Published online Jun 16, 2021. doi: 10.12998/wjcc.v9.i17.4415
Peer-review started: February 4, 2021
First decision: February 24, 2021
Revised: February 27, 2021
Accepted: March 25, 2021
Article in press: March 25, 2021
Published online: June 16, 2021
Processing time: 111 Days and 8.4 Hours
Immune checkpoint inhibitors (ICIs) can lead to immune-related hepatitis (IRH) and severe liver damage, which is life-threatening in the absence of specific treatment.
A 75-year-old man was admitted to our hospital complaining of loss of appetite, yellow urine, and abnormal liver function for the past 2 wk. Three months prior to admission, he was treated with two rounds of capecitabine in combination with camrelizumab for lymph node metastasis of esophageal cancer. Although liver function was normal before treatment, abnormal liver function appeared at week 5. Capecitabine and camrelizumab were discontinued. Ursodeoxycholic acid and methylprednisolone 40 mg daily were administered. Liver function continued to deteriorate. Prothrombin time and international normalized ratio were 19 s and 1.8, respectively. The patient was diagnosed with acute liver failure. A pathological analysis of liver biopsy indicated a strongly positive immunohistochemical staining of T8+ cells, thereby suggesting that drug-induced liver injury was related to IRH caused by camrelizumab. Subsequently, we performed sequential dual-molecule plasma adsorption system (DPMAS) treatment with plasma exchange (PE). After two rounds of treatment, the patient's appetite significantly improved, the yellow color of urine reduced, and liver function improved (total bilirubin level decreased) after five rounds of treatment. Liver function normalized 4 wk after discharge.
The use of sequential DPMAS with PE can reduce liver injury and systemic toxic reactions by clearing inflammatory mediators and harmful substances from blood, and regulate immune cell activity, which may be effective in the treatment of severe ICI-induced IRH.
Core Tip: Immune checkpoint inhibitors can lead to immune-associated hepatitis and severe liver damage that can be life-threatening without specific treatment. Corticosteroids and immunosuppressants do not show sufficient sensitivity and their use often leads to serious complications such as severe secondary infections. Here we report a case of severe liver damage caused by death protein 1 inhibitors and the first use of dual-molecule plasma adsorption combined with plasma exchange to achieve satisfactory results. In addition, the clinical biochemical indicators in this case were sufficient for diagnosing acute liver failure, while pathology provided an accurate diagnosis. Pathological diagnosis is a very important diagnostic tool for severe liver injury.
- Citation: Tan YW, Chen L, Zhou XB. Efficacy of artificial liver support system in severe immune-associated hepatitis caused by camrelizumab: A case report and review of the literature. World J Clin Cases 2021; 9(17): 4415-4422
- URL: https://www.wjgnet.com/2307-8960/full/v9/i17/4415.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i17.4415
Immune checkpoint inhibitors (ICIs) target programmed cell death protein 1 (PD-1)/programmed cell death protein ligand 1 (PD-L1); cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) monoclonal antibodies can enhance the anti-tumor immune response in the human body by blocking the inhibitory immunoregulation of the above-mentioned immune checkpoint pathway[1]. Since tumor and normal cells have similar antigens, an activated immune system can simultaneously kill the tumor and attack normal human tissues, causing immune-related adverse reactions in various systems of the body[2,3]. Immune-related adverse events (irAEs) can affect all organs[4]. ICI-related hepatitis (IRH) is a common irAE[5]. When the degree of liver injury is grade 1 or 2, most of them can relieve themselves[6]. Since corticosteroids and immunosuppressant treatment are difficult to achieve good curative effect in grades 3 and 4 liver injury, deaths are common[7]. We report a case wherein the first use of dual-molecule plasma adsorption system (DPMAS) combined with plasma exchange (PE) achieved satisfactory results in a patient with severe liver damage caused by PD-1 inhibitors.
A 75-year-old man with esophageal cancer was admitted to the hospital 1 year after surgery.
The patient presented with a 2 wk history of loss of appetite, yellow urine, and abnormal liver function. Three months prior, due to lymph node metastasis of esophageal cancer, the patient was placed on capecitabine (1.5 g) twice daily for 2 consecutive weeks, followed by a 1 wk interval, combined with 200 mg of camrelizumab (PD-1 inhibitor) twice every 2 wk.
There was no family history of viral hepatitis; no history of alcohol abuse, blood transfusion, use of blood products, or schistosomiasis; and no recent history of an unclean diet.
There was no history of contact with the novel coronavirus. The patient’s family members were healthy. He had no history of tuberculosis or other infectious diseases, and there was no family history of genetic disease.
Physical examination revealed the following: Blood pressure, 122/82 mmHg; heart rate, 67 beats/min; respiratory rate, 18 breaths/min; and body temperature, 36.5 °C. His skin was dark and dull, and yellowing of the skin and sclera was observed.
Other findings included: There was no palmar erythema, no spider nevi, and no obvious abnormal findings during cardiopulmonary auscultation; the abdomen was soft, non-distended, and non-tender; and there was no rebound tenderness. Mobility dullness was negative, and no edema was noted on either leg.
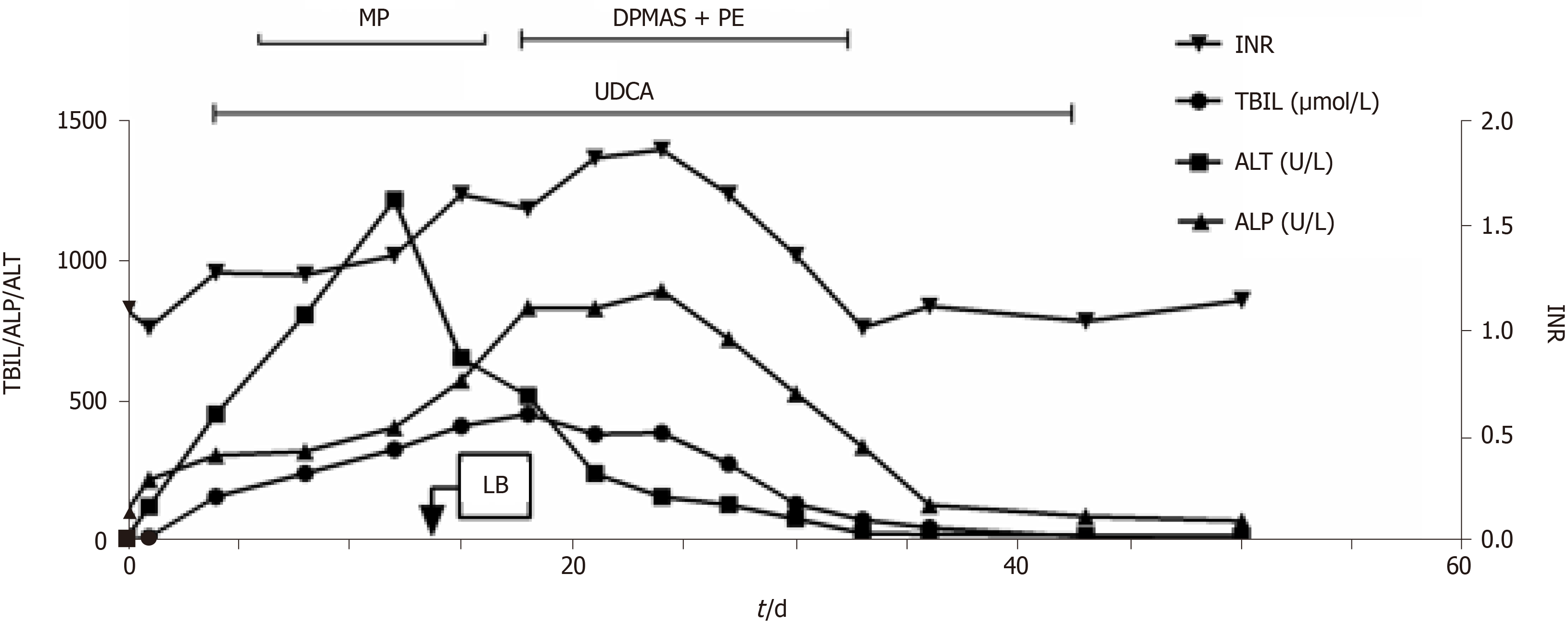
Liver function, monitored weekly, was normal in the first 4 wk and abnormal in the fifth week. The capecitabine and camrelizumab were discontinued; however, the patient’s liver function continued to deteriorate (Figure 1). The patient had no history of hepatitis A-E or alcoholism; analyses for Epstein-Barr, cytomegalovirus, and antinuclear and mitochondrial antibodies were negative.
Abdominal ultrasound and magnetic resonance cholangiopancreatography (MRCP) were performed to exclude biliary obstruction.
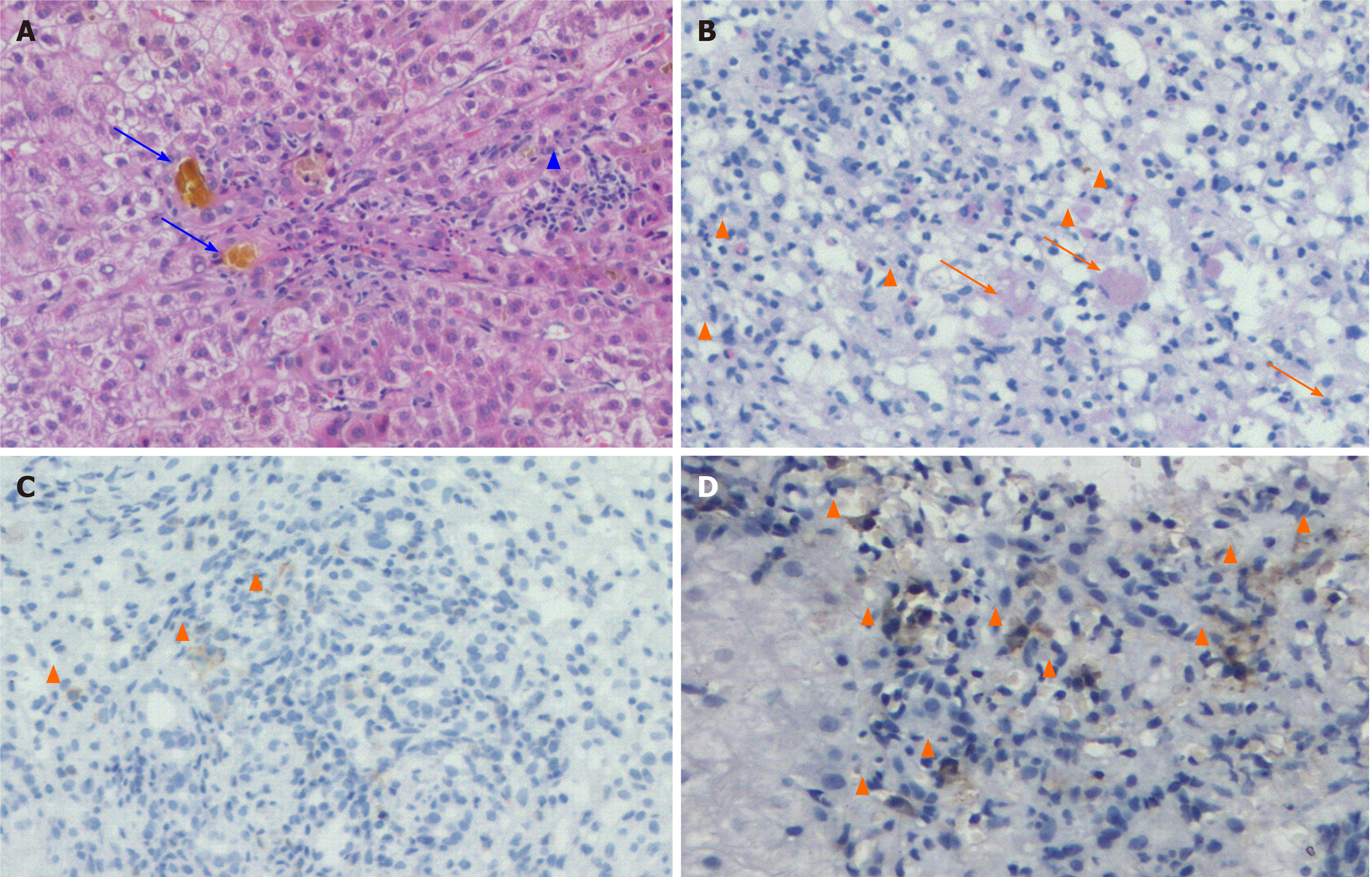
The patient was clinically diagnosed with acute liver failure. Pathological analysis of the liver biopsy showed extensive acinar inflammation and necrosis, cholestasis, bile duct injury, infiltration of a large number of eosinophils, and strong immunohistochemical staining of T8+ cells. Therefore, the patient was diagnosed with drug-induced liver injury (Figure 2) linked to IRH caused by camrelizumab.
The patient was administered ursodeoxycholic acid (UDCA) and methylprednisolone (MP) 40 mg daily. His alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels decreased; however, his serum total bilirubin (TBIL), alkaline phosph
After two rounds of DPMAS + PE, the patient's appetite significantly improved, the yellow color of urine was alleviated, and the UDCA treatment was continued. After five rounds of treatment. Liver function was normal at 4 wk after discharge. No abnormal liver function was found, and the capecitabine treatment was continued.
The incidences of IRH caused by CTLA-4 and PD-1/PD-L1 inhibitors are less than 10% and approximately 5%, respectively[8,9]. Its incidence with combined CTLA-4 inhibitor use is as high as 33%, and grade 3-4 liver injury accounted for approximately 14%[10,11]. IRH often develops more than 1-3 mo after administration, but it can appear at any time. The incidence of IRH was higher in hepatocellular carcinoma patients treated with two kinds of ICIs and CTLA-4 inhibitor combined with chemotherapy/targeted therapy than in those treated with PD-1 inhibitor alone[12].
The main clinical manifestations of liver injury associated with ICI include asymptomatic ALT and/or AST elevations, with or without TBIL elevation; moreover, some patients present with symptoms such as fever, fatigue, jaundice, and fullness. Most patients with mild hepatitis present normal imaging findings; furthermore, patients with moderate to severe hepatitis have non-specific manifestations such as hepatomegaly, periportal edema, and periportal lymphadenopathy. Common pathological features include lobular hepatitis, central inflammation, and necrosis; portal lesions are often mild[13,14]. CTLA-4 inhibitors cause similar CD4+ and CD8+ T cell infiltrations in liver tissue[15], and PD-1/PD-L1 inhibitors mainly lead to CD8+ T cell infiltration[16,17]. In this case report, we also showed strong CD8+ T cell positivity in liver tissue. Bile duct injury is also a common pathological manifestation, especially in patients with cholestasis and high jaundice[18]. We recently found the case of a patient with bile duct deficiency (unpublished). Some scholars have reported the existence of specific granulomas[18], such as fibrotic granulomas, but only in case reports[19,20].
In order to diagnose ICI-related hepatitis, it is necessary to exclude active viral hepatitis, alcoholic hepatitis, autoimmune hepatitis, thromboembolic events, metastatic disease progression, and other liver diseases, and to investigate the effect of the combination of drugs (such as acetaminophen, antibiotics, and chemotherapy drugs). In this case, we used capecitabine and camrelizumab simultaneously, and therefore it was difficult to determine whether IRH was caused by capecitabine. After liver function is repaired, there is no abnormal liver function after using capecitabine again. The Roussel Uclaf causality assessment method was used to score[21]. The total score of 1 (time to onset + 2; course, 0; risk factors, + 1; concomitant drug, - 1; search for non-drug cases, + 1; previous information on drug hepatotoxicity, 0; response to drug readministration, - 2) also excluded the correlation between liver injury occurr
The National Cancer Institute grades the severity of hepatotoxicity according to the common terminology criteria for adverse events version 4.03[22] (Table 1). Grade 1 [AST/ALT > 3 ×, ALP/GGT > 2.5 ×, total bilirubin (TB) > 1.5 × upper limit of normal (ULN), and TBIL < 1.5 × ULN] patients usually can continue to use ICIs. Grade 2 (AST/ALT > 3-5 ×, ALP/GGT > 2.5-5 ×, and TB > 1.5-3 × ULN) requires ICI discontinuation and oral prednisone intake 0.5-1 mg/kg/d, which is gradually reduced in one month; Grade 3 (AST/ALT/ALP/GGT > 5-20 × and TB > 3-10 × ULN) and Grade 4 (AST/ALT/ALP/GGT > 20 × and TB > 3-10 × ULN) require ICI discontinuation; if there is no improvement after 3 d, methylprednisone 0.5-1 g bid or antithymocyte globulin should be added[15]. Infliximab is not recommended because of its potential risk of liver failure[23,24].
| Grade | 1 mild | 2 moderate | 3 severe | 4 life-threatening |
| AST (× ULN) | > 1-3 × | > 3-5 × | > 5-20 × | > 20 × |
| ALT (× ULN) | > 1-3 × | > 3-5 × | > 5-20 × | > 20 × |
| ALP (× ULN) | > 1-2.5 × | > 2.5-5 × | > 5-20 × | > 20 × |
| GGT (× ULN) | > 1-2.5 × | > 2.5-5 × | > 5-20 × | > 20 × |
| Total bilirubin (× ULN) | > 1-1.5 × | > 1.5-3 × | > 3-10 × | > 10 × |
ICI-associated cholestatic hepatitis (mainly bilirubin, with ALP and gamma GGT levels elevation) has also been reported, and may be corticosteroid-resistant, with a poor prognosis[25]. However, abdominal ultrasound or MRCP is needed to exclude biliary obstruction factors. In a study of 387 patients from Japan who underwent ICI treatment, 56 developed corticotropin releasing hormone, 11 (19.6%) of the hepatocellular type and 34 (60.7%) of the cholestasis type, with cholestasis predomin
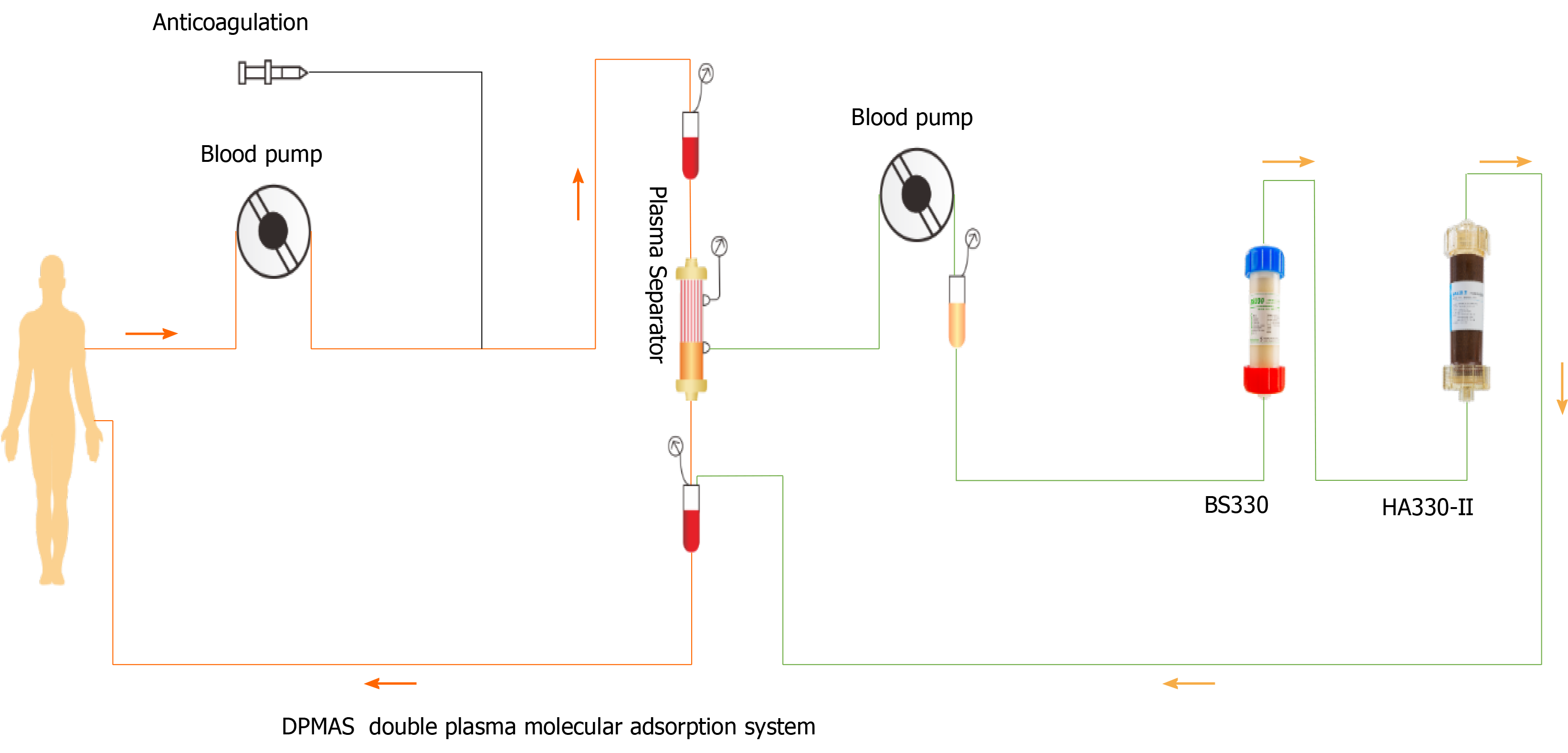
Blood adsorption technology is a type of blood purification method that uses different adsorbents to remove endogenous or exogenous poisons in blood[27]. In order to reduce adsorbent-induced blood cell damage, plasma separation technology can be combined with it to adsorb the separated plasma, namely, plasma adsorption. DPMAS can improve hyperbilirubinemia, clear inflammatory factors, reduce inflammatory response syndrome, block the occurrence and development of liver failure, and significantly improve the prognosis of liver failure[28,29]. DPMAS can process more than 6000 mL of plasma at one time. We previously used DPMAS and sequential PE for the treatment of thyroid crisis and severe liver injury[30]. Moreover, we used DPMAS to treat a patient with severe ICI-related liver injury. The patient's liver function improved for a while. However, due to long-term corticosteroid and multimode fiber (MMF) treatment, severe secondary infection and septic shock occurred, and the patient finally died of multiple organ failure. Nevertheless, it can be seen that this combination can conveniently repair liver injury with severe cholestasis.
A previous study reported that a 76-year-old patient with malignant melanoma developed liver function deterioration (abnormal TBIL and ALP levels) after using ipilimumab (anti-CTLA-4). Corticosteroids (2 mg/kg/d) and MMF (1.5 g/d) did not prevent the progression of liver failure. Finally, the patient was treated with PE for five times, and the corticosteroids and MMF were simultaneously reduced. DPMAS combined with PE has a curative effect on ICI-related liver injury because it can remove inflammatory factors, bilirubin, creatinine, and other small molecular substances from blood[31]. Other possible mechanisms include changes in lymphocyte proliferation and function, which may render lymphocytes sensitive to immunosuppressants and chemotherapy drugs, as well as changes in the immune system, including changes in the number and activation of B and T cells, the enhancement of T-suppressive function, and changes in the ratio of helper T cell type 1/2 (Th1/Th2)[32]. The changes in the number and function of these immunocompetent cells reduce the severity of ICI-related liver injury.
Recently, six cases were reported concerning patients with high-grade ICI-related hepatitis with spontaneous liver function improvement without corticosteroid therapy[15]. This showed the importance of individualized treatment according to biological and histological severity, with the possibility of avoiding unnecessary systemic cortisol treatment. In general, severe liver injuries caused by ICIs are rare. Most liver injuries of grades 3-4 or higher are sensitive to corticosteroid therapy, while mild liver injuries can be relieved without corticosteroid therapy[33].
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Manrai M S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Li JH
| 1. | Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 626] [Cited by in RCA: 767] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 2. | Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2870] [Cited by in RCA: 3583] [Article Influence: 358.3] [Reference Citation Analysis (0)] |
| 3. | Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, Rathmell WK, Ancell KK, Balko JM, Bowman C, Davis EJ, Chism DD, Horn L, Long GV, Carlino MS, Lebrun-Vignes B, Eroglu Z, Hassel JC, Menzies AM, Sosman JA, Sullivan RJ, Moslehi JJ, Johnson DB. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018;4:1721-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1850] [Article Influence: 264.3] [Reference Citation Analysis (0)] |
| 4. | Hahn AW, Gill DM, Pal SK, Agarwal N. The future of immune checkpoint cancer therapy after PD-1 and CTLA-4. Immunotherapy. 2017;9:681-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 5. | Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med. 2018;378:158-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2308] [Cited by in RCA: 3160] [Article Influence: 451.4] [Reference Citation Analysis (0)] |
| 6. | Huffman BM, Kottschade LA, Kamath PS, Markovic SN. Hepatotoxicity After Immune Checkpoint Inhibitor Therapy in Melanoma: Natural Progression and Management. Am J Clin Oncol. 2018;41:760-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 7. | Bhave P, Buckle A, Sandhu S, Sood S. Mortality due to immunotherapy related hepatitis. J Hepatol. 2018;69:976-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, Postow MA, Wolchok JD. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2016;27:1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 135] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 9. | Larkin J, Hodi FS, Wolchok JD. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:1270-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 570] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 10. | Valsecchi ME. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 570] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 11. | Parlati L, Vallet-Pichard A, Batista R, Hernvann A, Sogni P, Pol S, Mallet V; CERTIM group. Incidence of grade 3-4 Liver injury under immune checkpoints inhibitors: A retrospective study. J Hepatol. 2018;69:1396-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, Postow MA, Wolchok JD. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26:2375-2391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 978] [Cited by in RCA: 1070] [Article Influence: 107.0] [Reference Citation Analysis (0)] |
| 13. | Kimura H, Takeda A, Kikukawa T, Hasegawa I, Mino T, Uchida-Kobayashi S, Ohsawa M, Itoh Y. Liver injury after methylprednisolone pulse therapy in multiple sclerosis is usually due to idiosyncratic drug-induced toxicity rather than autoimmune hepatitis. Mult Scler Relat Disord. 2020;42:102065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Kleiner DE, Berman D. Pathologic changes in ipilimumab-related hepatitis in patients with metastatic melanoma. Dig Dis Sci. 2012;57:2233-2240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 15. | De Martin E, Michot JM, Papouin B, Champiat S, Mateus C, Lambotte O, Roche B, Antonini TM, Coilly A, Laghouati S, Robert C, Marabelle A, Guettier C, Samuel D. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J Hepatol. 2018;68:1181-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 380] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 16. | Johncilla M, Misdraji J, Pratt DS, Agoston AT, Lauwers GY, Srivastava A, Doyle LA. Ipilimumab-associated Hepatitis: Clinicopathologic Characterization in a Series of 11 Cases. Am J Surg Pathol. 2015;39:1075-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 17. | Zen Y, Yeh MM. Hepatotoxicity of immune checkpoint inhibitors: a histology study of seven cases in comparison with autoimmune hepatitis and idiosyncratic drug-induced liver injury. Mod Pathol. 2018;31:965-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 208] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 18. | Wu Z, Lai L, Li M, Zhang L, Zhang W. Acute liver failure caused by pembrolizumab in a patient with pulmonary metastatic liver cancer: A case report. Medicine (Baltimore). 2017;96:e9431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Simonelli M, Di Tommaso L, Baretti M, Santoro A. Pathological characterization of nivolumab-related liver injury in a patient with glioblastoma. Immunotherapy. 2016;8:1363-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Everett J, Srivastava A, Misdraji J. Fibrin Ring Granulomas in Checkpoint Inhibitor-induced Hepatitis. Am J Surg Pathol. 2017;41:134-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 21. | Becker MW, Lunardelli MJM, Tovo CV, Blatt CR. Drug and herb-induced liver injury: A critical review of Brazilian cases with proposals for the improvement of causality assessment using RUCAM. Ann Hepatol. 2019;18:742-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Peeraphatdit TB, Wang J, Odenwald MA, Hu S, Hart J, Charlton MR. Hepatotoxicity From Immune Checkpoint Inhibitors: A Systematic Review and Management Recommendation. Hepatology. 2020;72:315-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 172] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 23. | Simonaggio A, Michot JM, Voisin AL, Le Pavec J, Collins M, Lallart A, Cengizalp G, Vozy A, Laparra A, Varga A, Hollebecque A, Champiat S, Marabelle A, Massard C, Lambotte O. Evaluation of Readministration of Immune Checkpoint Inhibitors After Immune-Related Adverse Events in Patients With Cancer. JAMA Oncol. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 276] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 24. | Kok B, Lester ELW, Lee WM, Hanje AJ, Stravitz RT, Girgis S, Patel V, Peck JR, Esber C, Karvellas CJ; United States Acute Liver Failure Study Group. Acute Liver Failure from Tumor Necrosis Factor-α Antagonists: Report of Four Cases and Literature Review. Dig Dis Sci. 2018;63:1654-1666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Doherty GJ, Duckworth AM, Davies SE, Mells GF, Brais R, Harden SV, Parkinson CA, Corrie PG. Severe steroid-resistant anti-PD1 T-cell checkpoint inhibitor-induced hepatotoxicity driven by biliary injury. ESMO Open. 2017;2:e000268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 26. | Imoto K, Kohjima M, Hioki T, Kurashige T, Kurokawa M, Tashiro S, Suzuki H, Kuwano A, Tanaka M, Okada S, Kato M, Ogawa Y. Clinical Features of Liver Injury Induced by Immune Checkpoint Inhibitors in Japanese Patients. Can J Gastroenterol Hepatol. 2019;2019:6391712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 27. | Wu M, Zhang H, Huang Y, Wu W, Huang J, Yan D. Efficiency of Double Plasma Molecular Absorption System on the Acute Severe Cholestatic Hepatitis. Blood Purif. 2021;1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Chen G, Wu M, Wu B, Liu F, Liu J, Liu L. Effects of dual plasma molecular adsorption system on liver function, electrolytes, inflammation, and immunity in patients with chronic severe hepatitis. J Clin Lab Anal. 2019;33:e22926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Yan GS, Li LL, Jiang SL, Meng S, Wu CC. [Clinical study of different adsorbents with dual plasma molecular adsorption system in the treatment of hepatic failure]. Zhonghua Gan Zang Bing Za Zhi. 2019;27:51-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 30. | Tan YW, Sun L, Zhang K, Zhu L. Therapeutic plasma exchange and a double plasma molecular absorption system in the treatment of thyroid storm with severe liver injury: A case report. World J Clin Cases. 2019;7:1184-1190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Bernuau J. High volume plasma exchange in patients with acute liver failure. J Hepatol. 2016;65:646-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Reeves HM, Winters JL. The mechanisms of action of plasma exchange. Br J Haematol. 2014;164:342-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 189] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 33. | Jennings JJ, Mandaliya R, Nakshabandi A, Lewis JH. Hepatotoxicity induced by immune checkpoint inhibitors: a comprehensive review including current and alternative management strategies. Expert Opin Drug Metab Toxicol. 2019;15:231-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |