Published online Jun 16, 2021. doi: 10.12998/wjcc.v9.i17.4342
Peer-review started: February 12, 2021
First decision: March 14, 2021
Revised: March 22, 2021
Accepted: March 25, 2021
Article in press: March 25, 2021
Published online: June 16, 2021
Processing time: 102 Days and 19.1 Hours
Inflammatory bowel disease (IBD) is rare in patients with glycogen storage disease (GSD). In GSD patients, a decrease in the number of neutrophils leads to prolonged intestinal infection, leading to the formation of chronic inflammation and eventually the development of IBD. Minimally invasive surgery for patients with IBD has been proven to reduce inflammatory responses and postoperative risks and ultimately promote rapid recovery. Herein we discuss minimally invasive surgery and the perioperative management in a patient with GSD and IBD.
A 23-year-old male had GSD Ib associated with IBD-like disease for 10 years. Despite standard treatments, such as mesalazine, prednisone and adalimumab, the patient eventually developed colonic stenosis with incomplete ileus. After adequate assessment, the patient was treated with minimally invasive surgery and discharged in stable condition.
Minimally invasive surgery for patients with IBD and GSD is safe, feasible and effective.
Core Tip: With progression of inflammatory bowel disease (IBD), 70% of the patients undergo at least one surgical treatment in their lifetime, which can negatively impact physical and mental health. Glycogen storage disease is a rare genetic disease. Neutropenia in such patients leads to prolonged intestinal infections and chronic inflammation, eventually progressing to IBD. Minimally invasive surgery for IBD has the advantages of fewer injuries, less pain, more rapid recovery of gastrointestinal function and a shorter postoperative hospital stay. Specifically, minimally invasive surgery has obvious advantages for patients with glycogen storage disease and IBD due to impaired autoimmune function.
- Citation: Wan J, Zhang ZC, Yang MQ, Sun XM, Yin L, Chen CQ. Minimally invasive surgery for glycogen storage disease combined with inflammatory bowel disease: A case report. World J Clin Cases 2021; 9(17): 4342-4347
- URL: https://www.wjgnet.com/2307-8960/full/v9/i17/4342.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i17.4342
Glycogen storage disease (GSD) is an autosomal recessive disease caused by glucose-6-phosphate transporter deficiency. Neutropenia and impaired glucose homeostasis in GSD often cause cardiovascular, digestive, respiratory and immune system-related complications[1]. It is currently believed that the mechanism underlying GSD Ib combined with inflammatory bowel disease (IBD) is due to a decrease in neutrophils, which leads to prolonged intestinal infection, chronic inflammation and eventually the development of IBD[2,3]. For patients with intestinal strictures, obstruction, fistulas, perforation, bleeding, cancerization and drug treatment failure, surgical treatment must be performed to alleviate the symptoms. Minimally invasive surgery for IBD is safe, feasible, effective and promotes rapid recovery[4]. We discuss a patient with GSD and IBD who was treated with minimally invasive surgery.
The patient, a 23-year-old male, was hospitalized for abdominal pain and diarrhea of 10 years duration.
The patient developed diarrhea 10 years ago with a frequency of 7-8 times/d that was accompanied by abdominal pain. The patient was treated conservatively in the Department of Gastroenterology for a long time. In September 2020, a colonoscopy examination revealed ulcerative colonic lesions with strictures, and the patient was treated with prednisolone, mesalazine and adalimumab for the past 3 mo. In December 2020 the patient developed recurrent severe abdominal pain with an inability to pass gas or defecate, and he was hospitalized.
A genetic examination revealed GSD Ib due to a mutation in the LC37A4 gene. The patient was treated with oral cornstarch since childhood. The patient had a 20-year history of epilepsy and received long-term treatment with oxcarbazepine.
The patient had no remarkable family history.
The patient was 140 cm tall and weighed 44 kg (Body mass index, 19.6 kg/m2). The patient was small and had a childish face. The abdomen was distended, the lower margin of the liver was at the level of the umbilicus, and the spleen was palpable below the ribs. The patient had abdominal pain, but no rebound pain or muscle tension.
The clinical disease activity index score was 269, indicating that the patient had active IBD. The complete blood cell count revealed mild anemia with a hemoglobin concentration of 109 g/L and a normal platelet count. The patient had a high C-reactive protein level of 152.54 mg/L and a low white blood cell count (2.4 × 109/L). The transaminases were slightly elevated (alanine aminotransferase, 56.1 U/L; aspartate aminotransferase, 58.7 U/L). The urea nitrogen and creatinine levels were normal with the exception of elevated uric acid (667.9 μmol/m2). The calcitonin original, blood electrolytes, hematologic tumor markers, prothrombin and partial thromboplastin levels were normal. An electrocardiogram, echocardiography and pulmonary function tests were also normal.
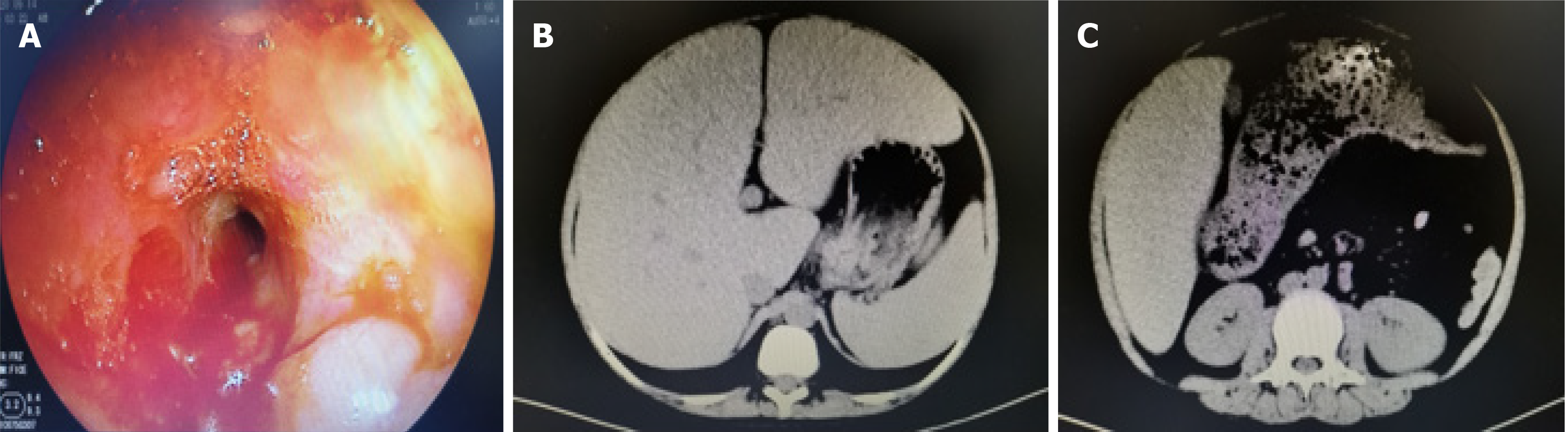
Computed tomography indicated changes in the left transverse colon wall and significant narrowing of the intestinal lumen, leading to proximal colonic obstruction and fecal accumulation. Magnetic resonance imaging showed multiple segmental intestinal lesions in the jejunum, ileum and colon with obvious intestinal strictures in the colon. Colonoscopy revealed hyperemia and edema in the colonic mucosa with multiple ulcers and intestinal strictures (Figure 1). The pathologic evaluation of a biopsy indicated acute and chronic colonic mucosal inflammation and granulation tissue formation.
The patient was diagnosed with GSD and IBD.
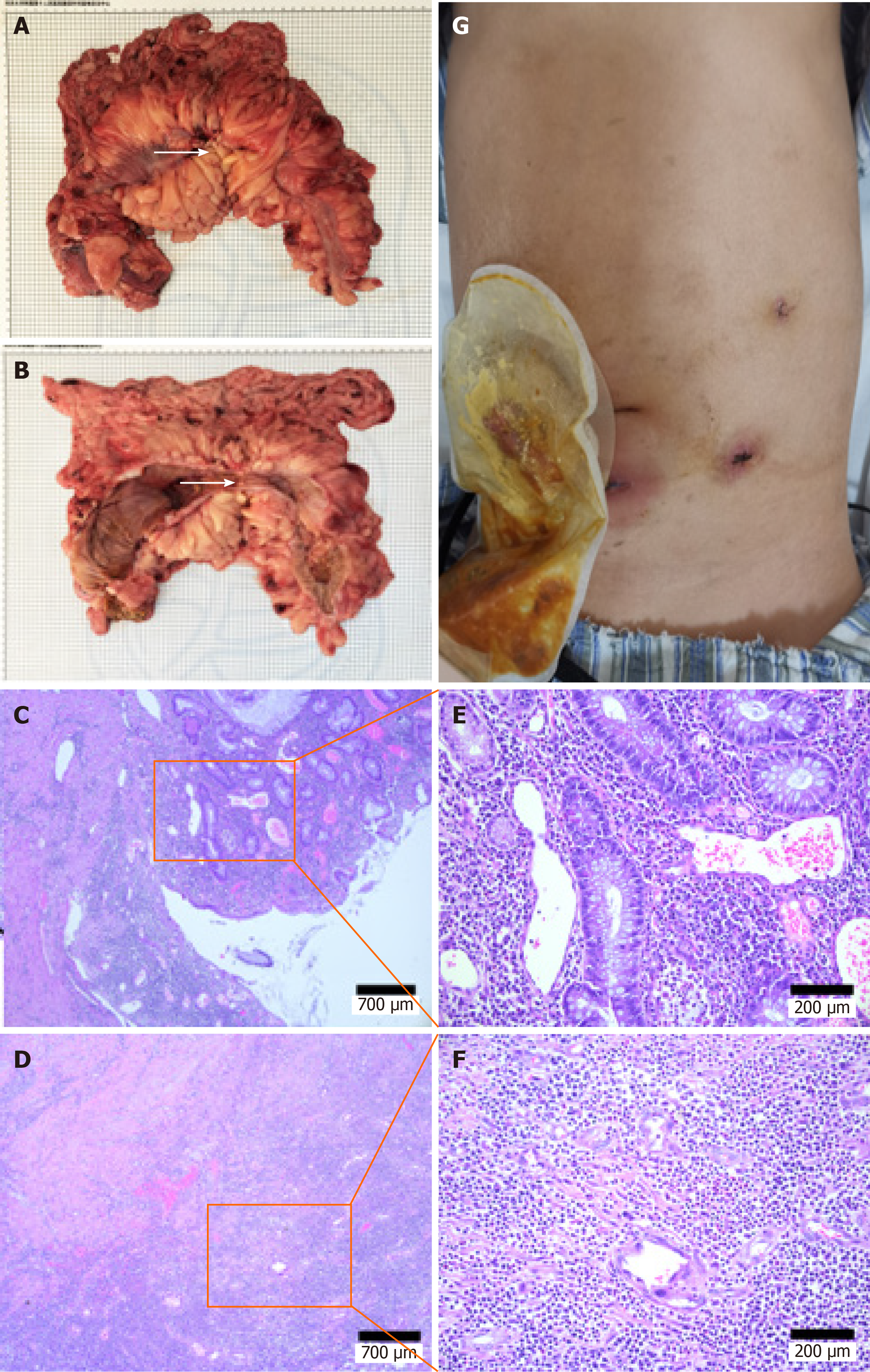
Intraoperatively the cecum was shown to extend to the lower margin of the liver, and the transverse colon was thickened and narrowed, leading to significant dilatation of the proximal intestine. The patient eventually underwent a laparoscopic subtotal colectomy and ileostomy. The postoperative pathologic evaluation revealed chronic ulcer and intestinal abscess formation in the colon with diffuse mesenteritis, which was consistent with IBD (Figure 2).
The patient was placed on a soft diet on the second postoperative day and resumed oral administration of cornstarch and antiepileptic drugs. The patient was discharged 1 wk postoperatively and continued treatment in the Gastroenterology Department.
A strong correlation has been shown to exist between GSD and IBD[5]. With improvements in diagnosis and therapy, GSD patient survival has greatly improved, and the incidence of IBD in GSD patients will be significantly increased in the future. Currently, the symptoms in patients with GSD can be alleviated with granulocyte colony-stimulating factor, antibiotics and appropriate dietary management. However, with respect to patients with GSD Ib, appropriate treatment rarely reverses the complications[6]. Studies have shown that the production of endogenous glucose by the glucose-6-phosphate transporter/glucose-6-phosphotase-β complex is the only energy source of mature neutrophils, which is essential for neutrophil function. Among patients with GSD Ib, neutrophils are unable to produce endogenous glucose due to lack of glucose-6-phosphate transporter, ultimately leading to increased neutrophil dysfunction and apoptosis[7,8]. In addition, the only neutrophils remaining are unable to infiltrate the intestinal mucosa normally in patients with GSD and IBD, thus resulting in the persistent presence of intestinal bacteria. At the same time, continuous infiltration of macrophages leads to the development of granulomas, which in turn leads to chronic inflammation of the intestine and eventually leads to intestinal fibrosis and fistula formation[9].
A recent study has shown that compared with infliximab therapy, minimally invasive surgery is a reasonable alternative in IBD patients with limited ileocecal Crohn’s disease without strictures in whom conventional therapy has failed[4]. The safety and feasibility of treating IBD have been confirmed with minimally invasive surgery in experienced IBD centers[10]. Regarding Crohn’s disease of colony type, the degree of transmural lesions was less than ileocolic colony type Crohn’s disease, and there were few obvious small intestinal strictures and obstruction. Therefore, the visual field was better exposed and lysis of adhesions was not difficult. The clinical efficacy of laparoscopic surgery in colony type Crohn’s disease is advantageous. Some studies have shown that compared with open surgery, the advantages of minimally invasive surgery include faster recovery, lower morbidity, shorter hospital stay, less pain and an earlier return to daily activities. Specifically, the risk of postoperative infection can be reduced in patients with IBD who frequently use immunosuppressants. For patients who require a prophylactic ostomy, the diseased bowel may be delivered through an incision at the ostomy without the need for an additional auxiliary incision[11,12].
The perioperative management of patients with GSD and IBD must focus on prevention of hypoglycemia and acidosis while maintaining metabolic balance. It has been reported that the absence of intra- and postoperative glucose infusion in GSD patients may lead to aggravation of a glucose metabolism disorder and metabolic acidosis. Perioperative blood glucose, electrolyte and lactic acid levels are more frequently detected, and blood glucose should be > 70 mg/dL[13]. It is necessary to fully communicate with the anesthesiologist to ensure adequate intraoperative glucose infusion to maintain a stable blood glucose concentration.
Postoperatively, if the patient’s condition is not abnormal, they should resume eating as soon as possible and resume oral administration of cornstarch. For the initial stage of eating, patients should also be administered maintenance levels of glucose intravenously at night. In the current case, the patient was given a liquid diet on the second day postoperatively, and the treatment of cornstarch was resumed. The patient was equipped with a flash glucose monitoring system to detect the 24 h blood glucose changes, and timely glucose supplementation treatment was provided as needed. To monitor postoperative infections in patients with GSD, the white blood cell count is often not high and has no great reference value. We adjusted the antibiotic treatment by monitoring C-reactive protein levels. Fortunately, the C-reactive protein of the patient was 60.5 mg/L on the third day postoperatively, and there were no signs of severe infections. The patient did not have postoperatively fevers, which suggested that the patient benefitted from minimally invasive surgery.
We suggest that patients with GSD and IBD undergo minimally invasive surgery, which is safe, feasible and effective given adequate preoperative preparation, which can facilitate a rapid and complete recovery.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Liu T, Yang W S-Editor: Fan JR L-Editor: Filipodia P-Editor: Li JH
| 1. | Biosse Duplan M, Hubert A, Le Norcy E, Louzoun A, Perry A, Chaussain C, Labrune P. Dental and periodontal manifestations of glycogen storage diseases: a case series of 60 patients. J Inherit Metab Dis. 2018;41:947-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Chou JY, Jun HS, Mansfield BC. Glycogen storage disease type I and G6Pase-β deficiency: etiology and therapy. Nat Rev Endocrinol. 2010;6:676-688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 3. | Wortmann SB, Van Hove JLK, Derks TGJ, Chevalier N, Knight V, Koller A, Oussoren E, Mayr JA, van Spronsen FJ, Lagler FB, Gaughan S, Van Schaftingen E, Veiga-da-Cunha M. Treating neutropenia and neutrophil dysfunction in glycogen storage disease type Ib with an SGLT2 inhibitor. Blood. 2020;136:1033-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 4. | Stevens TW, Haasnoot ML, D’Haens GR, Buskens CJ, de Groof EJ, Eshuis EJ, Gardenbroek TJ, Mol B, Stokkers PCF, Bemelman WA, Ponsioen CY; LIR!C study group. Laparoscopic ileocaecal resection vs infliximab for terminal ileitis in Crohn’s disease: retrospective long-term follow-up of the LIR! Lancet Gastroenterol Hepatol. 2020;5:900-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 5. | Lawrence NT, Chengsupanimit T, Brown LM, Weinstein DA. High Incidence of Serologic Markers of Inflammatory Bowel Disease in Asymptomatic Patients with Glycogen Storage Disease Type Ia. JIMD Rep. 2015;24:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Szymańska E, Lipiński P, Rokicki D, Książyk J, Tylki-Szymańska A. Over 20-Year Follow-up of Patients with Hepatic Glycogen Storage Diseases: Single-Center Experience. Diagnostics (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Chou JY, Jun HS, Mansfield BC. Neutropenia in type Ib glycogen storage disease. Curr Opin Hematol. 2010;17:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Jun HS, Weinstein DA, Lee YM, Mansfield BC, Chou JY. Molecular mechanisms of neutrophil dysfunction in glycogen storage disease type Ib. Blood. 2014;123:2843-2853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 9. | Levine AP, Segal AW. What is wrong with granulocytes in inflammatory bowel diseases? Dig Dis. 2013;31:321-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Moftah M, Nazour F, Cunningham M, Cahill RA. Single port laparoscopic surgery for patients with complex and recurrent Crohn’s disease. J Crohns Colitis. 2014;8:1055-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Seifarth C, Ritz JP, Kroesen A, Buhr HJ, Groene J. Effects of minimizing access trauma in laparoscopic colectomy in patients with IBD. Surg Endosc. 2015;29:1413-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Buchs NC, Bloemendaal ALA, Wood CPJ, Travis S, Mortensen NJ, Guy RJ, George BD. Subtotal colectomy for ulcerative colitis: lessons learned from a tertiary centre. Colorectal Dis. 2017;19:O153-O161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Kishnani PS, Austin SL, Abdenur JE, Arn P, Bali DS, Boney A, Chung WK, Dagli AI, Dale D, Koeberl D, Somers MJ, Wechsler SB, Weinstein DA, Wolfsdorf JI, Watson MS; American College of Medical Genetics and Genomics. Diagnosis and management of glycogen storage disease type I: a practice guideline of the American College of Medical Genetics and Genomics. Genet Med. 2014;16:e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 298] [Article Influence: 29.8] [Reference Citation Analysis (0)] |