Published online Jan 16, 2021. doi: 10.12998/wjcc.v9.i2.379
Peer-review started: May 28, 2020
First decision: November 14, 2020
Revised: November 24, 2020
Accepted: November 29, 2020
Article in press: November 29, 2020
Published online: January 16, 2021
Processing time: 224 Days and 18.5 Hours
Spinal metastases are common in patients with malignancies, but studies on those metastasized from unknown primaries are scarce due to the difficulty in treatment and the relatively poor prognosis. Knowledge of surgical complications, particularly perioperative mortality, in patients with spinal metastases from unidentified sources is still insufficient.
A 54-year-old man with chest-back pain was diagnosed with spinal metastasis in the seventh thoracic vertebra (T7). Radiographic examinations, as well as needle biopsy and immunohistochemical tests were performed to verify the characteristics of the lesion, resulting in an inconclusive diagnosis of poorly differentiated cancer from an unknown primary lesion. Therefore, spinal surgery was performed using the posterior approach to relieve symptoms and verify the diagnosis. Postoperative histologic examination indicated that this poorly differentiated metastatic cancer was possibly sarcomatoid carcinoma. As the patient experienced unexpectedly fast progression of the disease and died 16 d after surgery, the origin of this metastasis was undetermined. We discuss this case with respect to reported perioperative mortality in similar cases.
A comprehensive assessment prior to surgical decision-making is essential to reduce perioperative mortality risk in patients with spinal metastases from an unknown origin.
Core Tip: Studies on spinal metastases with unknown primary tumors (UPTs) are scarce due to the difficulty in diagnosis and treatment of this disease. Perioperative death, is one of the most serious complications and plays an important role in the prognostic outcome of spinal metastasis. Studies that directly analyze perioperative mortality in patients with unidentified origins of spinal metastases are still very rare. We describe a rare case of thoracic vertebral metastasis from an UPT who died in hospital after surgery due to dramatic deterioration of the disease. This uncertain diagnosis and rapid progression represent a highly unexpected disease presentation.
- Citation: Li XM, Jin LB. Perioperative mortality of metastatic spinal disease with unknown primary: A case report and review of literature. World J Clin Cases 2021; 9(2): 379-388
- URL: https://www.wjgnet.com/2307-8960/full/v9/i2/379.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i2.379
The spine is the most frequent skeletal site for metastatic deposits of cancers and approximately 50% to 60% of bone metastases occur in the spine[1,2]. Spinal metastases are detected in about 5% to 30% of patients with malignant neoplasms[3,4]. The common primary tumor types related to spinal metastases include lung, prostate, breast, kidney and thyroid carcinomas, whereas approximately 8% to 24% of patients have unknown primary lesions[5,6].
Survival after spinal metastasis is not very optimistic as no effective treatment has been established. Surgical intervention is one of the available choices that can relieve patients’ symptoms and improve their quality of life[7]. However, surgical treatment is often accompanied by complications with an incidence ranging from 20% to 75%[8,9], and re-admission rate and perioperative mortality are around 43% and 9%, respectively[10,11]. To date, only limited studies have analyzed the causes and the independent risk factors of postoperative death in patients with metastatic spinal disease. Furthermore, studies focusing on the portion of patients with spinal metastases from unknown primary tumors (UPTs) are rare.
Herein, we present an unusual case of a male patient who suffered from thoracic vertebral metastasis of a poorly differentiated cancer with an UPT. After surgical intervention, his health deteriorated rapidly and he died in hospital shortly afterwards. This unexpected disease presentation has made us aware that when dealing with such indeterminate metastatic diseases, special attention should be paid to lowering perioperative mortality and improving patient prognosis.
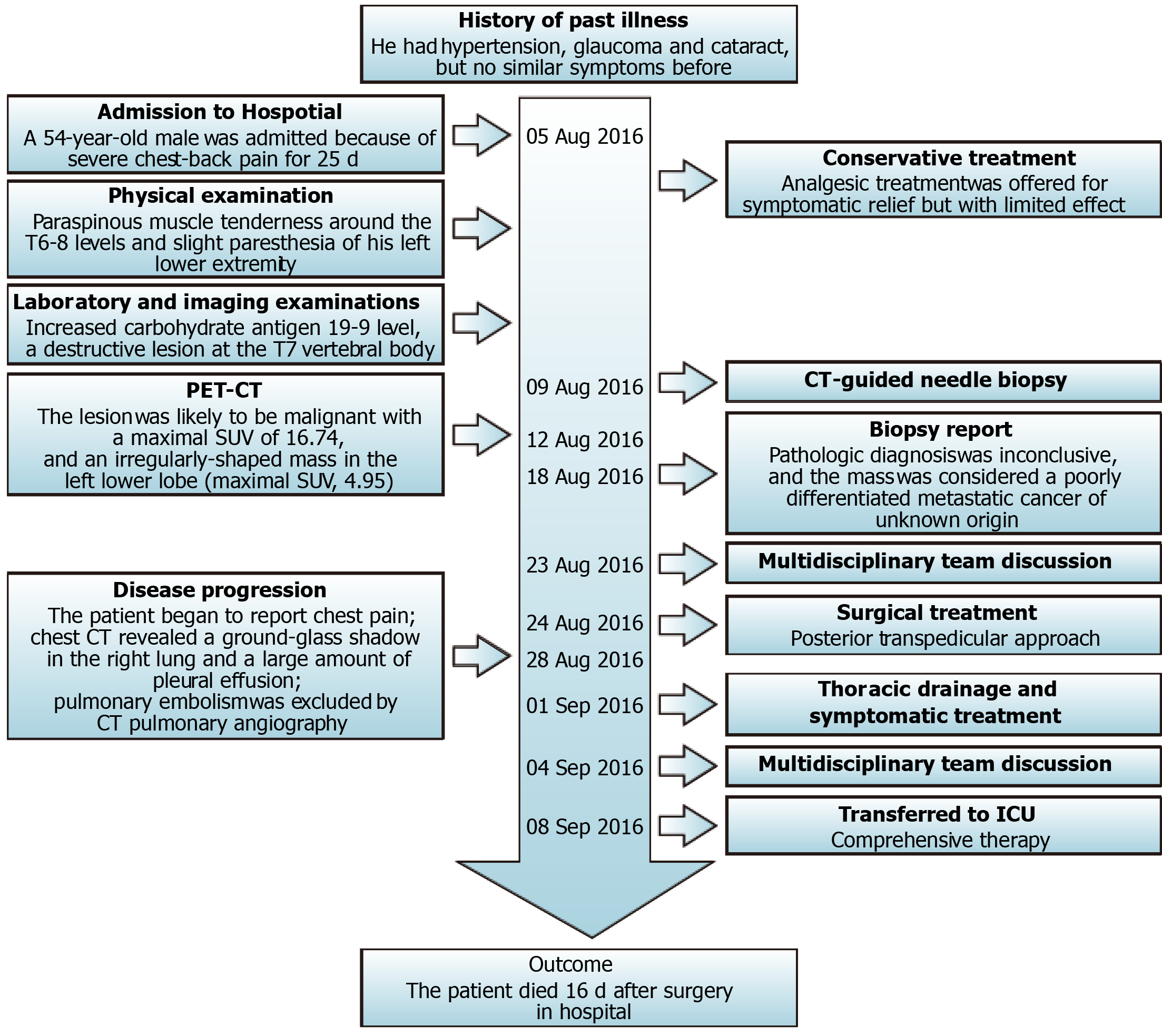
A 54-year-old male was admitted due to severe chest-back pain for 25 d, without trauma, fever, hemoptysis, numbness, or weakness of the limbs.
The patient had no previous similar symptoms.
His medical history included hypertension, glaucoma and cataract.
He denied a history of similar diseases in close relatives.
Physical examination demonstrated paraspinous muscle tenderness around the T6-8 levels and slight paresthesia of his left lower extremity. There were no abnormal findings in manual muscle test (Medical Research Council Scale), deep tendon reflexes, or pathological reflexes.
Except for an increased carbohydrate antigen 19-9 level (91.4 U/mL), which was almost 3 times higher than the normal value (< 37 U/mL), other tumor markers and routine blood examination were normal (Table 1). Hepatitis B surface antigen was positive.
| Examination | Result | Content |
| Serum tumor marker | Increased | CA19-9 |
| Normal | AFP, CA125, CA242, CEA, CYFRA 21-1, NSE, PSA, SCCA | |
| Needle biopsy | Diffusely positive | Vimentin |
| Partially positive | EMA, NSE, CK7, CK18, CK (AE1/AE3) | |
| Negative | AFP, CD31, CD34, CgA, CK20, HepPar-1, HMB45, Melan-A, Napsin, S-100, SMA, Syn, TTF-1 | |
| Postoperative pathology | Diffusely positive | Vimentin, Cam5.2 |
| Partially positive | CK5/6, CK (AE1/AE3), CD10, EMA, Ki-67 (30%) | |
| Negative | CD31, CD34, CD68, CD163, Desmin, HMB45, S-100, SMA |
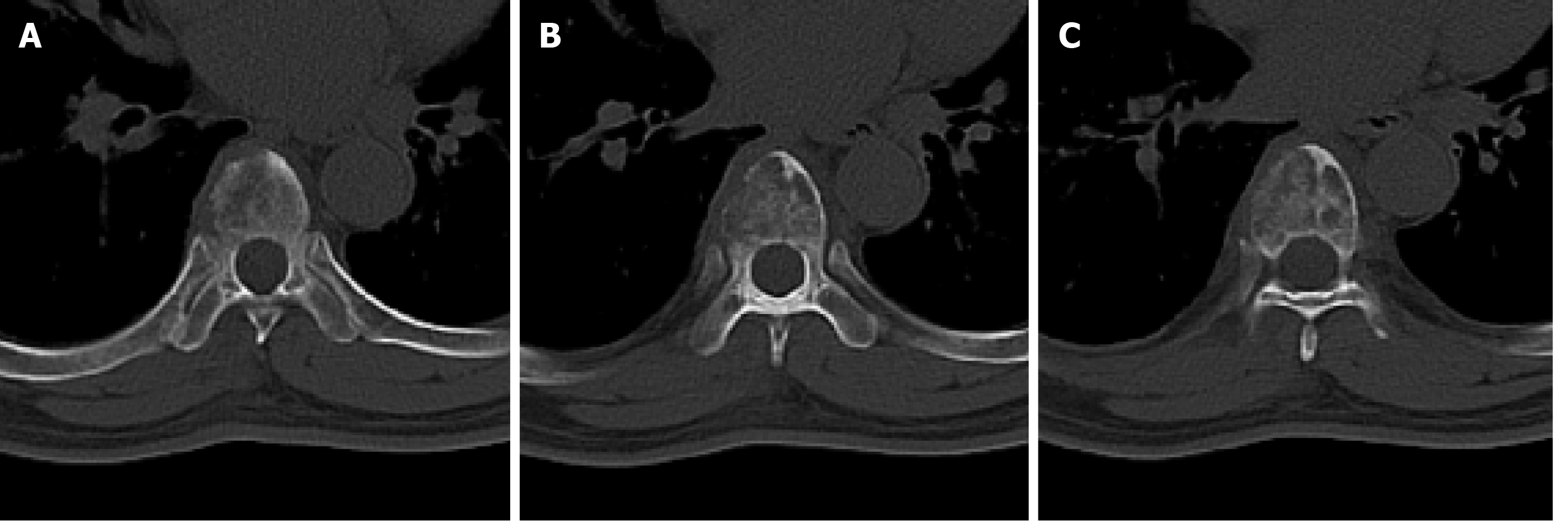
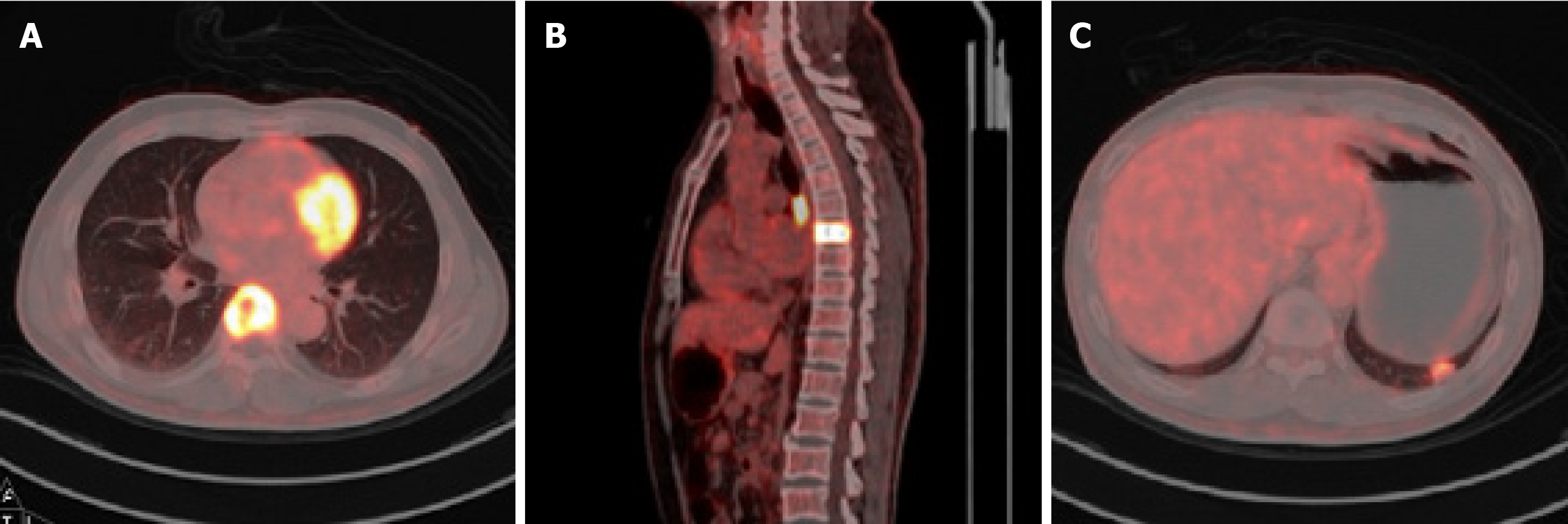
A computed tomography (CT) scan was performed, which revealed a destructive lesion at the T7 vertebral body (Figure 1A-C), and positron emission tomography-CT confirmed that the lesion was likely to be malignant with a maximal standard uptake value (SUV) of 16.74 (Figure 2A and B). An irregularly-shaped mass measuring 1.6 cm × 1.1 cm was also observed (maximal SUV, 4.95) in the left lower lobe close to the pleura (Figure 2C). The lesion was evaluated as grade D based on Frankel's grading system. Furthermore, his preoperative spine instability neoplastic score, modified Tokuhashi score, Tomita score, and Karnofsky performance scale score (KPS) were assessed to be 9, 10, 6, and 70, respectively. The lesion was localized at sectors 5-10 and layers A-C according to the Weinstein-Boriani-Biagini surgical staging system.
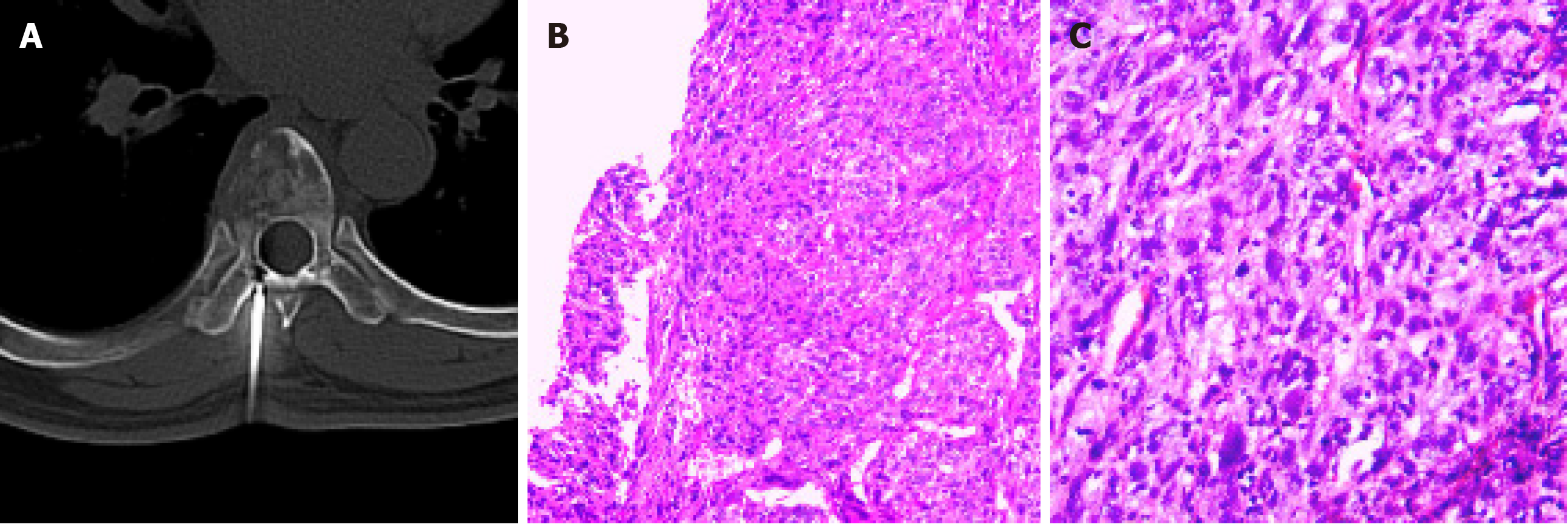
CT-guided needle biopsy and a series of immunohistochemical analyses revealed strong and diffuse immunoreactivity of the sarcomatoid component marker vimentin. In addition, partial positive staining of epithelial membrane antigen (EMA), neuron-specific enolase, CK7, CK18 and CK (AE1/AE3) was also detected (Figure 3A and B, Table 1).
Pathologic diagnosis was inconclusive, and the mass was considered a poorly differentiated metastatic cancer of unknown origin, which may have metastasized from a pulmonary malignancy.
Following admission, analgesic treatment was offered for symptomatic relief but with limited effect (Figure 4). Based on the above results and his intractable pain, the multidisciplinary team planned a strategy of palliative surgery; thus, a posterior transpedicular approach for T7 was performed. The tumor and the T7 vertebral body were completely resected piecewise followed by reconstruction with a titanium mesh cage and posterior fixation. Postoperative immunohistochemical examinations demonstrated that the tumor was positive for both the epithelial component marker CAM5.2 and the sarcomatoid component marker vimentin, partially positive for cytokeratin 5/6 (CK5/6), CK (AE1/AE3), CD10, and EMA, but negative for S-100, melanoma-related marker HMB45, smooth muscle actin, desmin, CD31, CD34, CD68 and CD163. The positive ratio of Ki-67 was around 30%. The staining pattern was considered to represent poorly differentiated metastatic cancer, partially consistent with sarcomatoid carcinoma, but the origin of the tumor was unclear (Figure 3C and Table 1).
Four days after surgery, the patient began to report chest pain, which was severe with VAS scores of 5-7. While pulmonary embolism was first excluded based on the results of an immediately arranged computed tomographic pulmonary angiography, a patchy area of ground-glass opacity in the anterior segment of the superior lobe of the right lung was observed, as well as bilateral hydrothorax and mediastinal lympha-denectasis. Although he was subsequently transferred to the intensive care unit for comprehensive therapy, his respiratory function declined progressively accompanied by dramatically increased pleural effusion. The patient died 16 d after surgery in hospital.
The prognosis is not optimistic for patients with spinal metastases, especially for those with UPTs. In general, the overall survival of patients suffering from spinal metastatic disease ranges from 2 to 21 mo[2], while the 1-, 2-, and 5-year survival rates after surgery are estimated to be 53%, 31%, and 10%, respectively[6]. Among all types of primary tumors with skeletal metastases, UPT is usually classified into the rapid growth group together with lung cancer, gastrointestinal cancer and pancreatic cancer[12,13]. Indeed, different studies in the literature showed various prognostic outcomes of spinal metastases with UPTs. Wang et al[14] in 2012 reported a prospective cohort study of 448 patients with spinal metastases and found that the median duration of survival in the UPT group was 11.4 mo, which was much better than that in the lung cancer group (3.0 mo), but worse than that in the breast cancer group (21.5 mo). In contrast, two independent studies in 2017 both mentioned that patients with UPTs had the shortest survival (3.5 mo and 4.6 mo, respectively) among all patients with metastatic spinal disease[15,16].
Perioperative death, is the most serious complication and plays an important role in the prognostic outcome of spinal metastasis. Studies that directly analyze the perioperative mortality in patients with unidentified origins of spinal metastases are rare. By searching the most recent English literature over the past five years in PubMed, we found a total of 13 publications involving the surgical treatment of spinal metastases with UPTs (Table 2). Nine of these 13 studies reported the perioperative mortality rate (ranging from 1.0% to 10.5%), but none of them clearly mentioned whether the deaths were in the UPT group, indicating that the attention paid to this particular group was limited. Three of these nine studies analyzed the causes of perioperative death. Lee et al[17] in 2014 reported 21 perioperative deaths, 12 of which were directly related to postoperative complications, including 5 due to pneumonia, 3 due to renal failure, 2 due to hepatic failure, 1 due to meningitis and 1 due to cerebral infarction. One patient with metastatic breast cancer died of pneumonia after surgery and was reported by Tan et al[18] in 2017, while in the same year Yang et al[19] reported another case of postoperative death caused by respiratory obstruction. After reviewing a total of 1266 patients with spinal metastases, Verlaan et al[5] claimed that disease progression, postoperative complications and unexplained causes were the three major reasons for postoperative mortality within 3 mo, and accounted for 84.4%, 4.0% and 11.6% of deaths, respectively. In our study, the patient died of disease progression accompanied by respiratory failure, which was in accordance with the common causes reported in the literature.
| No. | Ref. | Year | Period | Case, n | UPT, n (%) | Surgery indication | PC, n (%) | PM, n (%) |
| 1 | Morgen et al[33] | 2013 | 2005-2010 | 2321 | 549 (23.7) | NA | NA | NA |
| 2 | Lee et al[17] | 2014 | 2005-2010 | 200 | 5 (2.5) | Intractable pain | 33 (16.5) | 21 (10.5) |
| Neurologic deficits | ||||||||
| 3 | Quraishi et al[20] | 2014 | 2004-2009 | 285 | 17 (6.0) | MSCC | 67 (23.5) | NA |
| 4 | Fehlings et al[34] | 2016 | 2008-2013 | 142 | 17 (12.0) | Intractable pain | 42 (29.6) | 13 (9.0) |
| Neurologic deficits | ||||||||
| Spinal instability | ||||||||
| 5 | Wänman et al[15] | 2017 | 2003-2015 | 69 | 10 (14.5) | MSCC | 15 (21.7) | NA |
| 6 | Ragel et al[16] | 2017 | 2002-2010 | 45 | 4 (8.9) | NA | 19 (42.0) | 4 (8.9) |
| 7 | Tan et al[18] | 2017 | 2012-2014 | 19 | 2 (10.5) | Intractable pain | 3 (15.8) | 1 (5.3) |
| Neurologic deficits | ||||||||
| 8 | Yang et al[19] | 2017 | 2002-2014 | 39 | 1 (2.6) | Severe pain | 5 (12.8) | 1 (2.6) |
| Neurologic deficits | ||||||||
| Fracture with displacement | ||||||||
| Failure of nonoperative treatment | ||||||||
| Histological diagnosis | ||||||||
| 9 | Lau et al[29] | 2017 | 2005-2011 | 97 | 10 (10.3) | Intractable pain | 20 (20.6) | 1 (1.0) |
| Neurological deficit | ||||||||
| Spinal instability | ||||||||
| 10 | Uei et al[35] | 2018 | 2012-2015 | 55 | 1 (1.8) | Intractable pain | NA | 3 (5.5) |
| Neurologic deficits | ||||||||
| Spinal instability | ||||||||
| Radiation-resistant | ||||||||
| 11 | Wright et al[6] | 2018 | 1991-2016 | 1938 | 156 (8.0) | Symptomatic metastasis | NA | NA |
| 12 | Abdelbaky et al[36] | 2018 | 2008-2013 | 70 | 10 (14.3) | Intractable pain | 10 (14.3) | 3 (4.3) |
| Neurological deficit | ||||||||
| Histological diagnosis | ||||||||
| Spinal instability | ||||||||
| Radioresistant | ||||||||
| 13 | Czigléczki et al[37] | 2018 | 2008-2015 | 337 | 30 (8.9) | NA | 135 (40.1) | 26 (7.6) |
It is possible to obtain a definitive diagnosis of metastases from unknown sources after surgery. For instance, in the study published in 2014 by Quraishi et al[20], 10 of the 17 unidentified metastatic tumors were confirmed to be adenocarcinoma postoperatively, with 6 derived from pulmonary cancer and 4 from gastrointestinal cancer. However, most of the time, even though intraoperative bone biopsy confirmed the pathological features of metastatic lesions, up to the endpoint of the study their original sites might still not have been ascertained[15]. Similarly, in our case, although the thoracic vertebral lesion had undergone both preoperative and postoperative pathological analyses, due to the rapid deterioration of the disease and the absence of either pulmonary mass biopsy or autopsy, the relationship between the metastatic tumor and the pulmonary lesion remains dubious. Nevertheless, based on the patient’s clinical manifestations and pathological findings, his spinal metastasis was likely derived from pulmonary sarcomatoid carcinoma (PSC), which is a highly invasive and refractory group of non-small cell lung cancers (NSCLCs)[21,22]. The overall prognosis of PSC is worse than that of other types of NSCLCs due to its high risk of recurrence and distant metastasis after surgery, and its poor response to cisplatin-based chemotherapy. Furthermore, for patients with advanced PSC, the recurrence rate at the first assessment and the median progression-free survival were reported to be 72% and 2.7 mo, respectively[23].
Metastatic tumors from unknown sources are likely to retain the characteristics of their putative primary origins, therefore large differences in clinical manifestations are usually observed among patients with spinal metastases from UPT[24]. Nonetheless, several common signatures can be retrieved from the reported literature to depict this peculiar group of diseases: (1) Rapid progression and early dissemination, which contribute to the unidentified origin and aggressive presentation[24]; (2) Diversity of clinical and biological profiles due to the difference in origin[25]; (3) Relatively poor prognosis as non-selective empirical therapy rather than targeted management is conducted[26]; and (4) Traditional diagnostic indicators including tumor markers and immunohistochemical activity may be raised without any diagnostic or predictive value, and new methods such as NGS (next generation sequencing) may be suggested to improve diagnosis and prognosis[27].
There were several limitations in our management of this case, including uncertain diagnosis, underestimation of disease progression and inadequate preparation for perioperative death. Surgeons often have to rely on the malignancy of the primary tumor to assess the prognosis of patients with metastatic diseases, suggesting that survival prediction for those with UPTs is very challenging. Various prognostic scoring systems have been formulated to guide the management of patients with metastatic diseases[28]. During the decision-making process, we carried out comprehensive assessments consisting of the most classic Tomita score, modified Tokuhashi score and KPS, resulting in a prediction of moderate prognosis. Notwithstanding the predictable difficulty in treatment and poor prognosis, the dramatic progression of disease and death shortly after surgery was still unexpected. Possibly, the highly malignant characteristics of sarcomatoid carcinoma were involved in this unexpected disease presentation. Therefore, a more effective therapeutic strategy comprising both individualized evaluation and treatment is required for the management of patients with similar spinal metastases. In recent years, several novel scoring systems, such as the New England Spinal Metastasis Score and the Surgical Apgar Score[9,29], together with new therapeutic strategies, including stereotactic radiotherapy and tumor-targeting treatment[30-32], have been proposed to improve the prognosis and reduce the operation-related complications in metastatic patients. In the future, when surgical decisions are made on patients with unclear sources of spinal metastases, greater attention should be paid to the risk factors associated with perioperative mortality.
Surgical treatment for spinal metastases from unknown sources is mostly aimed at palliating symptoms, but has a high risk of complications including perioperative death. A comprehensive assessment prior to surgical decision-making is essential to lower the risk of perioperative mortality in such patients.
We thank The Department of Pathology in our hospital for the histological examinations in the patient. This work was conducted under the guidance and with the help of Professor Tao HM in our department.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wang DW S-Editor: Huang P L-Editor: Webster JR P-Editor: Wang LL
| 1. | Croucher PI, McDonald MM, Martin TJ. Bone metastasis: the importance of the neighbourhood. Nat Rev Cancer. 2016;16:373-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 337] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 2. | Spratt DE, Beeler WH, de Moraes FY, Rhines LD, Gemmete JJ, Chaudhary N, Shultz DB, Smith SR, Berlin A, Dahele M, Slotman BJ, Younge KC, Bilsky M, Park P, Szerlip NJ. An integrated multidisciplinary algorithm for the management of spinal metastases: an International Spine Oncology Consortium report. Lancet Oncol. 2017;18:e720-e730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 207] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 3. | Vanek P, Bradac O, Trebicky F, Saur K, de Lacy P, Benes V. Influence of the Preoperative Neurological Status on Survival After the Surgical Treatment of Symptomatic Spinal Metastases With Spinal Cord Compression. Spine (Phila Pa 1976). 2015;40:1824-1830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Claus EB. Neurosurgical management of metastases in the central nervous system. Nat Rev Clin Oncol. 2011;9:79-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Verlaan JJ, Choi D, Versteeg A, Albert T, Arts M, Balabaud L, Bunger C, Buchowski JM, Chung CK, Coppes MH, Crockard HA, Depreitere B, Fehlings MG, Harrop J, Kawahara N, Kim ES, Lee CS, Leung Y, Liu Z, Martin-Benlloch A, Massicotte EM, Mazel C, Meyer B, Peul W, Quraishi NA, Tokuhashi Y, Tomita K, Ulbricht C, Wang M, Oner FC. Characteristics of Patients Who Survived < 3 Months or > 2 Years After Surgery for Spinal Metastases: Can We Avoid Inappropriate Patient Selection? J Clin Oncol. 2016;34:3054-3061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 6. | Wright E, Ricciardi F, Arts M, Buchowski JM, Chung CK, Coppes M, Crockard A, Depreitere B, Fehlings M, Kawahara N, Lee CS, Leung Y, Martin-Benlloch A, Massicotte E, Mazel C, Oner C, Peul W, Quraishi N, Tokuhashi Y, Tomita K, Ulbricht C, Verlaan JJ, Wang M, Choi D. Metastatic Spine Tumor Epidemiology: Comparison of Trends in Surgery Across Two Decades and Three Continents. World Neurosurg. 2018;114:e809-e817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 7. | Tateiwa D, Oshima K, Nakai T, Imura Y, Tanaka T, Outani H, Tamiya H, Araki N, Naka N. Clinical outcomes and significant factors in the survival rate after decompression surgery for patients who were non-ambulatory due to spinal metastases. J Orthop Sci. 2019;24:347-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Bakar D, Tanenbaum JE, Phan K, Alentado VJ, Steinmetz MP, Benzel EC, Mroz TE. Decompression surgery for spinal metastases: a systematic review. Neurosurg Focus. 2016;41:E2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 9. | Schoenfeld AJ, Le HV, Marjoua Y, Leonard DA, Belmont PJ Jr, Bono CM, Harris MB. Assessing the utility of a clinical prediction score regarding 30-day morbidity and mortality following metastatic spinal surgery: the New England Spinal Metastasis Score (NESMS). Spine J. 2016;16:482-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 10. | Schoenfeld AJ, Ferrone ML, Sturgeon DJ, Harris MB. Volume-Outcome Relationship in Surgical Interventions for Spinal Metastases. J Bone Joint Surg Am. 2017;99:1753-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Hobbs JG, Patel AS, Chaker AN, Hasan N, Kumar P, Ramos E, Mehta AI. Steroid Use Associated With Increased Odds of 30-Day Mortality in Surgical Patients With Metastatic Spinal Tumors in the Setting of Disseminated Disease. Neurosurgery. 2019;85:394-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Katagiri H, Okada R, Takagi T, Takahashi M, Murata H, Harada H, Nishimura T, Asakura H, Ogawa H. New prognostic factors and scoring system for patients with skeletal metastasis. Cancer Med. 2014;3:1359-1367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 271] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 13. | Lei M, Li J, Liu Y, Jiang W, Liu S, Zhou S. Who are the Best Candidates for Decompressive Surgery and Spine Stabilization in Patients With Metastatic Spinal Cord Compression? Spine (Phila Pa 1976). 2016;41:1469-1476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Wang M, Bünger CE, Li H, Wu C, Høy K, Niedermann B, Helmig P, Wang Y, Jensen AB, Schättiger K, Hansen ES. Predictive value of Tokuhashi scoring systems in spinal metastases, focusing on various primary tumor groups: evaluation of 448 patients in the Aarhus spinal metastases database. Spine (Phila Pa 1976). 2012;37:573-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Wänman J, Grabowski P, Nyström H, Gustafsson P, Bergh A, Widmark A, Crnalic S. Metastatic spinal cord compression as the first sign of malignancy. Acta Orthop. 2017;88:457-462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Ragel BT, Mendez GA, Reddington J, Ferachi D, Kubicky CD, Philipp TC, Zusman NL, Klimo P, Hart R, Yoo J, Ching AC. Life Expectancy and Metastatic Spine Scoring Systems: An Academic Institutional Experience. Clin Spine Surg. 2017;30:335-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Lee BH, Park JO, Kim HS, Park YC, Lee HM, Moon SH. Perioperative complication and surgical outcome in patients with spine metastases: retrospective 200-case series in a single institute. Clin Neurol Neurosurg. 2014;122:80-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Tan T, Chu J, Thien C, Wang YY. Minimally Invasive Direct Lateral Corpectomy of the Thoracolumbar Spine for Metastatic Spinal Cord Compression. J Neurol Surg A Cent Eur Neurosurg. 2017;78:358-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Yang J, Jia Q, Peng D, Wan W, Zhong N, Lou Y, Cai X, Wu Z, Zhao C, Yang X, Xiao J. Surgical treatment of upper cervical spine metastases: a retrospective study of 39 cases. World J Surg Oncol. 2017;15:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Quraishi NA, Ramoutar D, Sureshkumar D, Manoharan SR, Spencer A, Arealis G, Edwards KL, Boszczyk BM. Metastatic spinal cord compression as a result of the unknown primary tumour. Eur Spine J. 2014;23:1502-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Li X, Wang D, Zhao Q, Ren D, Ren F, Chen G, Liu H, Chen J. Clinical Significance and Next-Generation Sequencing of Chinese Pulmonary Sarcomatoid Carcinoma. Sci Rep. 2017;7:3947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Boland JM, Mansfield AS, Roden AC. Pulmonary sarcomatoid carcinoma-a new hope. Ann Oncol. 2017;28:1417-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Ung M, Rouquette I, Filleron T, Taillandy K, Brouchet L, Bennouna J, Delord JP, Milia J, Mazières J. Characteristics and Clinical Outcomes of Sarcomatoid Carcinoma of the Lung. Clin Lung Cancer. 2016;17:391-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 24. | Varadhachary GR, Raber MN. Cancer of unknown primary site. N Engl J Med. 2014;371:757-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 187] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 25. | Argentiero A, Solimando AG, Brunetti O, Calabrese A, Pantano F, Iuliani M, Santini D, Silvestris N, Vacca A. Skeletal Metastases of Unknown Primary: Biological Landscape and Clinical Overview. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Tomuleasa C, Zaharie F, Muresan MS, Pop L, Fekete Z, Dima D, Frinc I, Trifa A, Berce C, Jurj A, Berindan-Neagoe I, Zdrenghea M. How to Diagnose and Treat a Cancer of Unknown Primary Site. J Gastrointestin Liver Dis. 2017;26:69-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | He B, Zhang Y, Zhou Z, Wang B, Liang Y, Lang J, Lin H, Bing P, Yu L, Sun D, Luo H, Yang J, Tian G. A Neural Network Framework for Predicting the Tissue-of-Origin of 15 Common Cancer Types Based on RNA-Seq Data. Front Bioeng Biotechnol. 2020;8:737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 28. | Hussain AK, Vig KS, Cheung ZB, Phan K, Lima MC, Kim JS, Kaji DA, Arvind V, Cho SK. The Impact of Metastatic Spinal Tumor Location on 30-Day Perioperative Mortality and Morbidity After Surgical Decompression. Spine (Phila Pa 1976). 2018;43:E648-E655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Lau D, Yee TJ, La Marca F, Patel R, Park P. Utility of the Surgical Apgar Score for Patients Who Undergo Surgery for Spinal Metastasis. Clin Spine Surg. 2017;30:374-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Tao R, Bishop AJ, Brownlee Z, Allen PK, Settle SH, Chang EL, Wang X, Amini B, Tannir NM, Tatsui C, Rhines LD, Brown PD, Ghia AJ. Stereotactic Body Radiation Therapy for Spinal Metastases in the Postoperative Setting: A Secondary Analysis of Mature Phase 1-2 Trials. Int J Radiat Oncol Biol Phys. 2016;95:1405-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Wang XS, Rhines LD, Shiu AS, Yang JN, Selek U, Gning I, Liu P, Allen PK, Azeem SS, Brown PD, Sharp HJ, Weksberg DC, Cleeland CS, Chang EL. Stereotactic body radiation therapy for management of spinal metastases in patients without spinal cord compression: a phase 1-2 trial. Lancet Oncol. 2012;13:395-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 217] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 32. | Tang J, Pearce L, O'Donnell-Tormey J, Hubbard-Lucey VM. Trends in the global immuno-oncology landscape. Nat Rev Drug Discov. 2018;17:922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 33. | Morgen SS, Lund-Andersen C, Larsen CF, Engelholm SA, Dahl B. Prognosis in patients with symptomatic metastatic spinal cord compression: survival in different cancer diagnosis in a cohort of 2321 patients. Spine (Phila Pa 1976). 2013;38:1362-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 34. | Fehlings MG, Nater A, Tetreault L, Kopjar B, Arnold P, Dekutoski M, Finkelstein J, Fisher C, France J, Gokaslan Z, Massicotte E, Rhines L, Rose P, Sahgal A, Schuster J, Vaccaro A. Survival and Clinical Outcomes in Surgically Treated Patients With Metastatic Epidural Spinal Cord Compression: Results of the Prospective Multicenter AOSpine Study. J Clin Oncol. 2016;34:268-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 155] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 35. | Uei H, Tokuhashi Y, Maseda M, Nakahashi M, Sawada H, Nakayama E, Soma H. Clinical results of multidisciplinary therapy including palliative posterior spinal stabilization surgery and postoperative adjuvant therapy for metastatic spinal tumor. J Orthop Surg Res. 2018;13:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Abdelbaky A, Eltahawy H. Neurological Outcome Following Surgical Treatment of Spinal Metastases. Asian J Neurosurg. 2018;13:247-249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Czigléczki G, Mezei T, Pollner P, Horváth A, Banczerowski P. Prognostic Factors of Surgical Complications and Overall Survival of Patients with Metastatic Spinal Tumor. World Neurosurg. 2018;113:e20-e28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |