Published online Jun 16, 2021. doi: 10.12998/wjcc.v9.i17.4400
Peer-review started: February 1, 2021
First decision: February 25, 2021
Revised: March 12, 2021
Accepted: April 8, 2021
Article in press: April 8, 2021
Published online: June 16, 2021
Processing time: 113 Days and 18.5 Hours
Pulmonary alveolar proteinosis (PAP) is a rare lung disease characterized by the accumulation of phospholipoproteinaceous material in the alveoli. Cases of PAP complicated with tuberculosis are much more complex and have rarely been well recorded.
We describe a 21-year-old Han Chinese patient with suspicious lung infection associated with mild restrictive ventilatory dysfunction and diffusion reduction. High resolution computed tomography revealed a “crazy-paving” appearance and multiple pulmonary miliary nodules around the bronchi. Bronchoalveolar lavage demonstrated a small amount of periodic acid-Schiff positive proteinaceous materials. A serological test for the presence of a Mycobacterium tuberculosis antibody and an interferon-gamma release assay were both positive. The patient received a standard course of first-line anti-tuberculosis treatment after diagnostic bronchoalveolar lavage. To date, clinical remission has been achieved and maintained for five years.
In summary, the diagnosis of PAP complicated with tuberculosis was supported by a combination of clinical manifestations, imaging, pulmonary function, laboratory examinations, bronchoalveolar lavage, etc. This case highlighted that diagnostic bronchoalveolar lavage in combination with anti-tuberculosis treatment is a safe and effective option for mild PAP patients with tuberculosis.
Core Tip: Pulmonary alveolar proteinosis (PAP) complicated with tuberculosis is a rare clinical situation. This case highlighted that diagnostic bronchoalveolar lavage in combination with anti-tuberculosis treatment is a safe and effective option for mild PAP patients with tuberculosis. Bronchoalveolar lavage did not induce the dissemination of tuberculosis because anti-tuberculosis drugs were used immediately.
- Citation: Bai H, Meng ZR, Ying BW, Chen XR. Pulmonary alveolar proteinosis complicated with tuberculosis: A case report. World J Clin Cases 2021; 9(17): 4400-4407
- URL: https://www.wjgnet.com/2307-8960/full/v9/i17/4400.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i17.4400
Pulmonary alveolar proteinosis (PAP) is an unusual diffuse lung disease characterized by the accumulation of large amounts of periodic acid-Schiff (PAS) positive phospholipoproteinaceous material in the alveoli, and the prevalence of PAP is approximately seven cases per million population[1]. The major symptoms of PAP are progressive dyspnea, cough, fatigue, weight loss, and low-grade fever, all of which may also occur in tuberculosis. However, when PAP is complicated with tuberculosis, it is much more complex and has rarely been well recorded. Here, we present a successfully diagnosed and treated case of PAP with tuberculosis and review the main characteristics of other previously reported cases of PAP complicated with tuberculosis to obtain a better understanding of this situation.
Nonproductive cough for 2 mo.
The patient was a 21-year-old Han Chinese man. He had no history of smoking, but he suffered from a nonproductive cough for 2 mo, accompanied by a low-grade fever (ranging from 37.4-37.8 ℃) and night sweats; however, he denied hemoptysis, chills, weight loss, dyspnea, and other symptoms. Then he developed paroxysmal chest stabbing pain for 1 wk.
The patient had no known history of a past illness.
The patient had no known personal and family history.
Physical examination showed normal auscultation of both lungs, oxygen saturation of 98%, blood pressure of 126/75 mmHg, a temperature of 36.5 ℃, respiratory rate of 20 times per minute, and pulse of 88 beats per minute. He had no clubbing, a pale complexion, or cyanosis.
Laboratory inspection revealed a white blood cell (WBC) count of 10.01 × 109/L with 79.10% neutrophils, and serological detection for Mycobacterium tuberculosis (M. tuberculosis) antibody was positive. The erythrocyte sedimentation rate (ESR), procalcitonin (PCT), and Fungitec G test results were normal.
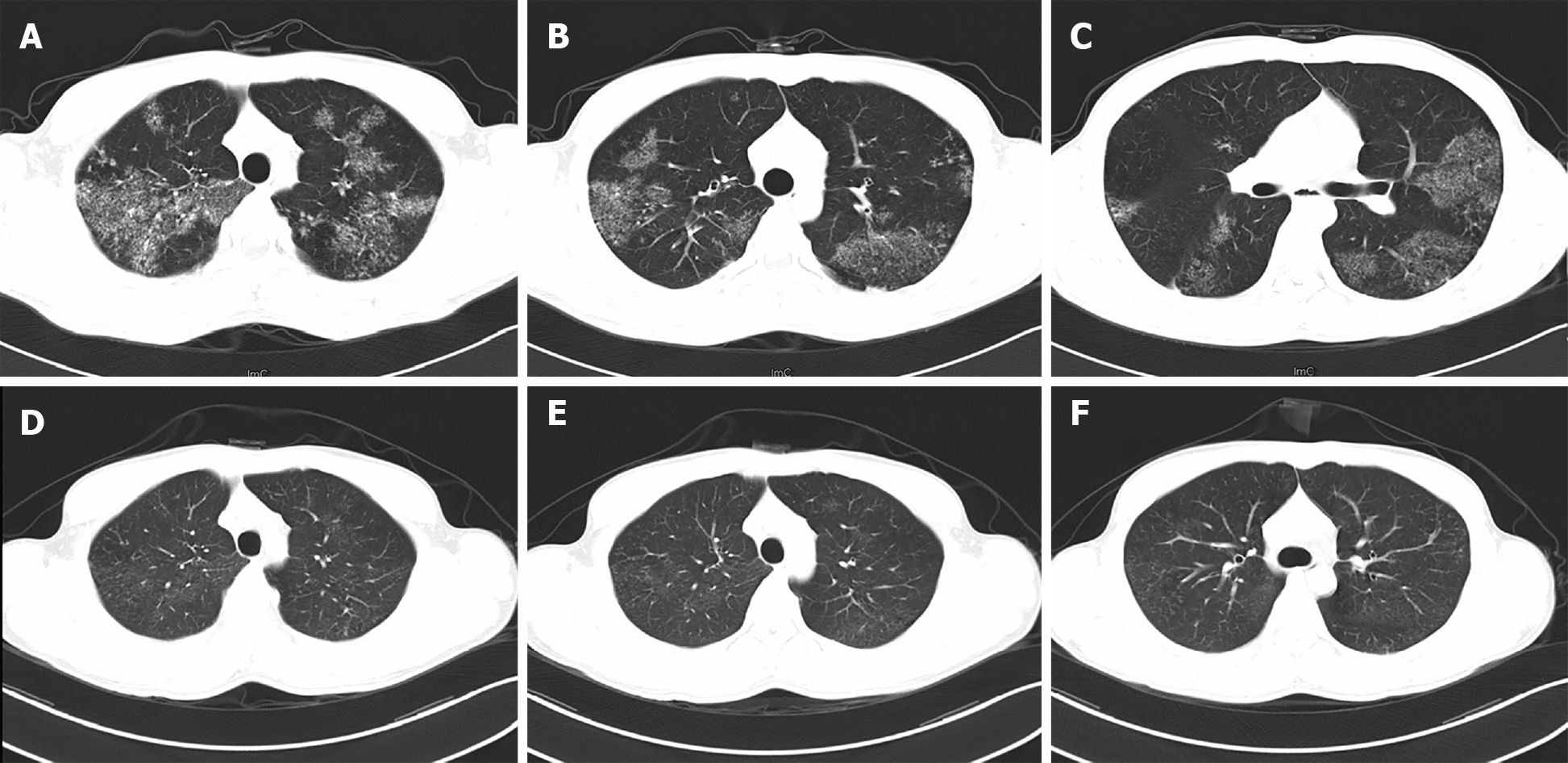
Computerized tomography of the chest demonstrated increased lung texture and multiple patchy enhanced densities of both lungs, especially in the upper lobes, with no obvious adhesion to the pleurae. There were no abnormities in the bronchi under the broncho fiberscope.
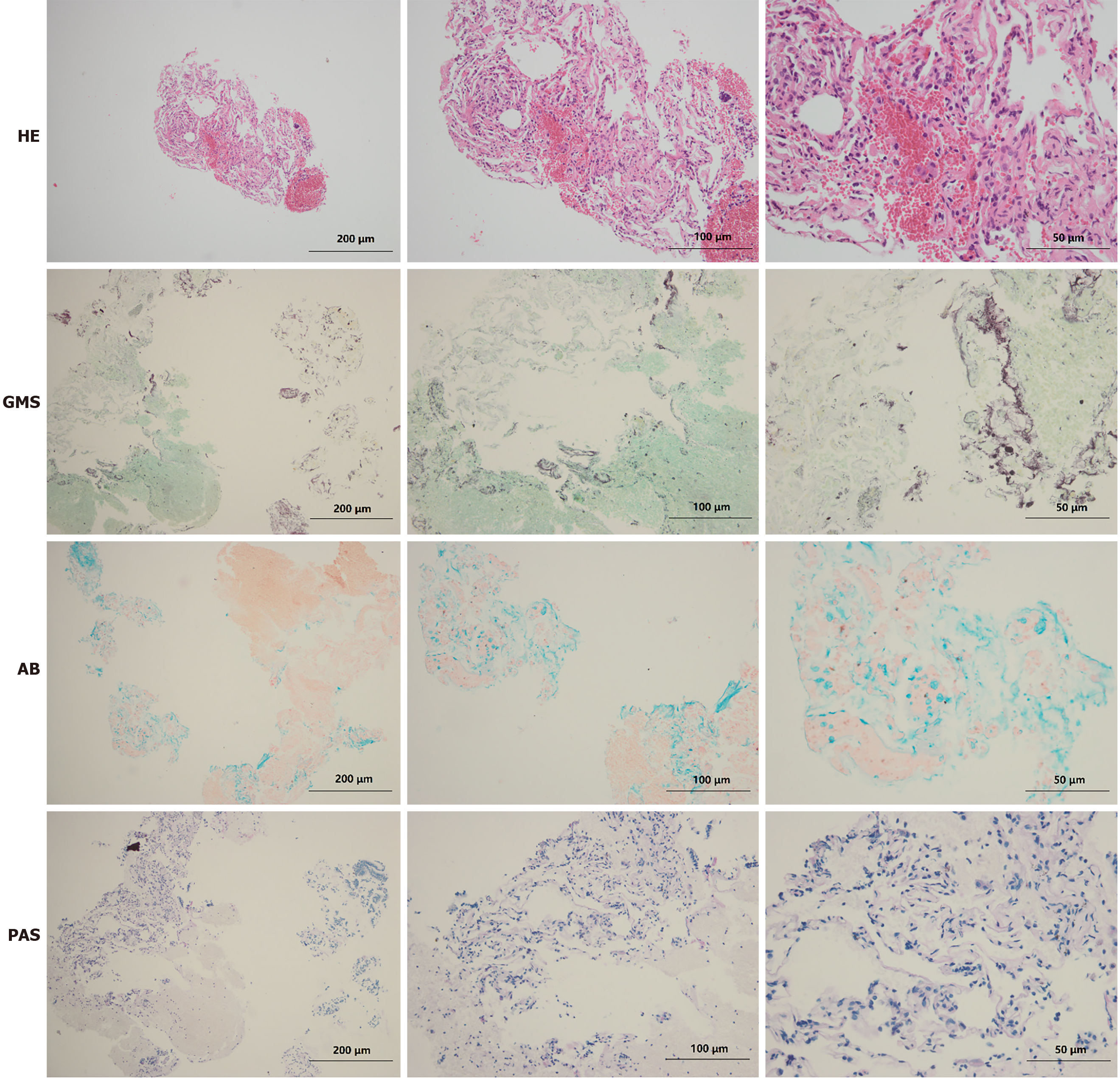
The patient was admitted to West China Hospital. During hospitalization, comprehensive examinations were performed. Laboratory analyses revealed WBC 5.74 × 109/L, C-reactive protein 15.60 mg/L, interleukin-6 19.26 pg/mL, PCT 0.05 ng/mL, and ESR 32.0 mm/h, and his interferon-gamma release assay was positive. Arterial blood gases showed SpO2 93.4%, PaO2 64.8 mmHg, PaCO2 42 mmHg, and pH 7.40 (the detailed laboratory results are presented in Table 1). Meanwhile, the pulmonary function test revealed mild restrictive ventilation dysfunction and diffuse dysfunction. High-resolution computerized tomography (HRCT) revealed multiple pulmonary miliary nodules distributed around the bronchi, which were flake-like, patchy aggregates, mainly located in the upper lobes (Figure 1). Furthermore, fiberoptic bronchoscopy with transbronchial lung biopsy and diagnostic bronchoalveolar lavage was performed. Grey-white lung tissue was obtained from the apical segment of the right upper lung, and the pathological examination of the lung tissue showed mild inflammation. The acid-fast staining and Gomori's methenamine silver staining of the lung tissue were negative, but it was positive for Alcian blue (AB) staining (Figure 2). More importantly, the bronchoalveolar lavage fluid from the upper lungs appeared light and milky, and additional positive AB and PAS staining (eosinophilic granular) of proteins was found in the bronchoalveolar lavage fluid.
| Laboratory examination | Result | Reference range |
| White blood cells (× 109/L) | 5.74 | 3.5-9.5 |
| Red blood cells (× 1012/L) | 5.28 | 4.3-5.8 |
| Platelets (× 109/L) | 227 | 100-300 |
| Prothrombin time (s) | 13.7 ↑ | 9.6-12.8 |
| International normalized ratio | 1.17 ↑ | 0.88-1.15 |
| Erythrocyte sedimentation rate (mm/h) | 32.0 ↑ | < 21 |
| C-reactive protein (mg/L) | 15.60 ↑ | < 5 |
| Interleukin-6 (pg/mL) | 19.26 ↑ | 0.00-7.00 |
| Procalcitonin (ng/mL) | 0.05 ↑ | < 0.046 |
| Arterial blood gases | ||
| SpO2 (%) | 93.4 ↓ | 95-98 |
| PaO2 (mmHg) | 64.8 ↓ | 80-100 |
| PaCO2 (mmHg) | 42 | 35-45 |
| pH | 7.40 | 7.35-7.45 |
| Interferon-gamma release assay | Positive | Negative |
| Mycobacterium tuberculosis antibody | Positive | Negative |
The final diagnosis of the patient was PAP complicated with tuberculosis. The diagnosis of PAP was mainly based on eosinophilic PAS-positive proteins found in his bronchoalveolar lavage fluid. The diagnosis of tuberculosis was mainly based on positive results of M. tuberculosis antibody and interferon-gamma release assays and was finally confirmed by anti-tuberculosis treatment.
Initially, the patient was diagnosed with a pulmonary infection and was treated with azithromycin (0.5 g, intravenous, once a day) and doxycycline (0.2 g, oral, once a day), after which his cough was reduced, but the shadows of the lungs remained the same. Then, we revised the diagnosis to PAP complicated with tuberculosis. The patient received standard anti-tuberculosis treatment for 6 mo (isoniazid, rifampicin, pyrazinamide, and ethambutol treatment for 2 mo, followed by isoniazid and rifampicin treatment for 4 mo).
As expected, all symptoms disappeared and the lesions of both lungs were significantly absorbed with a normal density demonstrated on HRCT (Figure 1) after 6 mo of anti-tuberculosis treatment. The patient has been followed up for 5 years, during which a clinical remission has been achieved and maintained. And the patient needs to visit the doctor if any symptom occurs again.
According to the pathogenesis and clinical features, PAP is divided into three categories: congenital, secondary, and idiopathic. Congenital PAP mainly occurs in infants and is caused by mutations in surfactant proteins (SP) and granulocyte-macrophage colony-stimulating factor (GM-CSF) receptor genes. Secondary PAP is associated with various underlying diseases, such as hematological malignancies, infections, and other diseases that cause severely low immune function or is associated with the inhalation of inorganic minerals or chemicals[2]. Approximately 90% of PAP cases are idiopathic, also known as autoimmune PAP, which is associated with the presence of anti-GM-CSF autoantibodies[3,4]. Of note, recent discoveries have suggested that there is an overlap between secondary PAP and idiopathic PAP, and impairments in the GM-CSF production pathway result in the majority of PAP cases[5,6].
With an insidious onset, the course of PAP lasts months to years. The clinical manifestations of PAP vary and are nonspecific, among which progressive dyspnea and nonproductive cough are the most common symptoms. Approximately one-third of patients are asymptomatic at presentation, while the majority present acutely with rapid progression to respiratory failure[7]. Physical examination often shows normal vital signs, although clubbing, cyanosis, and inspiratory crackles have been reported in individual cases. Typical lung dysfunction is restrictive ventilation dysfunction and diffuse dysfunction, and the imaging change is a “crazy-paving” pattern[8]. Although the “crazy-paving” pattern has been well-accepted to be associated with PAP, it is neither specific nor sensitive to diagnose PAP because several other diseases also have the same signs[9].
Notably, there are clear associations between PAP and tuberculosis. GM-CSF is critical for regulating alveolar macrophage function and maintaining homeostasis, by binding with macrophage surface receptors and then mediating signal transduction, macrophage terminal differentiation, intracellular lipid metabolism, surfactant catabolism, and pathogenic receptor expression[10]. Recent discoveries have suggested that individuals with deficient GM-CSF subsequently experience alveolar macrophage dysfunction and reduced elimination of both endogenous SP and exogenous M. tuberculosis; thus, these patients are susceptible to PAP and tuberculosis[11]. In PAP cases, gene mutation or GM-CSF antibodies lead to macrophage dysfunction and an imbalance in surfactants. Increases in SP-A and SP-D, on the one hand, promote macrophages to engulf M. tuberculosis and, on the other hand, they compromise the function of macrophages[12]. In contrast, tuberculosis may also be the primary event, stimulating type II pneumocytes to secrete excess surfactant, thus triggering the development of PAP[13]. In the present case, PAP and tuberculosis were found at the same time, so it is difficult to tell whether tuberculosis was involved as a superinfection of PAP or whether the PAP was secondary to the tuberculosis.
Tuberculosis may increase the risk of developing PAP, and PAP patients may also develop secondary tuberculosis. There are common symptoms of PAP and tuberculosis, which will lead to a missed diagnosis or misdiagnosis. However, cases of PAP complicated with tuberculosis have rarely been well recorded (Table 2)[13-23]. Empirically, we need to be vigilant about the following signals in PAP patients, which indicate the presence of tuberculosis infection. First, the patient suffers from symptoms such as hemoptysis, fever, or night sweats, which are common in tuberculosis but infrequent in PAP. Second, chest radiography shows nodules, fibrous stripes, or consolidation, which are hard to explain in PAP. Third, the pulmonary function test reveals obstructive ventilation dysfunction. Last, the PPD skin test is strongly positive, or the pulmonary lesions are absorbed after anti-tuberculosis treatment.
| Ref. | Country | Sex | Age | Main manifestations | Sequential order | Diagnosis method | Main treatment | Prognosis | Other diseases |
| Ramirez[14], 1967 | United States | Male | 48 | Pulmonary infiltrates | TB before PAP | Open lung biopsy | Bronchopulmonary lavage | Remission | None |
| Lathan et al[15], 1971 | United States | Female | 41 | Chills, fever, productive cough, diffuse pulmonary infiltrates, and progressive dyspnea | PAP before TB | Open lung biopsy | Single lung lavage | Remission | None |
| Reyes and Putong[13], 1980 | United States | Female | 32 | Fever, cough, and pulmonary cavities | TB before PAP | Open lung biopsy | Bronchopulmonary lavage | Unknown | Total gastrectomy and alcoholic hepatitis |
| Rekha et al[16], 1996 | India | Male | 26 | Dyspnea and dry cough | PAP before TB | Open lung biopsy | Anti-tuberculosis therapy | Remission | None |
| Pereira-Silva et al[17], 2002 | Brazil | Female | 35 | Dry cough, chest pain, mild dyspnea, and fever | At the same time | Open lung biopsy | Bronchoalveolar lavage | Unknown | Diabetes mellitus |
| Mayoralas Alises et al[18], 2003 | Spain | Female | 41 | Night sweats, dyspnea, productive cough, and weight loss | TB before PAP | Bronchoscopy | Bronchoalveolar lavage | Remission | None |
| Yin et al[19], 2008 | China | Female | 17 | Cough, expectoration, dyspnea on exertion, fever, and night sweats | At the same time | Transbronchial biopsy | Anti-tuberculous therapy and lung lavage | Improve | None |
| Tekgül et al[20], 2012 | Turkey | Male | 46 | Dyspnea, cough, and fever | PAP before TB | Transbronchial biopsy | Anti-tuberculosis therapy | Improve | None |
| Huang et al[21], 2012 | China | Male | 35 | Dyspnea and productive cough | TB before PAP | Bronchoalveolar lavage | Whole lung lavage | Improve | Partial gastrectomy and aspergilloma |
| Cheraghvandi et al[22], 2014 | Iran | Male | 29 | Progressive dyspnea, weakness, cough, fever, and chills | TB before PAP | Transbronchial biopsy | Anti-tuberculosis therapy | Died | Acute silicosis |
| Nimmatoori et al[23], 2020 | United States | Male | 32 | Progressive dry cough and breathlessness | TB before PAP | Bronchoalveolar lavage | Whole lung lavage | Remission | None |
The standard therapy for idiopathic PAP is therapeutic alveolar lavage, covering bronchoalveolar lavage and whole lung lavage. The basic theory underlying alveolar lavage is the reactivation of alveolar macrophages through mechanical clearance of deposited lipids, proteins, and materials. In addition, systemic replacement therapy based on exogenous GM-CSF has been investigated and even used as an alternative to alveolar lavage[24]. Depleting B lymphocytes by rituximab and plasmapheresis are two new approaches to reduce the number of GM-CSF autoantibodies, which offers another promising way to cure those who are unsuccessfully treated by alveolar lavage and exogenous GM-CSF[25].
However, the treatment is more complicated when PAP is accompanied by tuberculosis. A combination of alveolar lavage and anti-tuberculosis chemotherapy has been proven to be the most effective way to treat tuberculosis-associated PAP, but the implementation of alveolar lavage can disseminate the tuberculosis. To date, most reports have suggested that alveolar lavage should be performed early after anti-tuberculosis drugs are used, but the best lavage treatment opportunity remains to be explored. In this study, the correct diagnosis was not made until after diagnostic bronchoalveolar lavage was performed, and then anti-tuberculosis drugs were used immediately. We presented a successfully treated PAP case complicated with tuberculosis, thus providing a feasible treatment option for such a situation.
In summary, the diagnosis of PAP complicated with tuberculosis was supported by the combination of clinical manifestations, imaging, pulmonary function, laboratory examinations, bronchoalveolar lavage, etc. Diagnostic bronchoalveolar lavage in combination with anti-tuberculosis treatment is a safe and effective option for mild PAP patients with tuberculosis.
Manuscript source: Unsolicited manuscript
Specialty type: Respiratory system
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Singhania N, Strainiene S S-Editor: Gong ZM L-Editor: Wang TQ P-Editor: Li X
| 1. | Riley L, Wang T, Ataya A. Pulmonary Alveolar Proteinosis (PAP). Am J Respir Crit Care Med. 2019;200:P16-P17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Carey B, Trapnell BC. The molecular basis of pulmonary alveolar proteinosis. Clin Immunol. 2010;135:223-235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 150] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 3. | Sakagami T, Uchida K, Suzuki T, Carey BC, Wood RE, Wert SE, Whitsett JA, Trapnell BC, Luisetti M. Human GM-CSF autoantibodies and reproduction of pulmonary alveolar proteinosis. N Engl J Med. 2009;361:2679-2681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 4. | Sakagami T, Beck D, Uchida K, Suzuki T, Carey BC, Nakata K, Keller G, Wood RE, Wert SE, Ikegami M, Whitsett JA, Luisetti M, Davies S, Krischer JP, Brody A, Ryckman F, Trapnell BC. Patient-derived granulocyte/macrophage colony-stimulating factor autoantibodies reproduce pulmonary alveolar proteinosis in nonhuman primates. Am J Respir Crit Care Med. 2010;182:49-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Costabel U, Nakata K. Pulmonary alveolar proteinosis associated with dust inhalation: not secondary but autoimmune? Am J Respir Crit Care Med. 2010;181:427-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Cummings KJ, Donat WE, Ettensohn DB, Roggli VL, Ingram P, Kreiss K. Pulmonary alveolar proteinosis in workers at an indium processing facility. Am J Respir Crit Care Med. 2010;181:458-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 7. | Jouneau S, Ménard C, Lederlin M. Pulmonary alveolar proteinosis. Respirology. 2020;25:816-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 8. | Allwood BW, Bennji S. Crazy Paving in Pulmonary Alveolar Proteinosis. N Engl J Med. 2020;382:275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Rossi SE, Erasmus JJ, Volpacchio M, Franquet T, Castiglioni T, McAdams HP. "Crazy-paving" pattern at thin-section CT of the lungs: radiologic-pathologic overview. Radiographics. 2003;23:1509-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 190] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | Doerschuk CM. Pulmonary alveolar proteinosis--is host defense awry? N Engl J Med. 2007;356:547-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Rothchild AC, Stowell B, Goyal G, Nunes-Alves C, Yang Q, Papavinasasundaram K, Sassetti CM, Dranoff G, Chen X, Lee J, Behar SM. Role of Granulocyte-Macrophage Colony-Stimulating Factor Production by T Cells during Mycobacterium tuberculosis Infection. mBio. 2017;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 12. | Gaynor CD, McCormack FX, Voelker DR, McGowan SE, Schlesinger LS. Pulmonary surfactant protein A mediates enhanced phagocytosis of Mycobacterium tuberculosis by a direct interaction with human macrophages. J Immunol. 1995;155:5343-5351. [PubMed] |
| 13. | Reyes JM, Putong PB. Association of pulmonary alveolar lipoproteinosis with mycobacterial infection. Am J Clin Pathol. 1980;74:478-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Ramirez J. Pulmonary alveolar proteinosis. Treatment in a case complicated by tuberculosis. Am Rev Respir Dis. 1967;95:491-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Lathan SR Jr, Williams JD Jr, McLean RL, Ramirez J. Pulmonary alveolar proteinosis. Treatment of a case complicated by tuberculosis. Chest. 1971;59:452-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (1)] |
| 16. | Rekha C, Pralhad P, Pradeep V, Mahashur AA. Pulmonary alveolar proteinosis with pulmonary tuberculosis. Ind J Tub. 1996;43:27-29. [RCA] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Pereira-Silva JL, Marinho MM, Veloso TV, Coelho JC JC. Pulmonary alveolar proteinosis and tuberculosis in a diabetic patient: a rare or a seldom diagnosed association? Braz J Infect Dis. 2002;6:188-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Mayoralas Alises S, Gómez Carrera L, Díaz Lobato S. [Alveolar proteinosis or the importance of identifying concurrent infections]. Arch Bronconeumol. 2003;39:327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Yin WH, Chen XR, Zhu H, Su XY, Zhao LJ. [Pulmonary alveolar proteinosis with pulmonary tuberculosis: a case report and literature review]. Zhongguo Fanglao Zazhi. 2008;30:439-442. |
| 20. | Tekgül S, Bilaceroglu S, Ozkaya S, Coskun A, Komurcuoglu B, Cirak AK. Pulmonary alveolar proteinosis and superinfection with pulmonary tuberculosis in a case. Respir Med Case Rep. 2012;5:25-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Huang XY, Yu C, Xu XM, Yang WT, Zhang X, Liu PP, Wang LX. Pulmonary alveolar proteinosis associated with tuberculosis and aspergilloma formation. Chin Med J (Engl). 2012;125:3191-3192. [PubMed] |
| 22. | Cheraghvandi A, Fallah Tafti S, Talischi F, Seyedmehdi SM, Ghazanchaei E, Jebelli B, Pourabdollah M. Silicoproteino-tuberculosis: Three distinct entities or a unique entity: A case report and review of the literature. Med J Islam Repub Iran. 2014;28:23. [PubMed] |
| 23. | Nimmatoori DP, Bansal S, Singhania N, Singh AK, Sudigali VM. Milky fluid from the lungs: pulmonary alveolar proteinosis. Intern Emerg Med. 2021;16:781-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Antoniu SA, Rajnoveanu R, Grigore M, Antohe I. Pharmacotherapy options in pulmonary alveolar proteinosis. Expert Opin Pharmacother. 2020;21:1359-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Kumar A, Abdelmalak B, Inoue Y, Culver DA. Pulmonary alveolar proteinosis in adults: pathophysiology and clinical approach. Lancet Respir Med. 2018;6:554-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 110] [Article Influence: 15.7] [Reference Citation Analysis (0)] |