Published online Jun 16, 2021. doi: 10.12998/wjcc.v9.i17.4441
Peer-review started: February 12, 2021
First decision: March 25, 2021
Revised: April 5, 2021
Accepted: April 22, 2021
Article in press: April 22, 2021
Published online: June 16, 2021
Processing time: 103 Days and 2.5 Hours
Neuropathic pain management should aim at improving quality of life and daily living activities of patients; therefore, emphasis should be placed on pain management including understanding the pain patterns during daily activity. Therefore, lifestyle guidance should be based on a detailed understanding of pain; however, previous studies commonly evaluated pain intensity at a single point in time. We report a case on patient education intervention based on the relationship between pain circadian rhythms and detailed physical activity during the day.
A man in his 60s, who suffered a brachial plexus injury in a traffic accident, presented with neuropathic pain. Early assessment of the importance of daily living activities to the patient, pain rhythmicity, and physical activity, was performed. The early assessments showed that the pain intensity was lower on days when more light-intensity physical activity (LIPA) was performed, than on days when less LIPA was performed. Consequently, patient education focused on methods to decrease the pain intensity that tended to worsen in the afternoon, and encouraged behavioral changes by suggesting the patient to take walks,” which could be used to maintain LIPA in the afternoon. On reassessment, the afternoon LIPA, which had been the focus of attention, had increased and a change was noted in the circadian rhythm of pain.
Patient education based on a composite assessment elicited positive results in relation to the pain circadian rhythm and physical activity.
Core Tip: A thorough understanding of circadian pain rhythms can facilitate adjustments to patients’ lifestyle for better management of neuropathic pain. We evaluated the circadian pain rhythms and physical activity of a patient in his 60s. Based on the results of this evaluation, we provided pain neurophysiology education to the patient. We also discussed with him the relationship between his pain rhythmicity and physical activity and encouraged smooth behavioral changes for pain management by learning the importance of different types of daily life activities through dialogue.
- Citation: Tanaka Y, Sato G, Imai R, Osumi M, Shigetoh H, Fujii R, Morioka S. Effectiveness of patient education focusing on circadian pain rhythms: A case report and review of literature. World J Clin Cases 2021; 9(17): 4441-4452
- URL: https://www.wjgnet.com/2307-8960/full/v9/i17/4441.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i17.4441
Neuropathic pain (NP) is defined as “pain caused by a lesion or disease of the somatosensory nervous system”[1]. NP presents as a variety of sensory symptoms, such as electric shock pain, burning pain, and numbness[2]; the incidence of NP in developed countries is estimated to be 1%-7%[3,4]. NP is reportedly more severe than nociceptive pain in terms of pain intensity and psychological state[5], and causes a significant decline in the quality of life (QOL)[3,6]. In addition to the various sensory symptoms of neuropathic origin, psychosocial factors often linked to the pathophysiology of the disease, due to persistent pain[7-10], complicate its treatment.
Therefore, at present, NP management aims at improving the patients’ QOL and activities of daily living (ADL)[11]; therefore, instead of focusing on pain elimination, the emphasis is placed on pain management, which includes understanding the pain patterns during daily activities and physical exercise. Patient education is a method of intervention aimed at pain management. Moseley et al[12] reported the effectiveness of pain neurophysiology education (PNE), a teaching method that focuses on neurophy
Against this backdrop, it is important to use patient education to promote pain management in patients with NP, while addressing their daily activities and physical activity. However, specific and efficient lifestyle guidance should be based on a detailed understanding of pain. Previous studies have often evaluated pain intensity at a single point in time, and thus assessment on a detailed understanding of pain seems uncertain. PNE involves correcting the pain perception of people[16]; hence, underst
To clearly understand the characteristics of pain, attention should be given to the pain circadian rhythms in various diseases[17-21]. In a study on patients with NP, Gilron et al[22] reported that pain circadian rhythms caused increased pain perception in the evening and at night, as compared to the morning. A thorough understanding of these circadian rhythms can facilitate scheduling of daily activities and physical activity based on the time of the day, thereby, ensuring better pain management.
We evaluated the relationship between pain circadian rhythms and physical activity during the day in depth, in addition to assessing the current ADL in a patient with NP. Based on these assessments, patient education was used to explain the characteristics of the patient’s pain and physical activity, and to provide guidance on future pain management. The intervention resulted in an increase in the previously decreasing physical activity in the afternoon and a corresponding change in the circadian rhythm of pain.
A 68-year-old man presented with severe spontaneous pain in the affected upper limb with a feeling of electricity running through it.
The patient was in a car accident 8 years ago, which had resulted in complete damage to his right brachial plexus. Right after the car accident, he began to experience severe spontaneous pain in the affected upper limb with a feeling of electricity running through it. After the injury, the patient was admitted to a recovery-phase rehabilitation ward for 6 mo and received daily occupational and physical therapy. After discharge from the hospital, the patient returned to community life while receiving weekly occupational and physical therapy at a rehabilitation hospital. After about 1 year of hospital rehabilitation, the patient began receiving twice-weekly group rehabilitation at a seniors’ day care facility.
The patient had retired from work after the injury. Based on the description of his daily life during initial assessment, he utilized day care services for seniors twice a week in the morning and took a walk around the neighborhood on days when the day care service was not available. He spent most of his afternoons at home, often in front of the television, or pruning his garden plants and doing laundry. He had a strong desire to do more despite his pain, saying, “If only I could get rid of this pain, I could do more things”.
The patient gave written consent after being informed of the study procedure. The experimental protocol was approved by the Kio University Ethics Committee (approval number: H30-31).
From the car accident 8 years ago to the present, there were no illnesses that caused any problems.
The patient had no significant past history or family history.
He has continued to take pain medication regularly, in the morning and evening, to this day. The sensory-motor function of the affected upper limb had not changed significantly since the time of the injury; he had flaccid motor function with complete paralysis and a severely dulled sensory function. Tactile stimuli to the right shoulder, elbow, and fingers extremities were faintly perceptible but worsening of spontaneous pain with tactile stimuli was observed.
No abnormalities were found in the blood and urine tests.
The significance of the ADL for the patient, his pain characteristics, and psychological status were assessed beforehand. The pain circadian rhythms and physical activity during the day were also evaluated. All prior assessments were performed using questionnaires, which the patient was asked to answer on the spot.
The patient was asked to select five activities that he often did in his daily life, and to rate the importance of these activities on a scale of 0-10 (0: not important, 10: very important).
The NP diagnostic questionnaire (DN4) was used to determine NP, the Short-Form McGill Pain Questionnaire 2 (SFMPQ2) was used as a multidimensional assessment of pain intensity, and the NP symptom inventory (NPSI) was used to assess the severity of NP. Psychological states were assessed using the Hospital Anxiety and Depressions Scale (HADS) for depression and anxiety, and the pain catastrophizing scale (PCS-4) for pain catastrophizing thoughts.
DN4: DN4 is an evaluation scale developed to classify NP and non-NP[23]. It consists of 10 questions related to NP (e.g., “Do you have a burning pain?” “Do you have pain like an electric shock?”). If four or more items are applicable, the pain is judged as NP.
SFMPQ2: SFMPQ2 consists of 22 questions and can be categorized into four sub-items: Continuous pain, intermittent pain, affective descriptors, and NP[24]. Each item was answered on a numerical rating scale of 11 points, and higher the score, more severe was the pain experienced.
NPSI: The NPSI is a 10-item questionnaire related to NP (e.g., “Do you have burning spontaneous pain?”) (“Do you have attacks of pain, like a knife stabbing?”). It can be classified into the following sub-items: Spontaneous pain, attacks of pain, provoked pain, and abnormal sensations[25]. These symptoms were self-evaluated by the patient on an 11-point numeric rating scale by selecting the number that best described the average pain he had experienced within the last 24 h (range, 0 to 10, where 10 indicates maximum pain). The higher the total score, greater the severity of the symptoms of NP.
HADS: HADS contains 14 items and two subscales. The two subscales independently assess depression and anxiety[26]. Higher scores indicate more severe anxiety and depression.
PCS-4: PCS-4 is a shorter version of the 13-item PCS and contains four items. Higher scores indicate more severe catastrophic thinking[27].
The pain circadian rhythm was assessed over seven days. Pain intensity was measured at six time points per day (waking up, 9:00, 12:00, 15:00, 18:00, and 21:00 h) using a 10-cm visual analogue scale (VAS), written on paper. The assessment was done by the patient himself and in case of difficulty in conforming to the time of measurement, he was asked to complete the assessment in the range of one hour before and after of the scheduled time (e.g., if it was difficult to perform the assessment at 15:00 h, he was asked to do it between14:00 and 16:00 h).
An active style Pro HJA-750C activity monitor (OMRON, Kyoto, Japan) was used to assess the amount of physical activity during the day, and the patient was instructed to wear the device on his waist from the time of waking up to bedtime. The physical activity meter recorded the events every 10 s, and the composite acceleration was calculated from the vertical, longitudinal, and lateral accelerations by means of its 3-axis accelerometer. In order to separate walking from daily living activities, high-pass filtering at 0.7 Hz was used to calculate the ratio of the synthetic accelerations before and after the filtering, and walking and living activities were distinguished based on the synthetic accelerations calculated at 10-second intervals. The intensity of each activity was estimated in metabolic equivalents (METs)[28,29]. In the present study, we calculated the sedentary activity (SA) of less than 1.5 METs (e.g., watching TV or reading in the supine or seated position) and light-intensity physical activity (LIPA) 1.5-3.0 METs (e.g., standing and standing activities, such as housework and walking) within the wearing time. There were no missing data due to failing to wear the device during the survey period.
Pain characteristics and psychological status: The results of the questionnaire assessment revealed a DN4 score of 6, which exceeded the criterion for determining NP. The SFMPQ2 recorded a total value of 46 (subscale scores: continuous pain, 14/60; intermittent pain, 16/60; NP, 16/60; affective descriptors, 0/40). The NPSI showed moderate NP with a total value of 43 (subscale scores: spontaneous pain 17/30; attacks of pain 10/20; provoked pain 10/30; abnormal sensations 6/20). The HADS score was 9/21 for depression, 6/21 for anxiety, and 12/16 for PCS-4.
Importance of daily life activities: In the assessment of the importance of ADL, walking and day care services for seniors, which are mainly performed in the morning, was very important to the patient, who commented positively, “I feel less pain when I am moving.” In terms of afternoon activities, he commented, "I have no choice but to go because the pain bothers me if I sit still" (Table 1).
| Walking | Day care services for seniors | Laundry related work | Garden Maintenance | Watching TV |
| 8 | 6 | 4 | 4 | 2 |
| Patient statement | Walking: Mainly a morning routine. I feel better in pain when I‘m moving | |||
| Day care services for seniors: Just talking to a lot of people calms me down | ||||
| Watching TV: There's nothing else to do | ||||
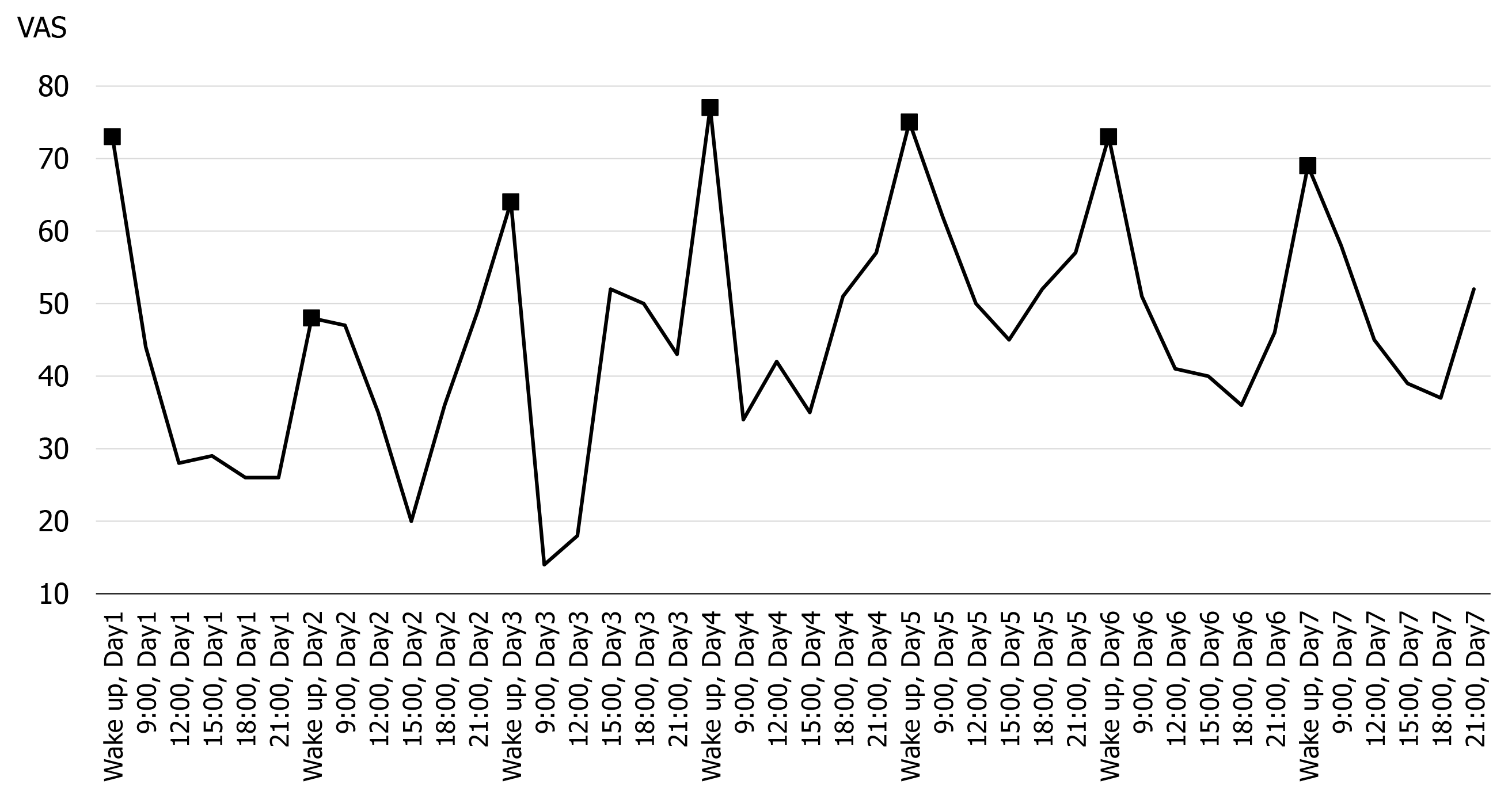
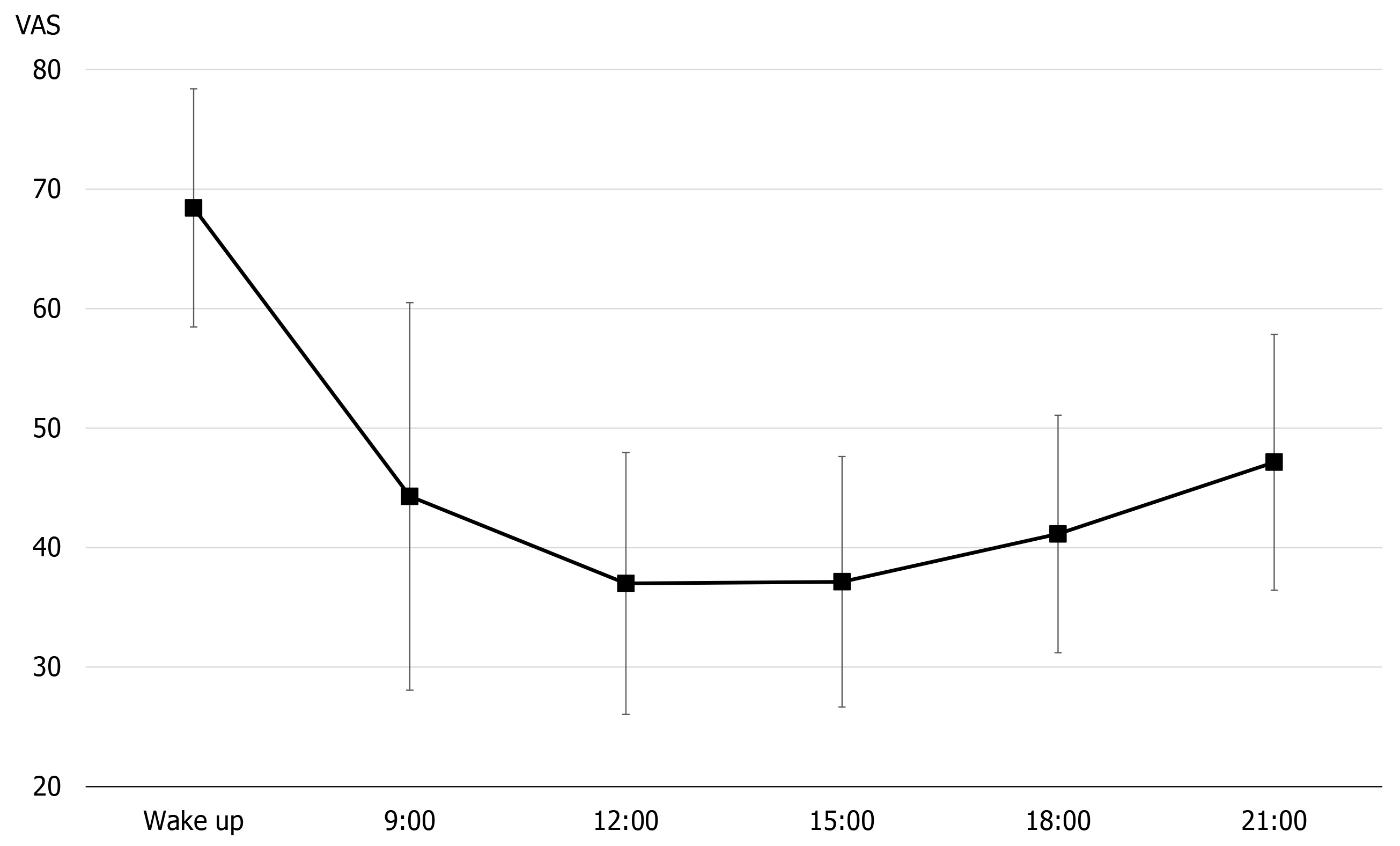
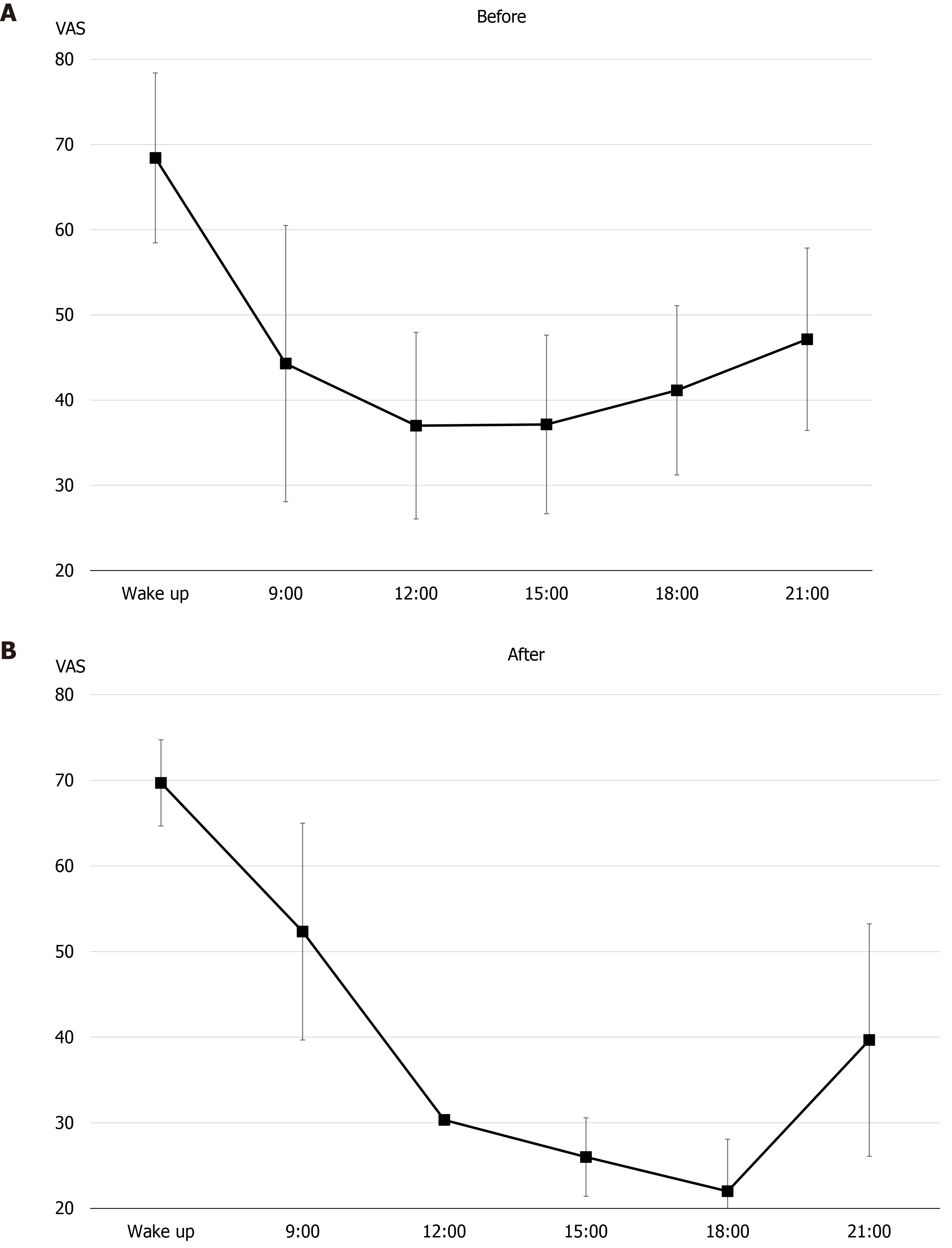
Pain circadian rhythm and physical activity: Figures 1 and 2 show the results of early assessment of the pain circadian rhythm. In terms of all observation points, there was little difference between days, at particular times of the day, and the VAS was consistently the highest on waking (Figure 1). In the 7-d average pain circadian rhythm, the VAS score decreased to a minimum value at 12:00 h, but then tended to worsen again over time (Figure 2).
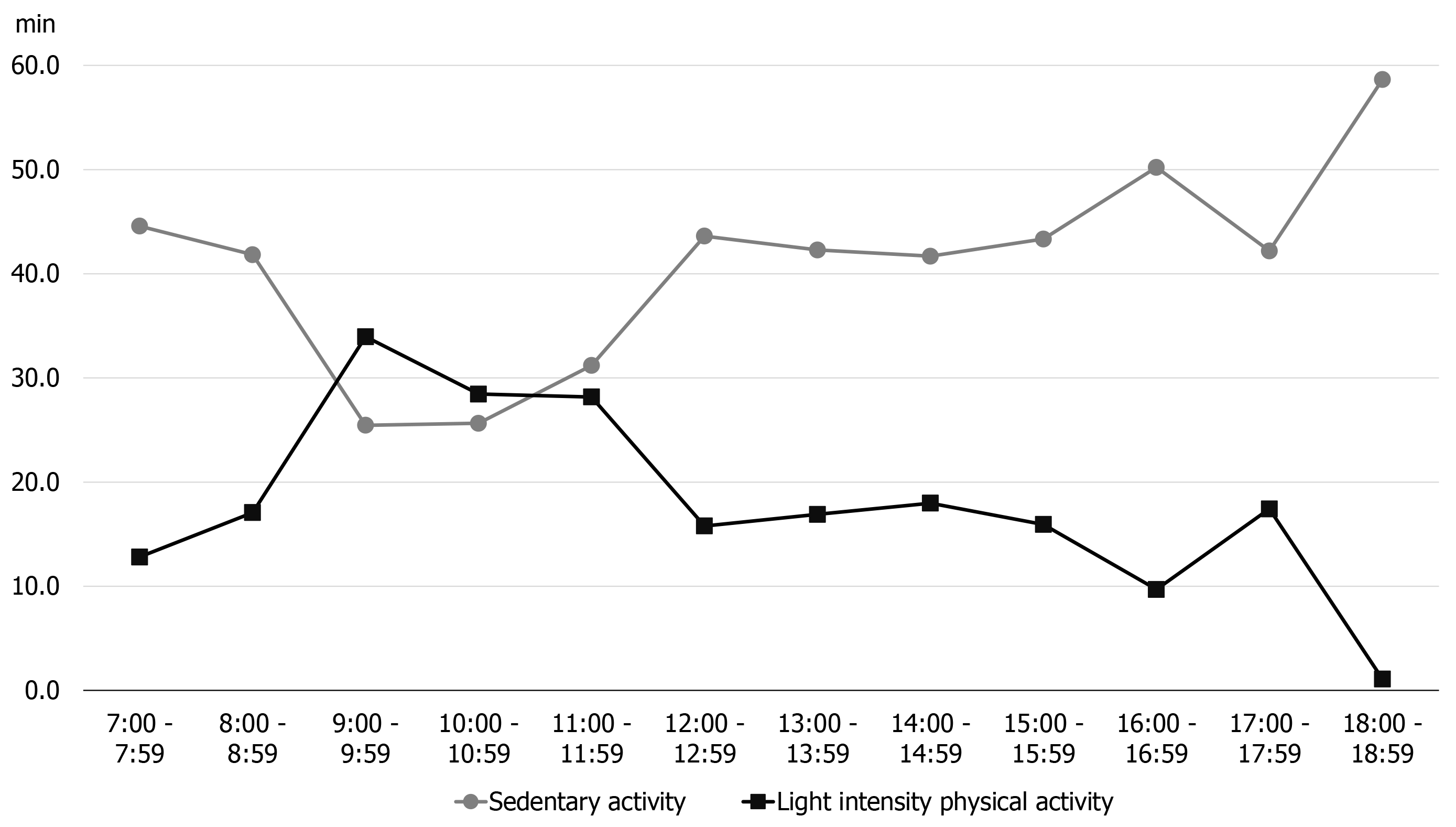
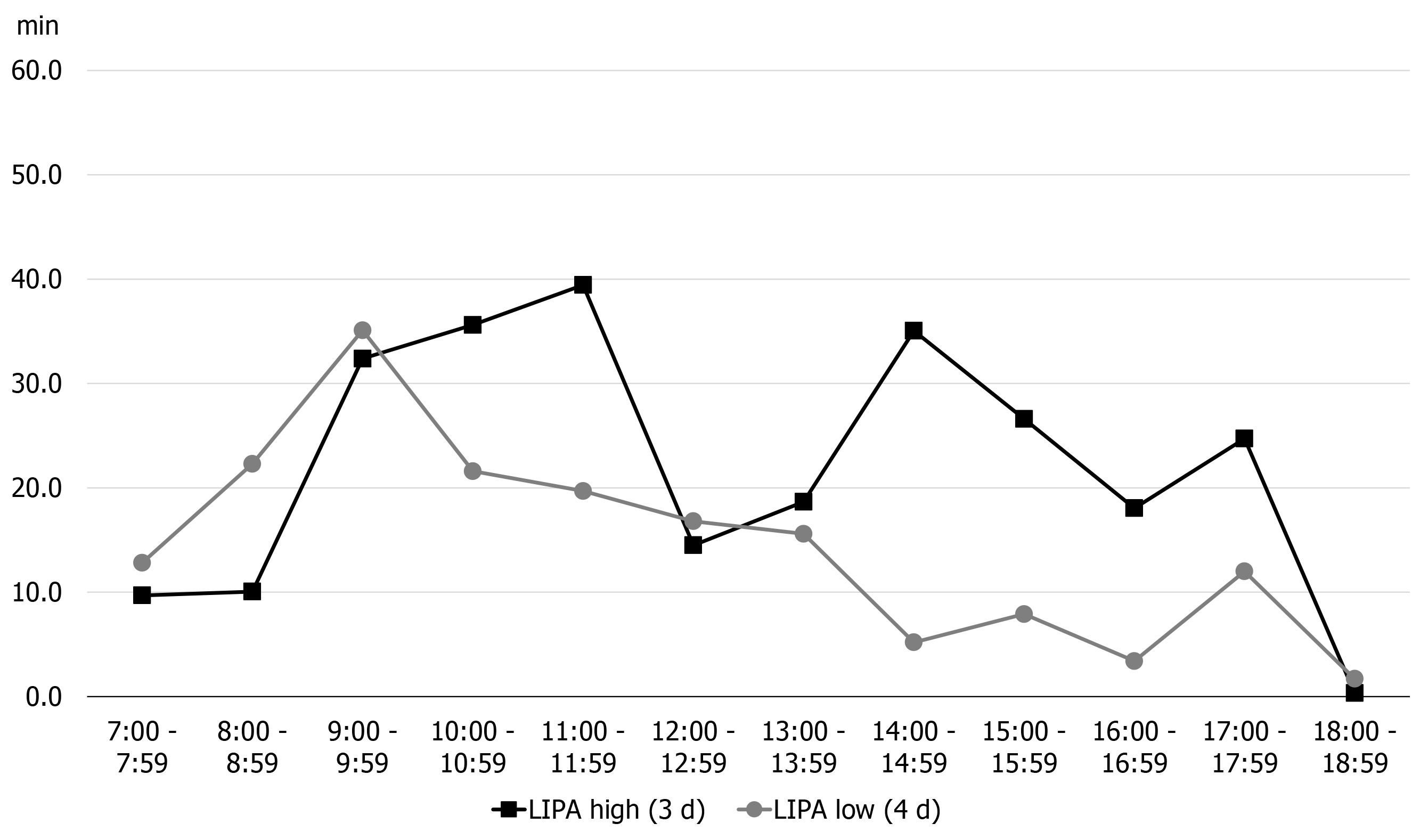
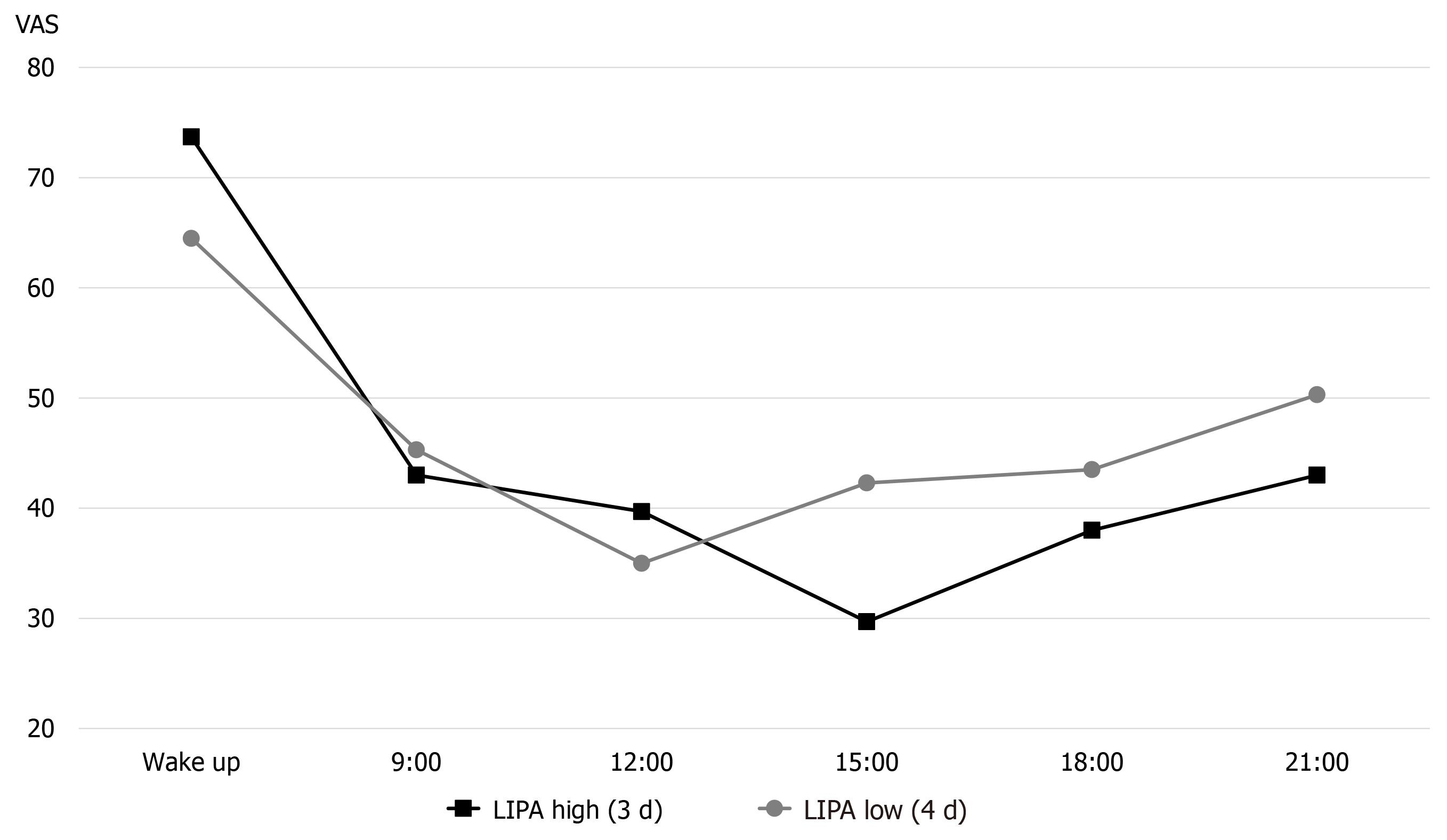
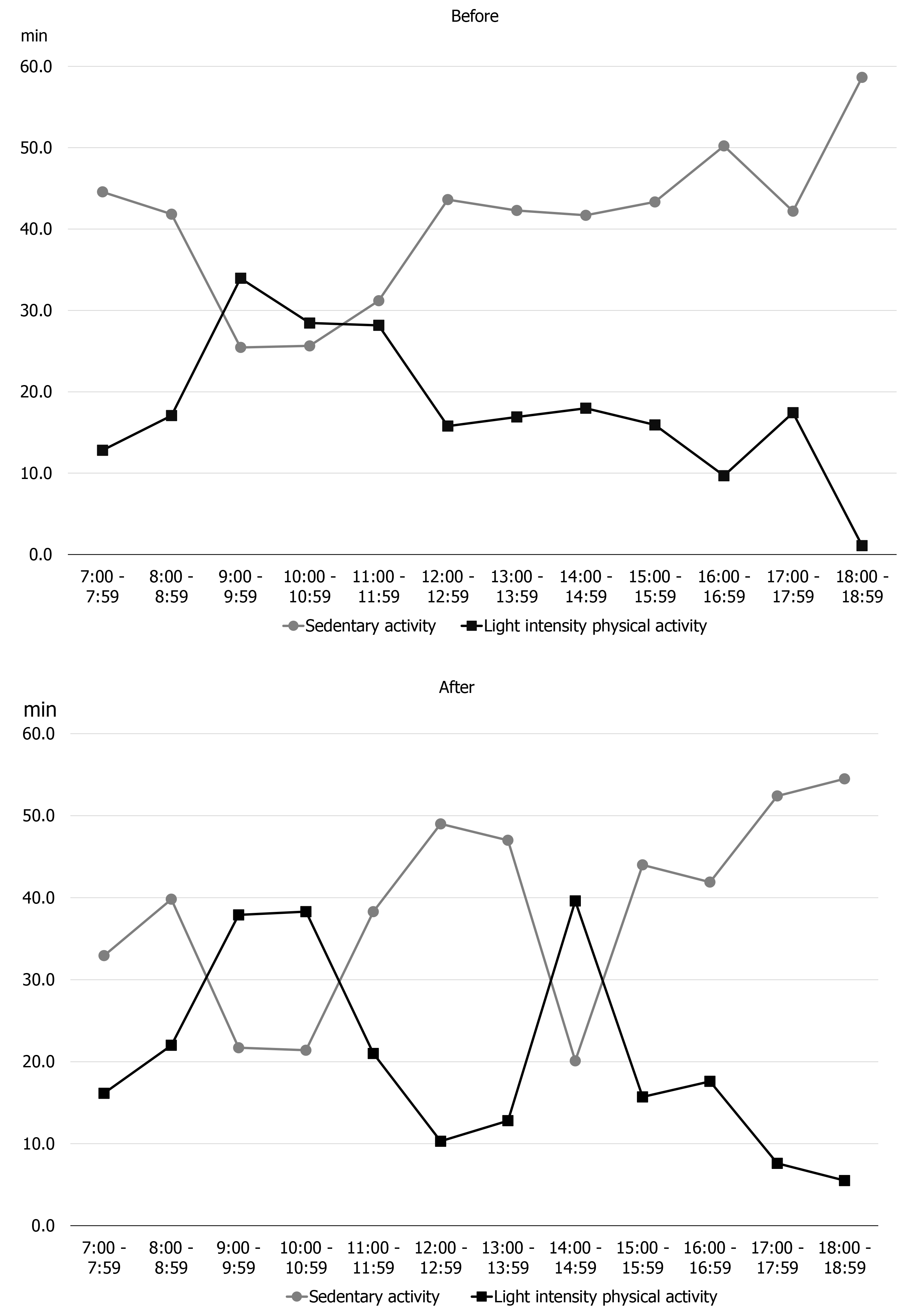
Figure 3 shows the results of early assessment of physical activity. The average physical activity during the 7 d, as described by the patient, involved marked LIPA in the morning, but afternoon onward, the LIPA decreased and SA increased. Furthermore, we classified the days on which the activity time was lower (4 d) and higher (3 d) than the 7-d average of LIPA, and confirmed the LIPA time and the pain circadian rhythm for each time period (Figures 4 and 5). On days with a longer LIPA duration, more time was spent in LIPA in the afternoon than on days with a shorter LIPA duration, and the VAS scores after 12:00 h were lower on days with a longer LIPA duration than on days with a shorter LIPA duration.
Based on the results of the early assessment, it was determined that the patient had NP.
The early assessment indicated that the patient had NP, which was moderate and persistent throughout the day. Additionally, walking and using day care services for seniors was important to the patient, and he was able to perform certain amount of physical activity. The comment, “I feel less pain when I move,” indicated that the patient had a positive attitude toward exercise. Based on the relationship between the pain circadian rhythm and physical activity, it seemed likely that, regardless of the ability to engage in LIPA, it was important in terms of pain management, and that the decreased LIPA time in the afternoon may be a causal factor in pain exacerbation.
Based on the results of the early assessment, we provided education and guidance to the patient on the following three points. First, PNE was provided, including the content of the neurophysiology of NP and the basic management of pain, with reference to the report of Gallagher et al[30]. The explanations were given in plain language so that patients without medical knowledge could understand them. We aimed to provide an accurate understanding of NP and the thought process regarding pain. Second, the results of the early assessment were explained in detail, introducing scientific evidence related to exercise-induced hypoalgesia and pain management. Specifically, a visual representation of the patient’s pain circadian rhythm and physical activity was presented, and an explanation that “walking” and “day services” are likely to be associated with the pain circadian rhythm was provided since they were considered very important to the patient and allowed him to maintain LIPA. Third, based on the previous explanations, we focused on the decrease in physical activity and increase in pain in the afternoon, and encouraged behavioral changes such as “taking walks” not only in the morning, but also in the afternoon, to the maximum extent possible. Thereby, we attempted to confirm a relationship between pain circadian rhythm and physical activity, and to provide a likely strategy for future pain management. Patient education on these three points was conducted after the completion of all early assessments and lasted approximately 1 h.
One month following patient education, a reassessment was conducted. In the reassessment, the pain circadian rhythm and physical activity were assessed for 3 d, considering the patient burden.
The reassessment questionnaire showed a DN4 score of 6 and a total SFMPQ2 score of 44 (subscale scores: continuous pain, 15/60; intermittent pain, 19/60; NP, 10/60; affective descriptors, 0/40), with some improvement in the NP sub-item. The NPSI also showed a slight improvement over the early assessment, with a total value of 38 (subscale scores: spontaneous pain, 15/30; attacks of pain, 11/20; provoked pain, 6/30; abnormal sensations, 6/20). The HADS score was 10/21 for depression and 6/21 for anxiety, and the PCS-4 score was 10/16, which had not changed significantly from the early assessment.
Figures 6 and 7 show reassessments of the pain circadian rhythm and physical activity. LIPA had increased somewhat during 14:00-16:00 h as compared to the early assessment (Figure 6). In terms of pain circadian rhythm, the VAS had decreased after 12:00 h as compared to the early assessment, and the minimum VAS score had moved from 12:00 h to 18:00 h (Figure 7).
In this study, we evaluated the NP of a patient following brachial plexus injury by means of a comprehensive evaluation, including an evaluation of the importance of the ADL, pain rhythmicity, physical activity, and their interrelationships.
During the early assessment, the patient had moderate NP, and from the patient's description, the presence of pain seemed to be a major problem for him in his daily life. The circadian rhythm of the patient’s pain showed the highest intensity pain upon waking on all 7 d, with a pattern of reduced pain during the day and pain exacerbation in the evening. It has been pointed out that the intensity of chronic pain fluctuates greatly from day to day[31], and that it is difficult to predict daily pain fluctuations. However, for this patient, a similar rhythm was observed throughout the 7 d, even at times other than waking up, and the circadian rhythm of pain was somewhat consistent. Furthermore, in relation to physical activity, the intensity of pain was lower on days when LIPA was more frequent than on days with less frequent LIPA. A previous study reported that conditioned pain modulation, a measure of descending pain control function, is more effective in patients with shorter SA and longer LIPA[32,33]. In this patient, it was highly likely that LIPA contributed to the reduction of pain intensity, and it is important to control LIPA based on the understanding of the pain circadian rhythm from the perspective of pain management.
Based on the interpretation of the early assessment, patient education focused on how to spend the afternoon, when the pain intensity tended to worsen, and encouraged behavioral changes in the afternoon by suggesting “walks,” which were very important to the patient and could be used to maintain LIPA. Reassessment showed no significant improvement in SFMPQ2 or NPSI scores, or psychological assessment, but there was an increase in LIPA in the afternoon, which was our focus, and in pain circadian rhythm, with the minimum pain intensity shifting from 12:00 h to 18:00 h. This result, as interpreted in the initial assessment, means that LIPA is likely to have contributed to the reduction in pain intensity in this patient.
Patient education has been shown to be effective to address pain intensity, psychological factors, such as catastrophic thoughts, and limitations in physical activity[13]. However, since most previous studies have provided long-term, continuous patient education, it was difficult to assess the effects on pain intensity and psychological factors with a once-off, short-term intervention such as used in the present patient. On the other hand, by assessing the pain circadian rhythm, even with a short-term intervention, it was easier for the patient to understand the relationship between the circadian rhythm of pain and physical activity, which may have led to an increase in physical activity and changes in the pain circadian rhythm during the target time.
We also assessed the importance of ADL and provided patient education in terms of “walking.” Pain intensity has been reported to vary with attention[34] and satisfaction with social participation[35]. Furthermore, positive emotions, such as a sense of accomplishment and relief from life goals, activate reward regions in the brain and stimulate a descending pain suppression mechanism[36]. In other words, participation in day-care activities for seniors and the practice of walking may itself have contributed to the reduction of pain intensity in this patient’s case. We were able to use “walking” as a concrete means of behavioral change by considering the importance of various activities of daily life, and this may have led to the positive result.
A limitation of this study is that it is based on a single case, and it is difficult to address the causal relationship between the decrease in pain and physical activity; the interpretation that the amount of activity increased simply because there was less pain cannot be ruled out. In addition, patient education should be individualized based on a composite assessment, and the strategy of “increasing physical activity” used in this case may not be applicable to all patients. In order to clarify the relationship between pain circadian rhythm and physical activity, we plan to use the same strategy for multiple cases in future.
In this study, patient education based on a composite assessment elicited positive results in relation to pain circadian rhythm and physical activity. For this patient, activities of great importance, such as “walking” and “participation in day-care services for seniors,” were also associated with the pain circadian rhythm. By considering the circadian rhythm of pain in the complex evaluation of patients with NP, it may be possible to examine the relationship between pain and ADL more specifically and to provide individualized treatment interventions based on each patient’s condition.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ge X, Ma L S-Editor: Gao CC L-Editor: A P-Editor: Li JH
| 1. | Jensen TS, Baron R, Haanpää M, Kalso E, Loeser JD, Rice ASC, Treede RD. A new definition of neuropathic pain. Pain. 2011;152:2204-2205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 841] [Cited by in RCA: 995] [Article Influence: 71.1] [Reference Citation Analysis (1)] |
| 2. | Soler MD, Moriña D, Rodríguez N, Saurí J, Vidal J, Navarro A, Navarro X. Sensory Symptom Profiles of Patients With Neuropathic Pain After Spinal Cord Injury. Clin J Pain. 2017;33:827-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Bouhassira D, Lantéri-Minet M, Attal N, Laurent B, Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain. 2008;136:380-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1083] [Cited by in RCA: 1129] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 4. | Gilron I, Watson CP, Cahill CM, Moulin DE. Neuropathic pain: a practical guide for the clinician. CMAJ. 2006;175:265-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 290] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 5. | Spahr N, Hodkinson D, Jolly K, Williams S, Howard M, Thacker M. Distinguishing between nociceptive and neuropathic components in chronic low back pain using behavioural evaluation and sensory examination. Musculoskelet Sci Pract. 2017;27:40-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Ataoğlu E, Tiftik T, Kara M, Tunç H, Ersöz M, Akkuş S. Effects of chronic pain on quality of life and depression in patients with spinal cord injury. Spinal Cord. 2013;51:23-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 7. | Meier ML, Stämpfli P, Humphreys BK, Vrana A, Seifritz E, Schweinhardt P. The impact of pain-related fear on neural pathways of pain modulation in chronic low back pain. Pain Rep. 2017;2:e601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Edwards RR, Kronfli T, Haythornthwaite JA, Smith MT, McGuire L, Page GG. Association of catastrophizing with interleukin-6 responses to acute pain. Pain. 2008;140:135-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 162] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 9. | Macdonald G, Leary MR. Why does social exclusion hurt? Psychol Bull. 2005;131:202-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 851] [Cited by in RCA: 687] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 10. | Eisenberger NI. The neural bases of social pain: evidence for shared representations with physical pain. Psychosom Med. 2012;74:126-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 215] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 11. | Wickson-Griffiths A, Kaasalainen S, Herr K. Interdisciplinary Approaches to Managing Pain in Older Adults. Clin Geriatr Med. 2016;32:693-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Moseley GL, Nicholas MK, Hodges PW. A randomized controlled trial of intensive neurophysiology education in chronic low back pain. Clin J Pain. 2004;20:324-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 340] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 13. | Louw A, Zimney K, Puentedura EJ, Diener I. The efficacy of pain neuroscience education on musculoskeletal pain: A systematic review of the literature. Physiother Theory Pract. 2016;32:332-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 436] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 14. | Sato G, Osumi M, Morioka S. Effects of wheelchair propulsion on neuropathic pain and resting electroencephalography after spinal cord injury. J Rehabil Med. 2017;49:136-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Tawashy AE, Eng JJ, Lin KH, Tang PF, Hung C. Physical activity is related to lower levels of pain, fatigue and depression in individuals with spinal-cord injury: a correlational study. Spinal Cord. 2009;47:301-306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Louw A, Butler DS, Diener I, Puentedura EJ. Development of a preoperative neuroscience educational program for patients with lumbar radiculopathy. Am J Phys Med Rehabil. 2013;92:446-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Bellamy N, Sothern RB, Campbell J, Buchanan WW. Rhythmic variations in pain, stiffness, and manual dexterity in hand osteoarthritis. Ann Rheum Dis. 2002;61:1075-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 79] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Stone AA, Broderick JE, Porter LS, Kaell AT. The experience of rheumatoid arthritis pain and fatigue: examining momentary reports and correlates over one week. Arthritis Care Res. 1997;10:185-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 170] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 19. | Hegarty RS, Treharne GJ, Stebbings S, Conner TS. Fatigue and mood among people with arthritis: Carry-over across the day. Health Psychol. 2016;35:492-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Allen KD, Coffman CJ, Golightly YM, Stechuchak KM, Keefe FJ. Daily pain variations among patients with hand, hip, and knee osteoarthritis. Osteoarthritis Cartilage. 2009;17:1275-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Bellamy N, Sothern RB, Campbell J. Rhythmic variations in pain perception in osteoarthritis of the knee. J Rheumatol. 1990;17:364-372. [PubMed] |
| 22. | Gilron I, Bailey JM, Vandenkerkhof EG. Chronobiological characteristics of neuropathic pain: clinical predictors of diurnal pain rhythmicity. Clin J Pain. 2013;29:755-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Bouhassira D, Attal N, Alchaar H, Boureau F, Brochet B, Bruxelle J, Cunin G, Fermanian J, Ginies P, Grun-Overdyking A, Jafari-Schluep H, Lantéri-Minet M, Laurent B, Mick G, Serrie A, Valade D, Vicaut E. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain. 2005;114:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1540] [Cited by in RCA: 1717] [Article Influence: 85.9] [Reference Citation Analysis (0)] |
| 24. | Maruo T, Nakae A, Maeda L, Takahashi K. Translation and reliability and validity of a Japanese version of the revised Short-Form McGill Pain Questionnaire (SF-MPQ-2). Pain Res. 2013;28:43-53. |
| 25. | Bouhassira D, Attal N, Fermanian J, Alchaar H, Gautron M, Masquelier E, Rostaing S, Lanteri-Minet M, Collin E, Grisart J, Boureau F. Development and validation of the Neuropathic Pain Symptom Inventory. Pain. 2004;108:248-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 889] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 26. | Hatta A, Higashi A, Yashiro H, Ozasa K, Hayashi K, Kiyota K, Inokuchi H, Ikeda J, Fujita K, Watanabe Y, Kawai K. A validation of the hospital anxiety and depression scale. Jpn J Psychosom Med. 1998;38:309-315. |
| 27. | Bot AGJ, Becker SJE, Bruijnzeel H, Mulders MA, Ring D, Vranceanu AM. Creation of the Abbreviated Measures of the Pain Catastrophizing Scale and the Short Health Anxiety Inventory: The PCS-4 and SHAI-5. J Musculoskel Pain. 2014;22:145-151. [RCA] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Oshima Y, Kawaguchi K, Tanaka S, Ohkawara K, Hikihara Y, Ishikawa-Takata K, Tabata I. Classifying household and locomotive activities using a triaxial accelerometer. Gait Posture. 2010;31:370-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 241] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 29. | Ohkawara K, Oshima Y, Hikihara Y, Ishikawa-Takata K, Tabata I, Tanaka S. Real-time estimation of daily physical activity intensity by a triaxial accelerometer and a gravity-removal classification algorithm. Br J Nutr. 2011;105:1681-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 310] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 30. | Gallagher L, McAuley J, Moseley GL. A randomized-controlled trial of using a book of metaphors to reconceptualize pain and decrease catastrophizing in people with chronic pain. Clin J Pain. 2013;29:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 31. | Bartley EJ, Robinson ME, Staud R. Pain and Fatigue Variability Patterns Distinguish Subgroups of Fibromyalgia Patients. J Pain. 2018;19:372-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 32. | Ellingson LD, Shields MR, Stegner AJ, Cook DB. Physical activity, sustained sedentary behavior, and pain modulation in women with fibromyalgia. J Pain. 2012;13:195-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 33. | Naugle KM, Ohlman T, Naugle KE, Riley ZA, Keith NR. Physical activity behavior predicts endogenous pain modulation in older adults. Pain. 2017;158:383-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 34. | Kong J, Gollub RL, Rosman IS, Webb JM, Vangel MG, Kirsch I, Kaptchuk TJ. Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. J Neurosci. 2006;26:381-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 282] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 35. | Farin E. The reciprocal effect of pain catastrophizing and satisfaction with participation in the multidisciplinary treatment of patients with chronic back pain. Health Qual Life Outcomes. 2015;13:163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Vachon-Presseau E, Martel MO, Roy M, Caron E, Albouy G, Marin MF, Plante I, Sullivan MJ, Lupien SJ, Rainville P. Acute stress contributes to individual differences in pain and pain-related brain activity in healthy and chronic pain patients. J Neurosci. 2013;33:6826-6833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |