Published online Jun 16, 2021. doi: 10.12998/wjcc.v9.i17.4453
Peer-review started: February 19, 2021
First decision: March 7, 2021
Revised: March 7, 2021
Accepted: March 29, 2021
Article in press: March 29, 2021
Published online: June 16, 2021
Processing time: 99 Days and 2.3 Hours
Schwannoma of the pancreas is extremely rare. We report a case of pancreatic schwannoma that was difficult to distinguish from pancreatic carcinoma before surgery.
A 66-year-old male underwent a right-lobe hepatectomy for hepatocellular carcinoma. Post-surgical computed tomography showed a 10 mm long solid mass with ischemia, with no expansion into the main pancreatic duct. Upon magnetic resonance cholangiopancreatography, the tumor had high signal intensity in diffusion weighted images, consistent with pancreatic carcinoma. Endoscopic ultrasound (EUS) was performed to obtain more information about the tumor, and showed a 14 mm solid and hypoechoic mass in the pancreatic body. Contrast enhanced EUS revealed that the tumor showed a hyperechoic mass in the early phase, and the contrasting effect continuation was very short; findings also consistent with pancreatic carcinoma. Thus, we preoperatively diagnosed his condition as a pancreatic carcinoma and performed distal pancreatectomy with splenectomy. Microscopic examination showed that the tumor was in fact a benign schwannoma. Histology showed a proliferation of spindle-shaped cell in a vague fascicular and haphazard pattern, with palisading arrangement.
Schwannoma of the pancreas is very rare, however, clinicians should consider schwannoma as the differential diagnosis for pancreatic tumors.
Core Tip: Schwannoma of the pancreas is extremely rare, with 68 cases reported in the literature. On the other hand, preoperative diagnosis of schwannomas can be difficult, as schwannomas have no specific imaging findings, thus the preoperative diagnosis is not easy to confirm. We have experienced a case of schwannoma in pancreatic body mimicking pancreatic carcinoma, and performed surgical treatment. Clinicians should consider nonepithelial tumors as a part of the differential diagnosis for pancreatic tumors despite their low frequency.
- Citation: Kimura K, Adachi E, Toyohara A, Omori S, Ezaki K, Ihara R, Higashi T, Ohgaki K, Ito S, Maehara SI, Nakamura T, Fushimi F, Maehara Y. Schwannoma mimicking pancreatic carcinoma: A case report. World J Clin Cases 2021; 9(17): 4453-4459
- URL: https://www.wjgnet.com/2307-8960/full/v9/i17/4453.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i17.4453
Schwannomas are usually benign nerve sheath tumors that originate from the Schwann cells and can arise in any aspect of the peripheral nerves[1]. Schwannomas are often found in the head and neck area, major nerve trunks. Intracavitary schwan
Herein, we present a review of the literature and a novel case of a pancreatic schwannoma that was initially diagnosed as a pancreatic carcinoma, in a patient who underwent a distal pancreatectomy with splenectomy.
A 66-year-old male who was followed-up after right lobe hepatectomy for hepatocellular carcinoma (pT2N0M0 stage II) secondary to a hepatitis B infection for ten years and six months. He was found pancreatic tumor accidentally in ultrasonography on a health examination.
Ultrasonography revealed a 10 mm tumor in the pancreas.
The patient had a history of right lobe hepatectomy for S5/6 hepatocellular (pathological stage: PT2N0M0 stage II carcinoma ten years and six months ago, and followed up for five years after hepatectomy.
There is no history of his family.
He had no physical abnormity, general condition was almost good.
Laboratory findings indicated that white blood counts were 4000/μL (reference range 3500-8400/μL), alkaline phosphatase 217 U/L (reference range 115-359 U/L), gamma-glutamyl transpeptidase 17 U/L (reference range 10-47 U/L), C-reactive protein 0.04 mg/dL (reference range < 0.2 mg/dL), carcinoembryonic antigen 2.0 ng/mL (reference range < 5.0 ng/mL), CA19-9 antigen 0.4 U/mL (reference range < 37.0 U/mL), and immunoglobulin G4 21 mg/dL (reference range 5-117 mg/dL).
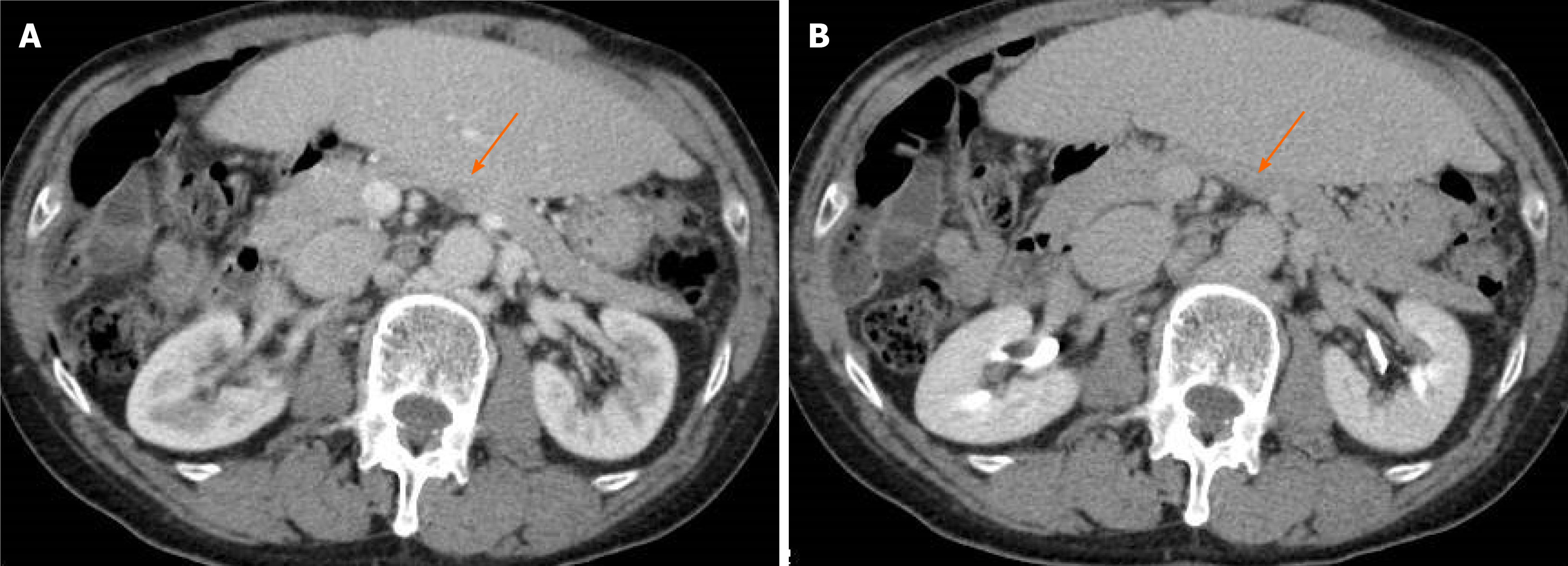
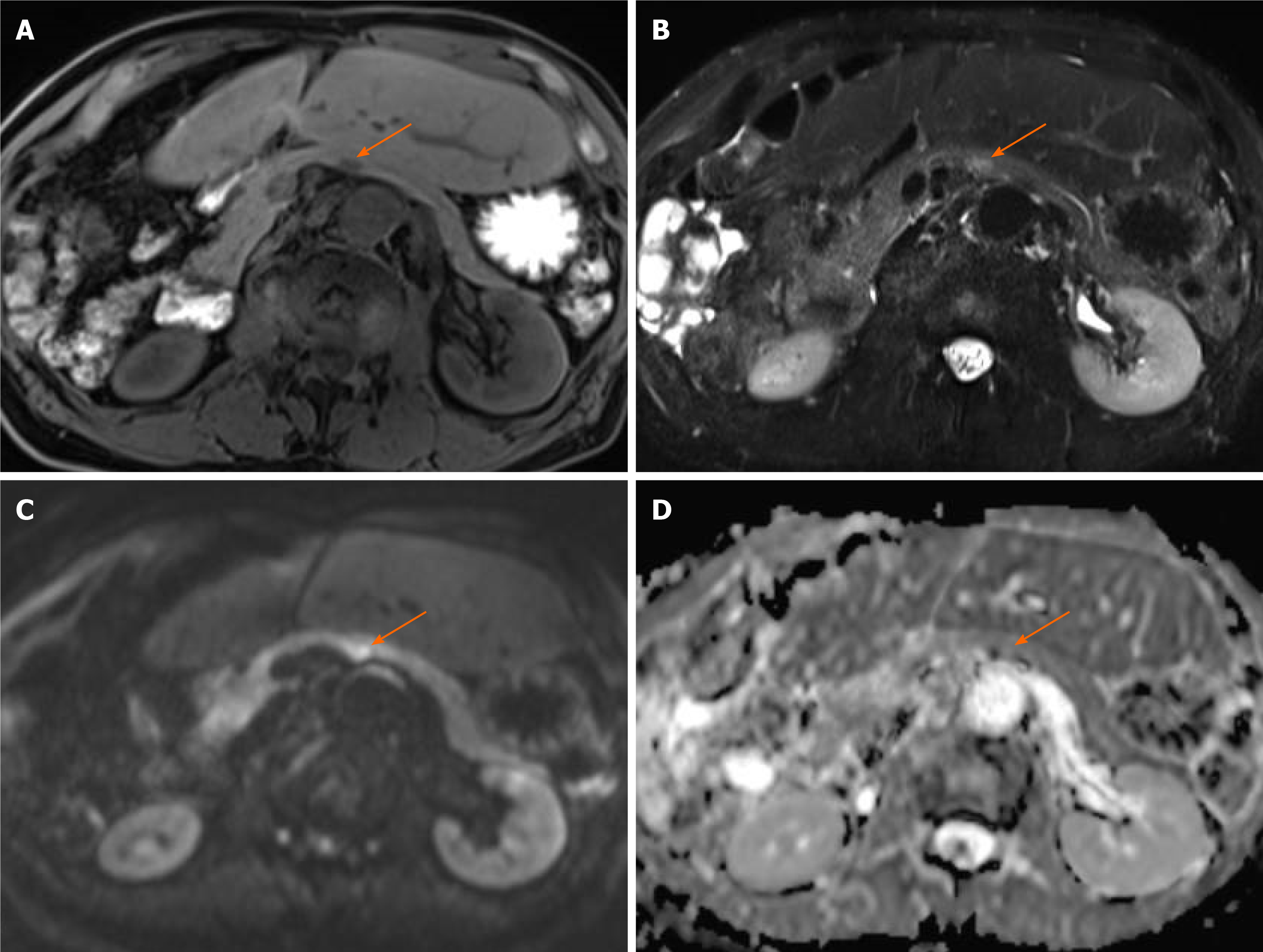
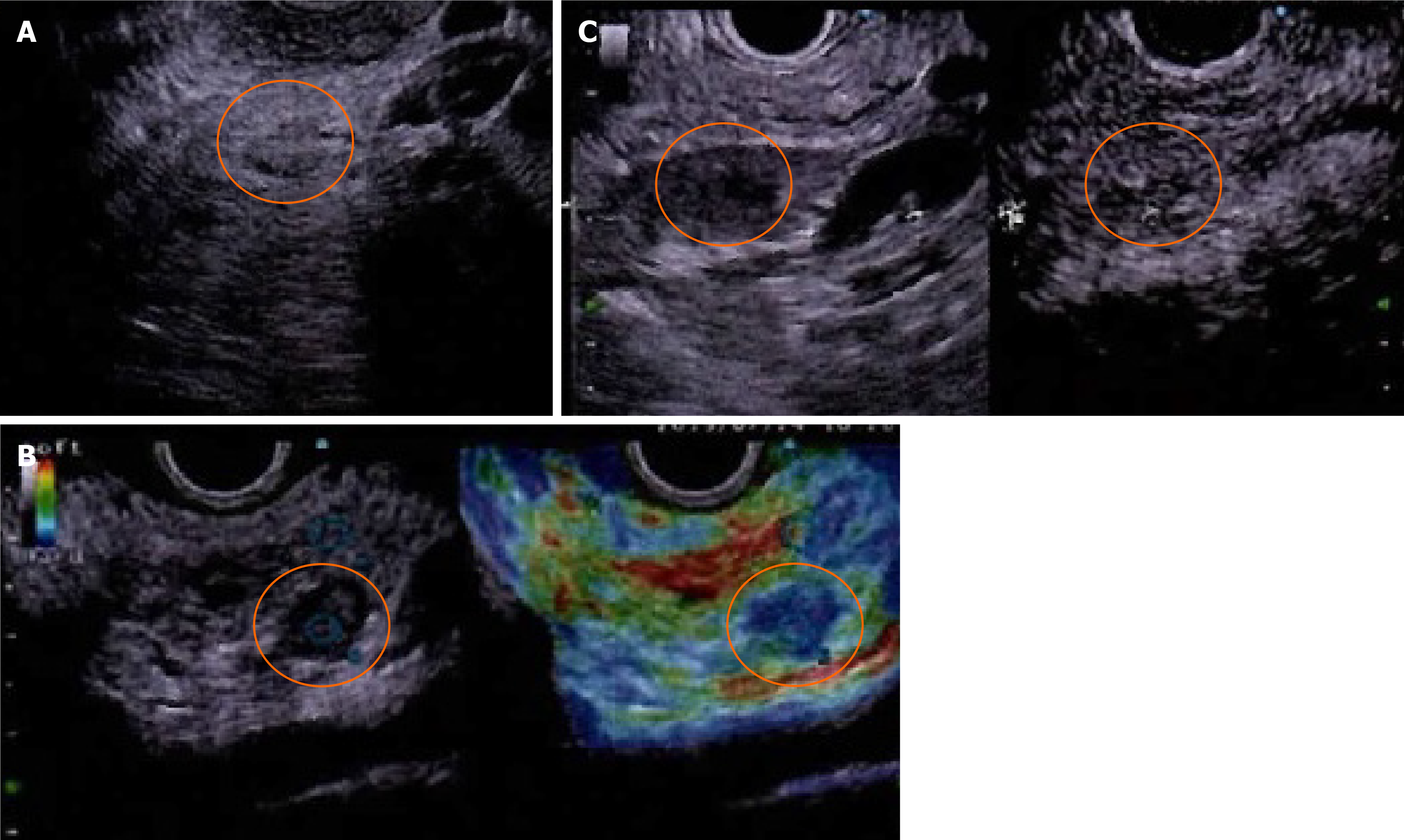
Computed tomography (CT) showed a 10 mm tumor with low density in the early phase (Figure 1A) and isodensity with pancreatic parenchyma in the late phase (Figure 1B). There was no expansion into the main pancreatic duct, and no swollen lymph nodes. Magnetic resonance cholangiopancreatography (MRI) revealed a 9 mm tumor in the pancreatic body. The tumor showed low intensity in T1-weighted images (Figure 2A) and showed slightly higher intensity in T2-weighted images (Figure 2B). The tumor also demonstrated high signal in diffusion weighted images (Figure 2C) and almost the same isodensity in an apparent diffusion coefficient-map phase (Figure 2D). Endoscopic ultrasound (EUS) was performed to obtain more information about the tumor. EUS images showed a 14 mm solid and low echoic mass in the pancreatic body (Figure 3A), EUS elastography showed a strain ratio < 0.05 (Figure 3B), and contrast enhanced EUS showed short term contrast effects in the early phase that washed out quickly (Figure 3C).
The patient was diagnosed with pancreatic carcinoma.
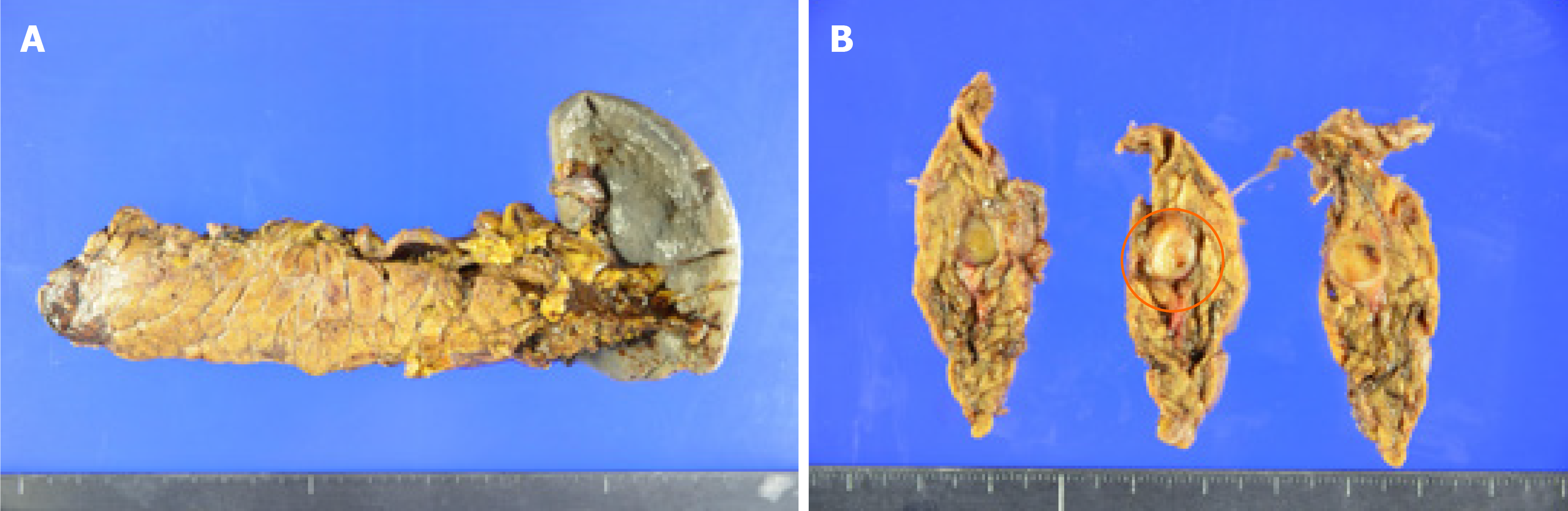
He underwent a distal pancreatectomy with splenectomy. During surgery, there was no ascites, no peritoneal or liver metastasis, and no macroscopic lymphadenopathy. Distal pancreatectomy with splenectomy and lymphadenectomy of lymph nodes 8, 9, 10, 11, 14, 15, 18 were performed (Figure 4).
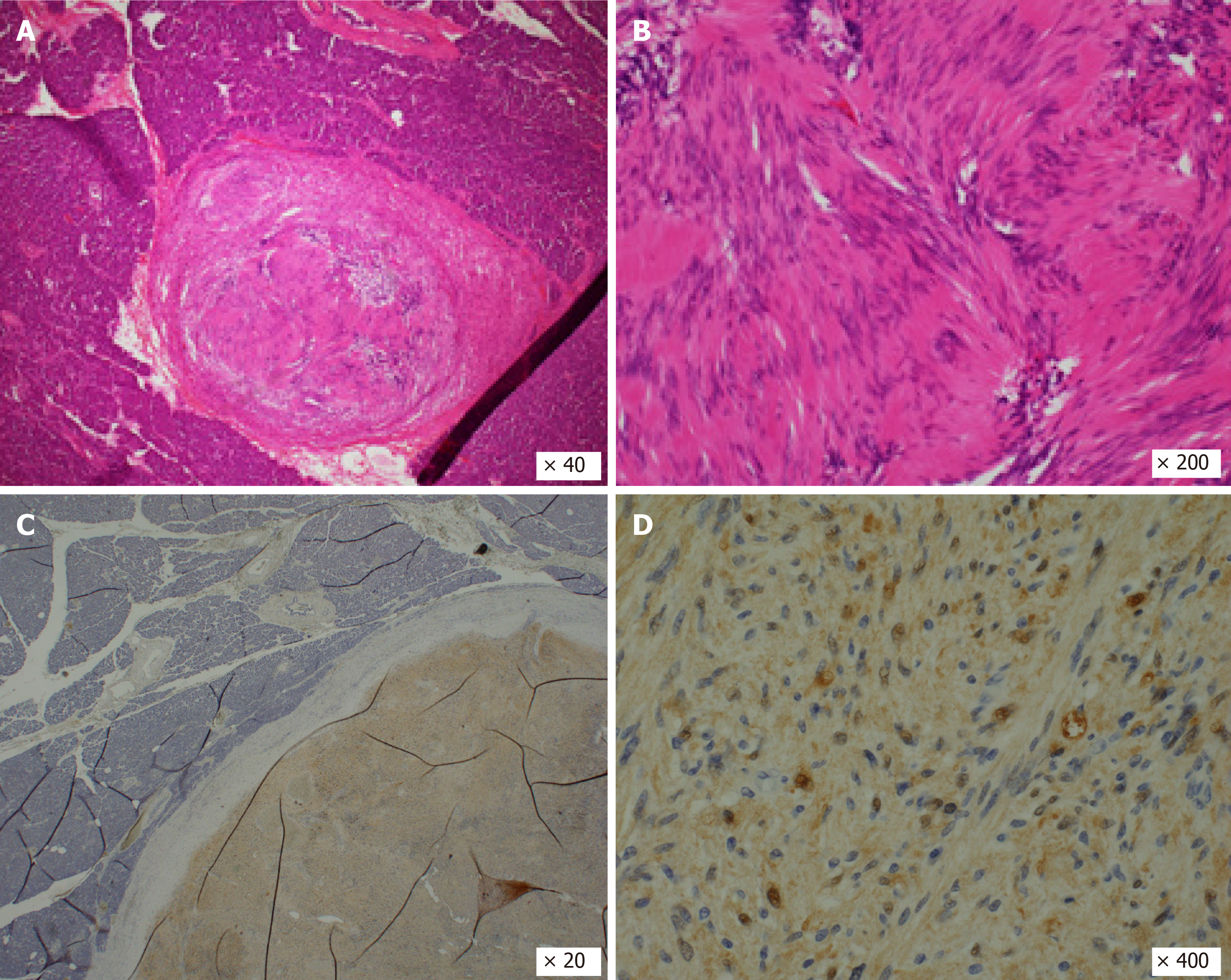
Histopathologic examination after surgery showed a proliferation of spindle-shaped cells in a vague fascicular and haphazard pattern, with palisading arrangement (Figure 5A and B). Mitotic figures were not evident. These features were consistent with schwannoma, not pancreatic carcinoma. There was in fact no evidence of malignancy. Immunohistochemical staining of S100 was positive (Figure 5C and D), and AE1/AE3, spinal muscular atrophy (SMA), and CD34 were all negative. There was no lymph node metastasis. As a result, the final diagnosis of the tumor proved to be a pancreatic schwannoma (11 mm × 8 mm). Although the patient developed a grade B pancreatic fistula after surgery, this was resolved by conservative treatment.
We present a patient with a pancreatic schwannoma who underwent a distal pancreatectomy and splenectomy with a working diagnosis of pancreatic carcinoma. In the abdominal cavity, the retroperitoneum and stomach are the most frequently involved sites for schwannomas. On the other hand, other intraperitoneal organ schwannomas have been previously reported, such as in the gallbladder[7] and intrahepatic duodenal ligament[8]. To the best of our knowledge, less than 70 cases of schwan
Preoperative diagnosis of schwannomas can be difficult, as schwannomas have no specific imaging findings, thus the preoperative diagnosis is not easy to confirm. Several imaging modalities such as ultrasonography, CT, MRI, and EUS may be useful in diagnosing. Contrast enhanced CT shows well-defined hypodense lesions with encapsulation and/or cystic degeneration[8], and MRI shows hypointensity on T1-weighted images and lack of homogeneity and hyperintensity on T2-weighted images[10]. Crinò et al[11] reported that EUS is useful to diagnose pancreatic schwan
Most pancreatic schwannomas are benign, however there are some reports that show malignant transformation[4,12]. Some researchers have attempted to correlate the characteristics of the schwannomas on imaging examination with its malignant potential. Ma et al[3] suggested that larger tumors correlated with greater malignant potential. The management of schwannomas in pancreas should be decided by location and histological findings. Most pancreatic schwannomas are benign and malignant transformation is extremely rare. As such, enucleation is often the first-line procedure if tumor pathology is confirmed pre-operation. In cases of large tumors expressing malignant behavior, such as malignancy in frozen sections or invasion to major vessels, an oncological resection is recommended.
Incidental detection of pancreatic schwannoma is predicted to increase due to the widespread use of CT and MRI. It is important to consider this tumor type in the differential diagnosis of pancreatic tumors. Pancreatic schwannoma is extremely rare, but in centers performing large numbers of pancreatic surgeries, the possibility of this diagnosis is still reasonable.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhu CF S-Editor: Fan JR L-Editor: A P-Editor: Li JH
| 1. | Pilavaki M, Chourmouzi D, Kiziridou A, Skordalaki A, Zarampoukas T, Drevelengas A. Imaging of peripheral nerve sheath tumors with pathologic correlation: pictorial review. Eur J Radiol. 2004;52:229-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 204] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 2. | Ercan M, Aziret M, Bal A, Şentürk A, Karaman K, Kahyaoğlu Z, Koçer HB, Bostancı B, Akoğlu M. Pancreatic schwannoma: A rare case and a brief literature review. Int J Surg Case Rep. 2016;22:101-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Ma Y, Shen B, Jia Y, Luo Y, Tian Y, Dong Z, Chen W, Li ZP, Feng ST. Pancreatic schwannoma: a case report and an updated 40-year review of the literature yielding 68 cases. BMC Cancer. 2017;17:853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Stojanovic MP, Radojkovic M, Jeremic LM, Zlatic AV, Stanojevic GZ, Jovanovic MA, Kostov MS, Katic VP. Malignant schwannoma of the pancreas involving transversal colon treated with en-bloc resection. World J Gastroenterol. 2010;16:119-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 5. | Witkowski G, Kołos M, Nasierowska-Guttmejer A, Durlik M. Neuroma (schwannoma). A rare pancreatic tumor. Pol Przegl Chir. 2019;92:48-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Aggarwal G, Satsangi B, Shukla S, Lahoti BK, Mathur RK, Maheshwari A. Rare asymptomatic presentations of schwannomas in early adolescence: three cases with review of literature. Int J Surg. 2010;8:203-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Tajiri T, Hayashi H, Higashi T, Yamao T, Takematsu T, Uemura N, Yamamura K, Imai K, Yamashita YI, Baba H. Coexisting schwannoma of the gallbladder and sarcoidosis: a case report. Surg Case Rep. 2020;6:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Xu SY, Sun K, Xie HY, Zhou L, Zheng SS, Wang WL. Schwannoma in the hepatoduodenal ligament: A case report and literature review. World J Gastroenterol. 2016;22:10260-10266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Joshi R. Learning from eponyms: Jose Verocay and Verocay bodies, Antoni A and B areas, Nils Antoni and Schwannomas. Indian Dermatol Online J. 2012;3:215-219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Rha SE, Byun JY, Jung SE, Chun HJ, Lee HG, Lee JM. Neurogenic tumors in the abdomen: tumor types and imaging characteristics. Radiographics. 2003;23:29-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 264] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 11. | Crinò SF, Bernardoni L, Manfrin E, Parisi A, Gabbrielli A. Endoscopic ultrasound features of pancreatic schwannoma. Endosc Ultrasound. 2016;5:396-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Liegl B, Bodo K, Martin D, Tsybrovskyy O, Lackner K, Beham A. Microcystic/reticular schwannoma of the pancreas: a potential diagnostic pitfall. Pathol Int. 2011;61:88-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |