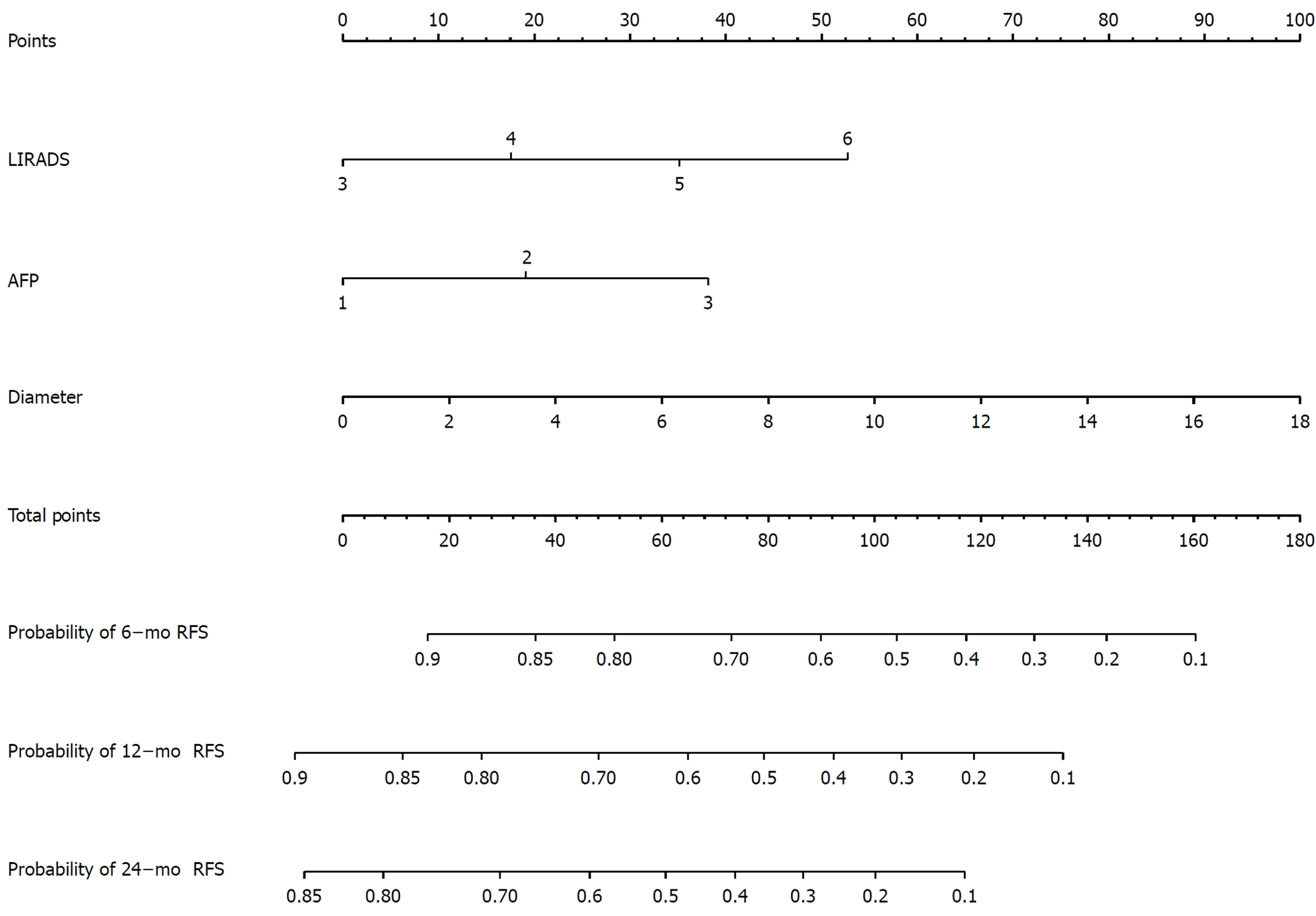
The nomogram is shown in Figure 4. Every patient’s score was calculated and divided into three subgroups: 6, 12, and 24 mo.
For the 6-mo recurrence, 100 was the optimal cut-off. It can distinguish the high-risk group from the low-risk group (Figure 5A). The C-index was 0.748 (95%CI: 0.683–0.813), and the calibration curve was satisfactory (Figure 6A). For the 12-mo recurrence, 80 was the optimal cut-off. It can distinguish the high-risk group from the low-risk group (Figure 5B). The C-index was 0.762 (95%CI: 0.704–0.820), and the calibration curve was insufficient (Figure 6B). For the 24-mo recurrence, 50 was the optimal cut-off. It can distinguish the high-risk group from the low-risk group (Figure 5C). The C-index was 0.762 (95%CI: 0.706–0.819), and the calibration curve was insufficient (Figure 6C).
This study aimed to construct and validate an early recurrence risk model before surgery, called the CEUS model, based on CEUS and serology for HCC. The CEUS model showed satisfactory results. The C-index at 6, 12, and 24 mo was 0.683–0.820. The calibration at 6 mo is good, although a 12- and 24-mo improvement is still needed. The CEUS model showed lower prediction error than a single indicator, with an integrated Brier score of 0.197. In addition, at the three study time points, using appropriate cut-off value can well distinguish high-risk groups from low-risk groups.
Resection for patients with HCC is still plagued by high recurrence, especially early recurrence[2]. This rate is approximately 30%–50% in early reports[13,14]; similar to a previous report, tumor relapse occurred in approximately half of the patients in the present study. Gene signatures may promote prognosis scoring; however, they are not used in routine clinical process[15]. Conversely, the potential and power of radiological image data, which include CT, MRI, PET, and ultrasound, are increasingly recognized in the field of oncology[16-19]. Meanwhile, serology indicators, such as albumin-to-alkaline phosphatase ratio[7], AFP[20], and neutrophil–lymphocyte ratio[21], are also widely used in this domain. In our study, parameters that may predict recurrence pre-operation were collected, rigorous statistical analysis was performed, and unimportant parameters were excluded. Finally, LIRADS, AFP level, and the maximum tumor diameter were confirmed as independent hazard factors. These factors were combined, and a novel model named the CEUS model was established.
In the past, many imaging techniques have been used for the noninvasive prediction of postoperative recurrence, and satisfactory results have been obtained. However, the use of ultrasound combined with serological biomarkers to build a prediction model is rare. The present study fully explored the advantages of CEUS and serological biomarkers and constructed the CEUS model through a series of rigorous test. We used LI-RADS because of its better accuracy compared with most previous noninvasive prediction models[8]. The overall prediction ability in the present study was as anticipated and was similar to that in previous studies[14,22]. However, the 1- and 2-year calibration degree was slightly insufficient. The main reasons are as follows: (1) The subjects included in the two studies are different. Ji et al‘s study only included patients who met the Milan criteria, but our study included all patients with radical resection. Thus, our model may have a wider applicable population; and (2) Ji et al‘s study recruited external validation queue, while only internal resampling was performed in our study. Although the calibration was insufficient, we used LI-RADS as the operation specification, which was more objective and convenient.
CEUS can dynamically display microvascular and tissue perfusion in real time, and it has good resolution and simple operation, without risk of nephrotoxicity and radiation. It is widely used in the diagnosis of clinical liver diseases. Different contrast patterns reveal varying tumor differentiation[23]. Qin et al found that the faster the washout, the higher the recurrence rate, and the C-index of this parameter was 0.756[24]. In our study, the difference in the washout time and type was not statistically significant. This result may be attributed to the following reasons: (1) The primary study end point was different. Qin et al defined early recurrence as 1 year, and in our study, the early recurrence was 2 years; (2) The definition of washout rate was different. Qin et al divided the washout rate into four grades (120 s, 60–120 s, 30–60 s, and < 30 s), but in our study, the washout time was less than 30 s and defined as fast-out in contrast to others that were non-fast-out; (3) The research subjects were different; Qin et al’s study enrolled the patients who were first discovered and after radical resection, which was pathologically confirmed; and (4) Qin et al’s study only enrolled patients with a single tumor, but our study enrolled patients with single and multiple tumors.
In the past, CEUS evaluation was mainly based on the subjective evaluation of an operator, and it lacked objective criteria. Thus, the American College of Radiology (ACR) convened a working group of international experts to develop the ACR CEUS Liver Imaging Reporting and Data System (LI-RADS) in 2014, which was implemented in 2016[10]. CEUS LI-RADS is highly specific for HCC, enabling its use for a confident non-invasive diagnosis[25]. In our study, we divided patients into different categories according to CEUS LI-RADS, which is objective and suitable for general applications. Our study also showed that LI-RADS can be used as an independent hazard factor for recurrence. The higher the LI-RADS grade, the higher the recurrence rate.
Tumor diameter can be used as an independent risk factor for postoperative recurrence and has been proven by many previous studies[26-28]; tumor size was significantly related to the outcomes in our cohort with an HR per centimeter of 1.12 for recurrence. Our findings are similar to those of Agopian et al[29], and this result supports the use of the Milan and UCSF criteria, which include the tumor size. The increase in tumor diameter suggests that the local proliferation of tumor is in an active state, resulting in the recurrence of liver cancer. The increase in tumor diameter prolongs the whole operation process, affecting the immune system of patients, heightening the response state of the body to tumor cells, and further accelerating recurrence.
AFP is an established and routine tumor marker in patients with HCC and is readily available for patients who are AFP-positive before surgery. AFP values > 1000 ng/mL before surgery have been associated with the risk of HCC recurrence after hepatectomy. High AFP serum levels may be a surrogate parameter for vascular invasion and is a well-characterized predictor of HCC recurrence after surgery[20]. A high serum AFP level suggests that HCC is highly invasive. Elevated preoperative AFP levels indicate an increased risk of tumor cell metastasis via blood route. Therefore, predicting the malignant characteristics and prognosis of HCC through the pre
As this work was a single-center and retrospective cohort study, some limitations should be noted due to the inherent defects of the study design. First, selection and management bias is unavoidable. In general, if liver ultrasound examination is not enough, CEUS examination is also insufficient. In addition, the quality of an exa
In summary, early recurrence of liver cancer after operation seriously affects the health of patients. Our non-invasive prediction model based on CEUS and serological biomarkers can achieve a high prediction efficiency with few indicators and is helpful in planning an appropriate treatment schedule.
Surgery is the main treatment for hepatocellular carcinoma (HCC). However, 30%-50% of patients develop recurrence within 2 years, which is the main cause of death.
Screening patients with high recurrence risk plays an important role in making reasonable clinical decisions.
To combine contrast-enhanced ultrasound (CEUS) liver imaging reporting and data system (LIRADS) with serology biomarkers to construct a non-invasive model predicting the early recurrence of HCC, and verify the model.
Records of 744 consecutive patients undergoing first-line curative surgery for HCC in one institution from 2016–2018 were reviewed, and 292 local patients were selected for analysis. General characteristics, CEUS liver imaging reporting and data system (LIRADS) parameters, and serology biomarkers were collected. Univariate analysis and multivariate analyses were performed to evaluate the independent prognostic factors for tumor recurrence. Then, a nomogram called CEUS model was constructed. The CEUS model was then used to predict recurrence at 6 mo, 12 mo, and 24 mo.
Tumor diameter, preoperative alpha-fetoprotein (AFP) level, and LIRADS were identified to be independent hazard factors, with a hazard ratio of 1.123 (95% confidence interval [CI]: 1.041-1.211), 1.547 (95%CI: 1.245-1.922), and 1.428 (95%CI: 1.059-1.925), separately. A nomogram based on them was constructed; the cut-off value at 6 mo, 12 mo, and 24 mo was 100, 80, and 50, and the C-index was 0.748 (95%CI: 0.683-0.813), 0.762 (95%CI: 0.704-0.820), and 0.762 (95%CI: 0.706-0.819), separately. The calibration at 6 mo was desirable; however, the calibration at 12 and 24 mo should be improved.
The CEUS model can screen out patients who have a high recurrence risk, it is helpful for making reasonable treatment strategy.
A multi-center study, with an expanded sample size and prospective verification, is required.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Chen Y, Hashimoto N S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Zhang YL
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64461] [Article Influence: 16115.3] [Reference Citation Analysis (176)] |
| 2. | Chan AW, Chan SL, Wong GL, Wong VW, Chong CC, Lai PB, Chan HL, To KF. Prognostic Nutritional Index (PNI) Predicts Tumor Recurrence of Very Early/Early Stage Hepatocellular Carcinoma After Surgical Resection. Ann Surg Oncol. 2015;22:4138-4148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 169] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 3. | Lv J, Yin H, Mao W, Shi H. Investigating the value of pre-treatment 18F-FDG PET/CT in predicting the pathological characteristic of hepatocellular carcinoma and recurrence after liver transplantation. Abdom Radiol (NY). 2021;46:2490-2497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Ang SF, Ng ES, Li H, Ong YH, Choo SP, Ngeow J, Toh HC, Lim KH, Yap HY, Tan CK, Ooi LL, Cheow PC, Chung AY, Chow PK, Foo KF, Tan MH. The Singapore Liver Cancer Recurrence (SLICER) Score for relapse prediction in patients with surgically resected hepatocellular carcinoma. PLoS One. 2015;10:e0118658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Shim JH, Jun MJ, Han S, Lee YJ, Lee SG, Kim KM, Lim YS, Lee HC. Prognostic nomograms for prediction of recurrence and survival after curative liver resection for hepatocellular carcinoma. Ann Surg. 2015;261:939-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 189] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 6. | Huang S, Huang GQ, Zhu GQ, Liu WY, You J, Shi KQ, Wang XB, Che HY, Chen GL, Fang JF, Zhou Y, Zhou MT, Chen YP, Braddock M, Zheng MH. Establishment and Validation of SSCLIP Scoring System to Estimate Survival in Hepatocellular Carcinoma Patients Who Received Curative Liver Resection. PLoS One. 2015;10:e0129000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Zhang F, Lu SX, Hu KS, Gan YH, Chen Y, Ge NL, Yang BW, Zhang L, Chen RX, Ren ZG, Yin X. Albumin-to-alkaline phosphatase ratio as a predictor of tumor recurrence and prognosis in patients with early-stage hepatocellular carcinoma undergoing radiofrequency ablation as initial therapy. Int J Hyperthermia. 2021;38:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Ji GW, Zhu FP, Xu Q, Wang K, Wu MY, Tang WW, Li XC, Wang XH. Radiomic Features at Contrast-enhanced CT Predict Recurrence in Early Stage Hepatocellular Carcinoma: A Multi-Institutional Study. Radiology. 2020;294:568-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 190] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 9. | Lyshchik A, Kono Y, Dietrich CF, Jang HJ, Kim TK, Piscaglia F, Vezeridis A, Willmann JK, Wilson SR. Contrast-enhanced ultrasound of the liver: technical and lexicon recommendations from the ACR CEUS LI-RADS working group. Abdom Radiol (NY). 2018;43:861-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 10. | Kono Y, Lyshchik A, Cosgrove D, Dietrich CF, Jang HJ, Kim TK, Piscaglia F, Willmann JK, Wilson SR, Santillan C, Kambadakone A, Mitchell D, Vezeridis A, Sirlin CB. Contrast Enhanced Ultrasound (CEUS) Liver Imaging Reporting and Data System (LI-RADS®): the official version by the American College of Radiology (ACR). Ultraschall Med. 2017;38:85-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 11. | Lei Z, Li J, Wu D, Xia Y, Wang Q, Si A, Wang K, Wan X, Lau WY, Wu M, Shen F. Nomogram for Preoperative Estimation of Microvascular Invasion Risk in Hepatitis B Virus-Related Hepatocellular Carcinoma Within the Milan Criteria. JAMA Surg. 2016;151:356-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 449] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 12. | Cohen ME, Ko CY, Bilimoria KY, Zhou L, Huffman K, Wang X, Liu Y, Kraemer K, Meng X, Merkow R, Chow W, Matel B, Richards K, Hart AJ, Dimick JB, Hall BL. Optimizing ACS NSQIP modeling for evaluation of surgical quality and risk: patient risk adjustment, procedure mix adjustment, shrinkage adjustment, and surgical focus. J Am Coll Surg. 2013;217:336-46.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 453] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 13. | Chan AWH, Zhong J, Berhane S, Toyoda H, Cucchetti A, Shi K, Tada T, Chong CCN, Xiang BD, Li LQ, Lai PBS, Mazzaferro V, García-Fiñana M, Kudo M, Kumada T, Roayaie S, Johnson PJ. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J Hepatol. 2018;69:1284-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 398] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 14. | Ning P, Gao F, Hai J, Wu M, Chen J, Zhu S, Wang M, Shi D. Application of CT radiomics in prediction of early recurrence in hepatocellular carcinoma. Abdom Radiol (NY). 2020;45:64-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 15. | Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2066] [Cited by in RCA: 3161] [Article Influence: 526.8] [Reference Citation Analysis (37)] |
| 16. | Bell M, Turkbey EB, Escorcia FE. Radiomics, Radiogenomics, and Next-Generation Molecular Imaging to Augment Diagnosis of Hepatocellular Carcinoma. Cancer J. 2020;26:108-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Hu Z, Yu N, Wang H, Li S, Yan J, Zhang G. Pre-radiofrequency ablation MRI imaging features predict the local tumor progression in hepatocellular carcinoma. Medicine (Baltimore). 2020;99:e23924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Morio K, Kawaoka T, Aikata H, Namba M, Uchikawa S, Kodama K, Ohya K, Fujino H, Nakahara T, Murakami E, Yamauchi M, Tsuge M, Hiramatsu A, Imamura M, Nakamura Y, Akagi M, Awai K, Kobayashi T, Ohdan H, Chayama K. Preoperative PET-CT is useful for predicting recurrent extrahepatic metastasis of hepatocellular carcinoma after resection. Eur J Radiol. 2020;124:108828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Nakamura I, Hatano E, Tada M, Kawabata Y, Tamagawa S, Kurimoto A, Iwama H, Toriguchi K, Sueoka H, Iida K, Yoshida M, Nishimura T, Iijima H. Enhanced patterns on intraoperative contrast-enhanced ultrasonography predict outcomes after curative liver resection in patients with hepatocellular carcinoma. Surg Today. 2021;51:764-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Koch C, Bette T, Waidmann O, Filmann N, Schrecker C, Trojan J, Weiler N, Vermehren J, Schnitzbauer AA, Bechstein WO, Zeuzem S, Herrmann E, Welker MW. AFP ratio predicts HCC recurrence after liver transplantation. PLoS One. 2020;15:e0235576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Wong L, Bozhilov K, Hernandez B, Kwee S, Chan O, Ellis L, LeMarchand L. Underlying liver disease and advanced stage liver cancer are associated with elevated neutrophil-lymphocyte ratio. Clin Mol Hepatol. 2019;25:305-316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Zhang Z, Jiang H, Chen J, Wei Y, Cao L, Ye Z, Li X, Ma L, Song B. Hepatocellular carcinoma: radiomics nomogram on gadoxetic acid-enhanced MR imaging for early postoperative recurrence prediction. Cancer Imaging. 2019;19:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 101] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 23. | Wang Y, Liao J, Qi W, Xie L, Li Y. Predictive Value of Conventional Ultrasound and Contrast-Enhanced Ultrasound in Early Recurrence of Hepatocellular Carcinoma after Surgical Resection. Ultrasound Med Biol. 2016;42:1042-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Xiachuan Q, Xiang Z, Xuebing L, Yan L. Predictive Value of Contrast-enhanced Ultrasound for Early Recurrence of Single Lesion Hepatocellular Carcinoma After Curative Resection. Ultrason Imaging. 2019;41:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Terzi E, Iavarone M, Pompili M, Veronese L, Cabibbo G, Fraquelli M, Riccardi L, De Bonis L, Sangiovanni A, Leoni S, Zocco MA, Rossi S, Alessi N, Wilson SR, Piscaglia F; CEUS LI-RADS Italy study group collaborators:. Contrast ultrasound LI-RADS LR-5 identifies hepatocellular carcinoma in cirrhosis in a multicenter restropective study of 1,006 nodules. J Hepatol. 2018;68:485-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 211] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 26. | Liu S, Li H, Guo L, Zhang B, Zhou B, Zhang W, Zhou J, Fan J, Ye Q. Tumor Size Affects Efficacy of Adjuvant Transarterial Chemoembolization in Patients with Hepatocellular Carcinoma and Microvascular Invasion. Oncologist. 2019;24:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 27. | Goh BK, Teo JY, Chan CY, Lee SY, Jeyaraj P, Cheow PC, Chow PK, Ooi LL, Chung AY. Importance of tumor size as a prognostic factor after partial liver resection for solitary hepatocellular carcinoma: Implications on the current AJCC staging system. J Surg Oncol. 2016;113:89-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 28. | Xu XF, Xing H, Han J, Li ZL, Lau WY, Zhou YH, Gu WM, Wang H, Chen TH, Zeng YY, Li C, Wu MC, Shen F, Yang T. Risk Factors, Patterns, and Outcomes of Late Recurrence After Liver Resection for Hepatocellular Carcinoma: A Multicenter Study From China. JAMA Surg. 2019;154:209-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 394] [Article Influence: 65.7] [Reference Citation Analysis (0)] |
| 29. | Agopian VG, Harlander-Locke M, Zarrinpar A, Kaldas FM, Farmer DG, Yersiz H, Finn RS, Tong M, Hiatt JR, Busuttil RW. A novel prognostic nomogram accurately predicts hepatocellular carcinoma recurrence after liver transplantation: analysis of 865 consecutive liver transplant recipients. J Am Coll Surg. 2015;220:416-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 198] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 30. | Kim YI, Kim HS, Park JW. Higher Ratio of Serum Alpha-Fetoprotein Could Predict Outcomes in Patients with Hepatitis B Virus-Associated Hepatocellular Carcinoma and Normal Alanine Aminotransferase. PLoS One. 2016;11:e0157299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |