Published online Jan 16, 2021. doi: 10.12998/wjcc.v9.i2.509
Peer-review started: October 3, 2020
First decision: November 3, 2020
Revised: November 18, 2020
Accepted: November 29, 2020
Article in press: November 29, 2020
Published online: January 16, 2021
Processing time: 97 Days and 0.8 Hours
Inguinal hernia repair is one of the most common general surgical operations worldwide. We present a case of indirect inguinal hernia containing an expanded portosystemic shunt vessel.
We report a 72-year-old man who had a 4 cm × 4 cm swelling in the right inguinal region, which disappeared with light manual pressure. Abdominal-pelvic computed tomography (CT) revealed a right inguinal hernia containing an expanded portosystemic shunt vessel, which had been noted for 7 years due to liver cirrhosis. We performed Lichtenstein’s herniorrhaphy and identified the hernia sac as being indirect and the shunt vessel existing in the extraperitoneal cavity through the internal inguinal ring. Then, we found two short branches between the expanded shunt vessel and testicular vein in the middle part of the inguinal canal and cut these branches to allow the shunt vessel to return to the extraperitoneal cavity of the abdomen. The hernia sac was returned as well. We encountered no intraoperative complications. After discharge, groin seroma requiring puncture at the outpatient clinic was observed.
If an inguinal hernia patient has portal hypertension, ultrasound should be used to determine the contents of the hernia. When atypical vessels are visualized, they may be shunt vessels and additional CT is recommended to ensure the selection of an adequate approach for safe hernia repair.
Core Tip: We present the case of indirect inguinal hernia containing portosystemic shunt vessel. If an inguinal hernia patient has portal hypertension, for example due to liver cirrhosis, ultrasound and/or computed tomography should be used to determine the contents of the hernia. Shunt vessels present in the inguinal canal may be connected to surrounding tissues or communicate with extraperitoneal vessels, such as the peripheral testicular vein. Careful preoperative diagnosis is important to ensure that an adequate approach for safe hernia repair is selected.
- Citation: Yura M, Yo K, Hara A, Hayashi K, Tajima Y, Kaneko Y, Fujisaki H, Hirata A, Takano K, Hongo K, Yoneyama K, Nakagawa M. Indirect inguinal hernia containing portosystemic shunt vessel: A case report. World J Clin Cases 2021; 9(2): 509-515
- URL: https://www.wjgnet.com/2307-8960/full/v9/i2/509.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i2.509
Inguinal hernia repair is one of the most frequently performed surgeries in general surgery departments worldwide[1]. Many surgical methods are available: Anterior or posterior, open or laparoscopic, and tension-inducing or tension-free[1-7]. With increasing aging populations, candidates for inguinal hernia repair are often elderly, have comorbidities, or have a history of surgery. Thus, the procedure must be chosen with consideration of the patient’s condition. Lichtenstein’s herniorrhaphy is a type of anterior transversalis fascia repair[8] that is preferred worldwide because it is technically easy, effective, and associated with a low recurrence rate[9]. It is also most often selected surgical method in inguinal hernia repair in patients with liver cirrhosis[10].
Here, we present a patient with indirect inguinal hernia containing an expanded portosystemic shunt vessel existing in the extraperitoneal cavity, attributable to liver cirrhosis, entered the inguinal canal. We chose the Lichtenstein’s herniorrhaphy to ensure safe repair after a careful preoperative diagnosis. There are no reports of a case of indirect inguinal hernia with contents including an extraperitoneal portosystemic venous shunt.
Right inguinal swelling and pain.
A 72-year-old male complained of a right inguinal swelling that had gradually increased in size over the past five years and caused significant discomfort and pain of the right inguinal region when walking.
He had liver cirrhosis due to non-alcoholic steatohepatitis, as well as diabetes mellitus and histories of endovascular aortic repair of an abdominal aortic aneurysm (AAA) and of laparoscopic cholecystectomy after percutaneous transhepatic gallbladder drainage for acute cholecystitis.
No family history to note.
Physical examination revealed a 4 cm × 4 cm sized swelling, which disappeared with light manual pressure. No tenderness was noted at the time of physical examination. His scrotum was normal. It was thought to be typical physical findings of inguinal hernia.
Laboratory tests revealed a hemoglobin level of 13.9 g/dL, white blood cell count of 4200 cells/μL, and platelet count of 132 × 103/μL. The creatinine level was 0.58 mg/dL, total bilirubin level 1.5 mg/dL, direct bilirubin level 0.5 mg/dL, glutamic oxaloacetic transaminase level 35 IU/L, glutamic pyruvate transaminase level 35 IU/L, alkaline phosphatase level 1085 IU/L, albumin level 3.6 g/dL, and hemoglobin A1c level 6.7%. The prothrombin level was 74.9%, the international normalized ratio was 1.18, and the activated partial thromboplastin time was 34.9 s. No encephalopathy or ascites were observed, so the Child–Pugh liver function score was 5 (grade A; mild liver dysfunction).
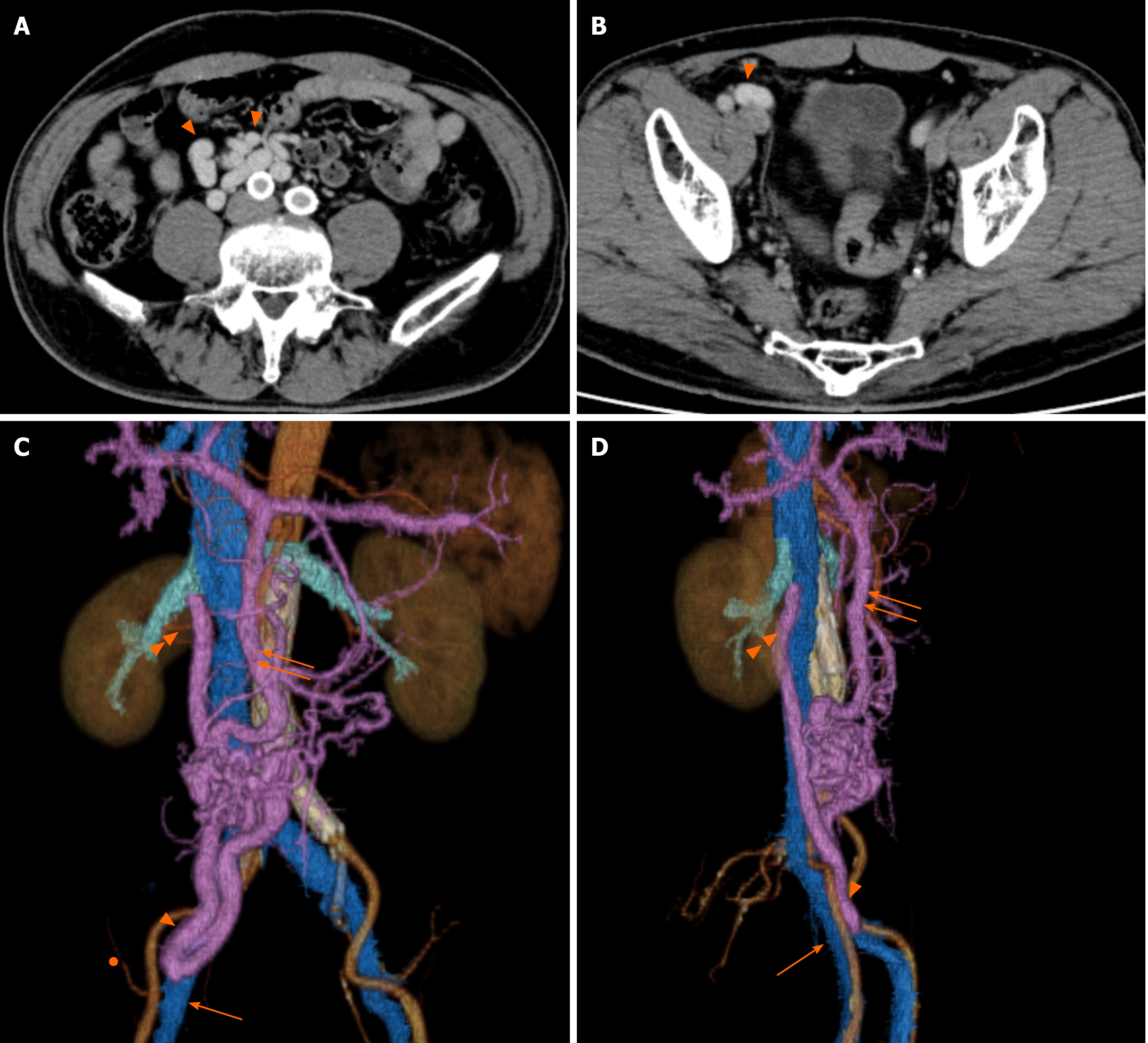
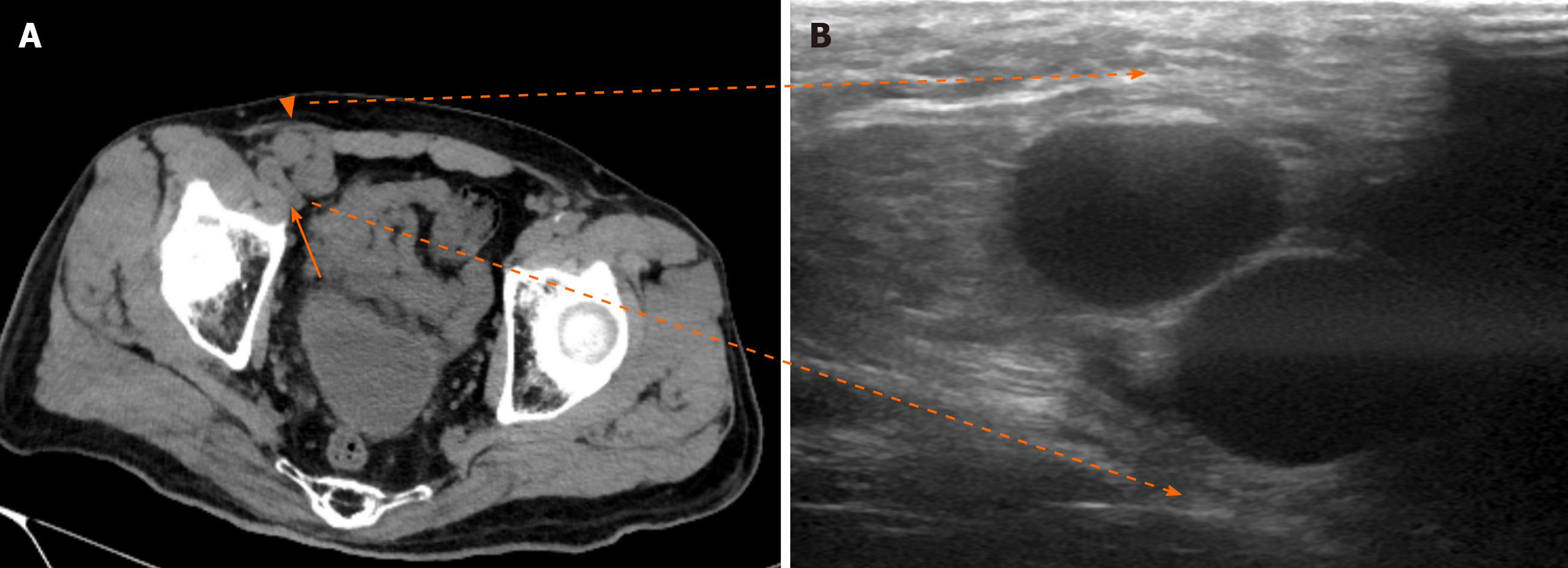
The patient had a portosystemic venous shunt that had been detected 7 years earlier by contrast abdominal-pelvic computed tomography (CT) during routine postoperative assessment of an AAA repair. A shunt had formed between the ileocolic vein, which is peripheral to the superior mesenteric vein, and the testicular vein, whose venous return finally flows into the inferior vena cava (Figure 1A-D). Preoperative non contrast abdominal-pelvic CT revealed a typical right indirect inguinal hernia and also suggested that an expanded portosystemic shunt vessel (diameter of 2 cm) attributable to liver cirrhosis may be a content of the inguinal hernia (Figure 2A). Furthermore, the CT showed that the shunt vessel was located just inside the ventral part of the femoral vein at the internal inguinal ring, which means that it did not move away from the right inguinal internal ring even in the supine position (Figure 2A). We could not perform contrast CT before the inguinal hernia surgery because the patient had become allergic to the iodine contrast agent.
Ultrasound (US) imaging performed in a standing position (Figure 2B) showed that the shunt vessel was in the same region; although it became flattened with light pressure of the probe in the supine posture, it remained in same place. Based on these findings, we speculated that the prolapsed shunt vessel might exist in the extraperitoneal cavity, not in the peritoneal cavity, and was not reducible due to adhesion with the surrounding tissues or vascular communications in the inguinal canal. The patient wanted surgical treatment, and the Child–Pugh score of 5 was considered acceptable for surgery.
Thus, we made a diagnosis of primary indirect hernia < 3 cm (PL2) according to the European Hernia Society Classification System[4].
Surgical treatment was scheduled one month after the first visit. We chose the Lichtenstein’s herniorrhaphy because it does not require entry into the extraperitoneal cavity and allows the contents of the inguinal hernia in the inguinal canal to be easily handled.
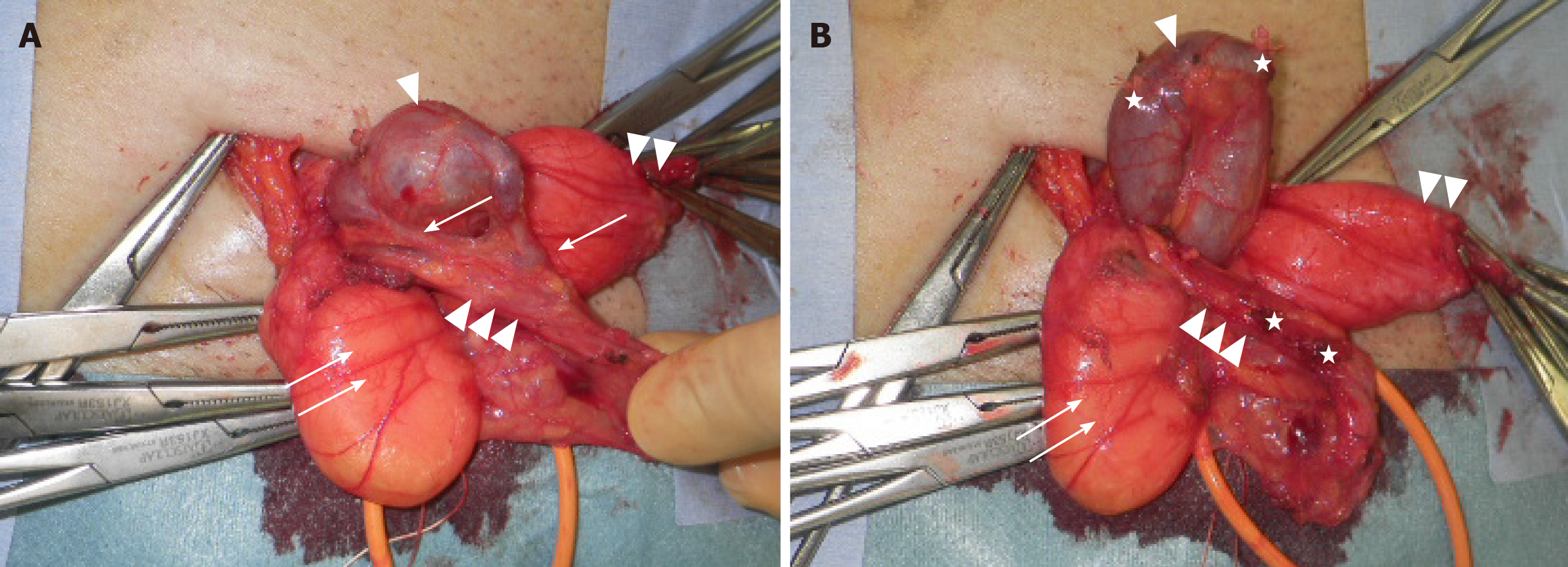
Surgical strategy: We exposed the spermatic cord to establish circumferential access, and then explored the cord structures to identify the hernial sac and made a diagnosis of indirect hernia. We then identified the expanded (diameter of 2 cm) shunt vessel existing in the extraperitoneal cavity, which entered the inguinal canal through the internal inguinal ring, and carefully dissected it from the surrounding connective tissue. We also found two short branches between the expanded shunt vessel and the testicular vein in the middle part of the inguinal canal (Figure 3A); we cut these branches (Figure 3B) to allow the shunt vessel to return to the extraperitoneal cavity through the internal inguinal ring without injuring the vessels. Next, we opened the hernia sac and identified omental adhesion in the peritoneal cavity, which could be detached until the level of the internal inguinal ring. The hernia sac was ligated with the double transfixing method and also returned to the extraperitoneal cavity through the internal inguinal ring. We found no direct hernia. Finally, we repaired the indirect hernia using on-lay mesh (Parietex ProGripTM, Medtronic) and encountered no intraoperative complications.
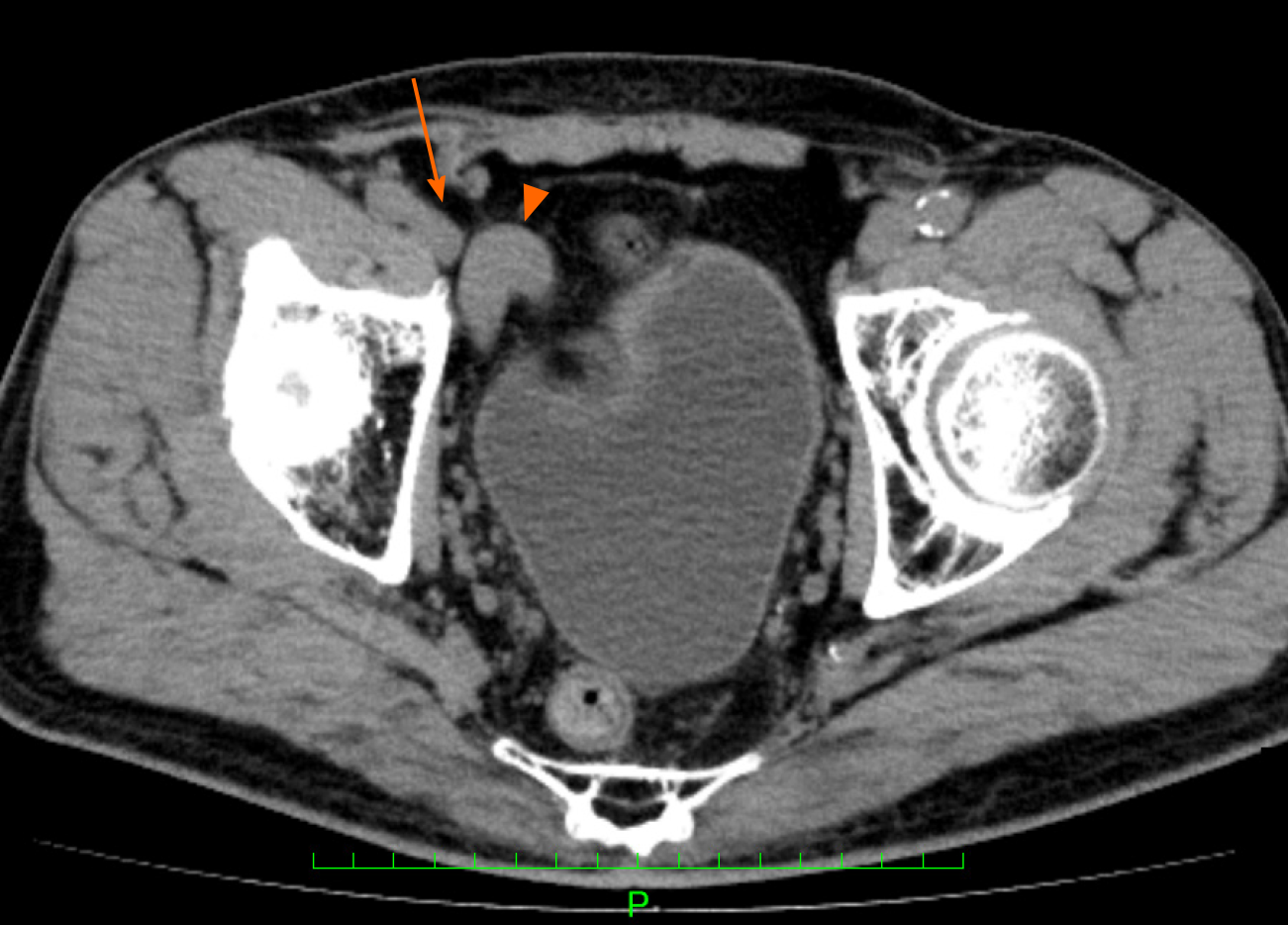
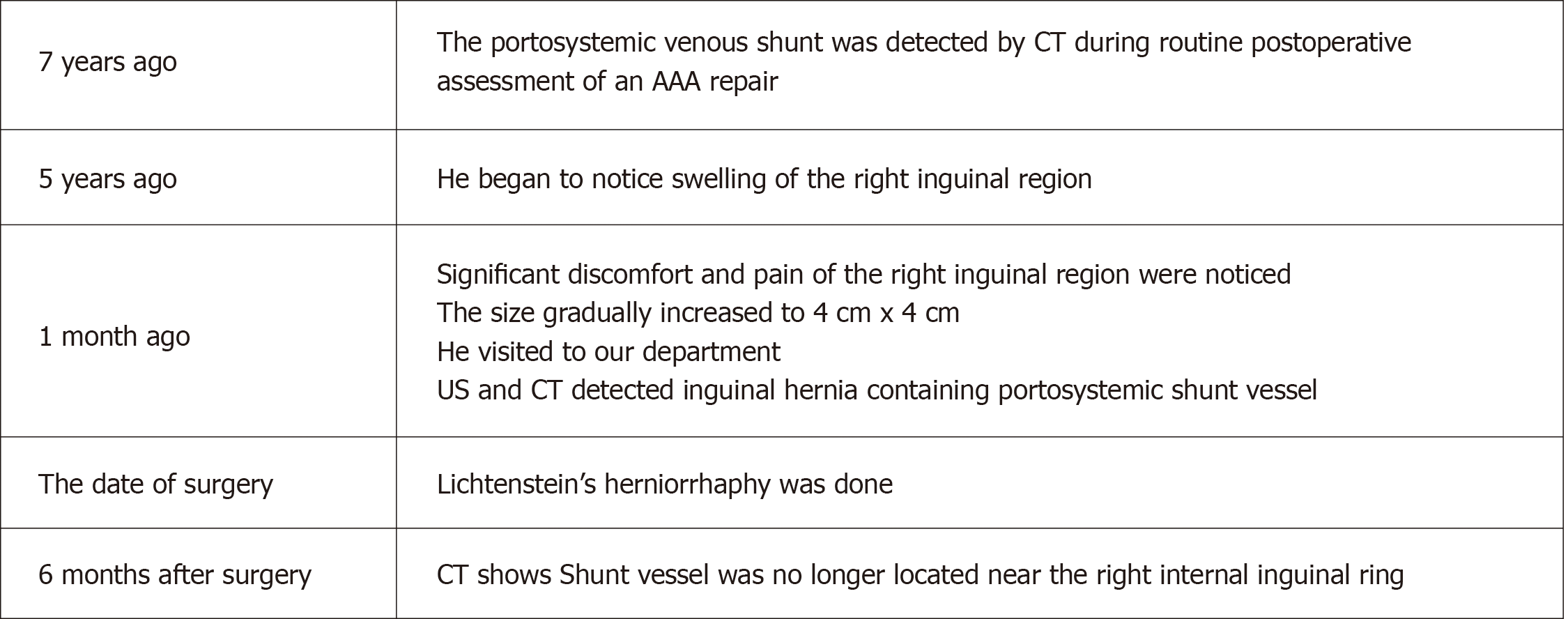
The patient recovered well and was discharged to home on postoperative day 3 after passing our routine clinical inspection. After discharge, groin seroma was diagnosed by palpation and US imaging and removed by puncture on days 8 (30 mL), 12 (25 mL), 15 (10 mL), and 19 (10 mL) at an outpatient clinic. Thereafter, the patient was followed-up for 12 mo. We observed no recurrence or other complications (chronic pain, infection, swelling, or discomfort) that compromised daily life during the follow-up. After the surgery, the patient continued to undergo CT scans for evaluation of the AAA. One year after hernia surgery, CT scans indicated that the shunt vessel was no longer located near the right internal inguinal ring, and it had separated from the femoral vein (Figure 4). The timeline from diagnosis to treatment is shown in Figure 5.
We successfully treated an indirect inguinal hernia containing an expanded portosystemic venous shunt vessel communicated with the peripheral testicular vein in the middle part of the inguinal canal. Use of the Lichtenstein’s herniorrhaphy was the key factor in the successful treatment.
Portosystemic venous shunts are usually attributable to portal hypertension accompanied by hepatic fibrosis in cirrhotic cases. Our patient had a history of liver cirrhosis due to non-alcoholic steatohepatitis, and a portosystemic venous shunt had been detected 7 years earlier during routine postoperative CT assessment of his AAA repair. On physical examination, his inguinal swelling was similar to that of a typical inguinal hernia, being soft and disappearing with light manual pressure, even though a prolapsed shunt vessel was also included in the hernia contents. Zahir et al[11] reported unilateral varicocele mimicking inguinal hernia which showed painless compressible soft swelling of inguinoscrotal region, and Afzal et al[12] also reported thrombosis of congenital portosystemic shunt simulating inguinal hernia incarceration. From these cases, including our case, port systemic shunt deviation similar to inguinal hernia should be considered as a differentiation of inguinal swelling. However, one of the other two cases received only anticoagulant therapy for thrombosis, and the other did not undergo intervention at the patient's request. Only our case is accompanied by intestinal prolapse and has been successful treatment with surgical intervention for inguinal hernia.
By adding the results of preoperative CT and US, we could predict the presence of shunt vessel in the inguinal canal. According to the International Hernia Guidelines[1], US is now widely available but rarely magnetic resonance imaging, CT and herniography may play a role as well. Also, there is no description of additional recommended tests for patients with liver cirrhosis. However, we think that patients with cirrhosis should undergo US to screen for the presence of atypical blood vessels associated with inguinal hernia and should undergo contrast-enhanced CT if atypical blood vessels are suggested by US. Contrast-enhanced CT scans provide reliable visualization of blood vessels and are recommended for safe surgery in these cases.
In this case, another important preoperative finding was that the content of the hernia: A shunt vessel remained in the inguinal canal even in the supine position. We suspected that prolapsed shunt vessel existed in the extraperitoneal cavity, not in the peritoneal cavity and had adhesion in the inguinal canal. Therefore, we planned open surgery. In fact, it was necessary to detach the connective tissue around the shunt vessel, and we found that the shunt vessel had venous communications with the peripheral testicular veins in the middle part of the inguinal canal. These venous branches had to be dissected and cut to return the shunt vessel to the extraperitoneal cavity through the internal inguinal ring. These procedures were expected to be difficult to perform with laparoscopic surgery via the internal inguinal ring. In this case, another important aspect with respect to the preoperative diagnosis was that the extraperitoneal expanded shunt vessels faced the myopectineal orifice widely. Dissection of the whole inguinal area and development of a sufficiently large mesh with laparoscopic surgery might have been difficult because of the shunt vessel, which is why we chose the Lichtenstein’s herniorrhaphy.
If an inguinal hernia patient has portal hypertension, US should be used to determine the contents of the hernia. When atypical vessels are visualized, they may be shunt vessels and additional CT scan is recommended to ensure the selection of an adequate approach for safe hernia repair.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Gram-Hanssen A, Raissi D, Ruiz-Jasbon F S-Editor: Huang P L-Editor: A P-Editor: Li JH
| 1. | HerniaSurge Group. International guidelines for groin hernia management. Hernia. 2018;22:1-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1355] [Cited by in RCA: 1276] [Article Influence: 182.3] [Reference Citation Analysis (1)] |
| 2. | Nordin P, Bartelmess P, Jansson C, Svensson C, Edlund G. Randomized trial of Lichtenstein versus Shouldice hernia repair in general surgical practice. Br J Surg. 2002;89:45-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Amid PK. Lichtenstein tension-free hernioplasty: its inception, evolution, and principles. Hernia. 2004;8:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 166] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 4. | Simons MP, Aufenacker T, Bay-Nielsen M, Bouillot JL, Campanelli G, Conze J, de Lange D, Fortelny R, Heikkinen T, Kingsnorth A, Kukleta J, Morales-Conde S, Nordin P, Schumpelick V, Smedberg S, Smietanski M, Weber G, Miserez M. European Hernia Society guidelines on the treatment of inguinal hernia in adult patients. Hernia. 2009;13:343-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 949] [Cited by in RCA: 901] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 5. | Wu JJ, Way JA, Eslick GD, Cox MR. Transabdominal Pre-Peritoneal Versus Open Repair for Primary Unilateral Inguinal Hernia: A Meta-analysis. World J Surg. 2018;42:1304-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Bullen NL, Massey LH, Antoniou SA, Smart NJ, Fortelny RH. Open versus laparoscopic mesh repair of primary unilateral uncomplicated inguinal hernia: a systematic review with meta-analysis and trial sequential analysis. Hernia. 2019;23:461-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 101] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 7. | Mitura K, Garnysz K, Michałek I. Long-term follow-up of a randomized controlled trial of Lichtenstein repair vs the Valenti technique for inguinal hernia. Hernia. 2019;23:547-554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Lichtenstein IL, Shulman AG, Amid PK, Montllor MM. The tension-free hernioplasty. Am J Surg. 1989;157:188-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 796] [Cited by in RCA: 740] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 9. | EU Hernia Trialists Collaboration. Mesh compared with non-mesh methods of open groin hernia repair: systematic review of randomized controlled trials. Br J Surg. 2000;87:854-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 232] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 10. | Li J, Qin C, Lai D, Hu Y, Wang L. Safety and effectiveness of inguinal hernia repair in patients with liver cirrhosis: a retrospective study and literature review. Hernia. 2020;24:489-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Zahir M, Al Muttairi HR, Upadhyay SP, Mallick PN. Unilateral Giant Varicocele Mimicking Inguinal Hernia Resulting from Portosystemic Shunt without Evidence of Portal Hypertension: An Unusual Case Report. Case Rep Surg. 2013;2013:709835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Afzal S, Nair A, Grainger J, Latif S, Rehman AU. Spontaneous thrombosis of congenital extrahepatic portosystemic shunt (Abernethy malformation) simulating inguinal hernia incarceration. Vasc Endovascular Surg. 2010;44:508-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |