Published online Jun 16, 2021. doi: 10.12998/wjcc.v9.i17.4336
Peer-review started: January 19, 2021
First decision: February 11, 2021
Revised: February 19, 2021
Accepted: March 15, 2021
Article in press: March 15, 2021
Published online: June 16, 2021
Processing time: 126 Days and 23.5 Hours
Primitive neuroectodermal tumors (PNETs) are rare, sporadic malignant tumors of the peripheral nervous system, bone, or soft tissues. However, to the best of our knowledge, only three cases of PNET in the pericardium have been reported in the English literature, and their magnetic resonance imaging findings have not previously been described.
A 3-year-old boy was hospitalized with a 1-wk history of recurrent vomiting and weakness. Detailed history-taking revealed no evidence of heart disease. Computed tomography demonstrated a soft tissue mass in the left pericardial cavity with heterogeneous contrast enhancement. The border between the mass and the heart was poorly defined. Thoracotomy revealed a mass invading the left ventricle, with a high risk of bleeding. The mass was considered inoperable. A biopsy was performed, and the histological and immunohistochemical findings confirmed the diagnosis of primary PNET of the pericardium. The patient received four cycles of standard chemotherapy. Chest magnetic resonance imaging 3 mo after the initiation of chemotherapy revealed that the tumor in the pericardium still existed, but its volume had slightly decreased. The patient was lost to follow-up, and the final outcome was therefore unknown.
Medical imaging plays an important role in defining the pericardial origin of PNET and understanding its characteristics. Magnetic resonance imaging can provide more information on the tumor than computed tomography and may thus aid therapeutic planning.
Core Tip: Primitive neuroectodermal tumors (PNETs) are rare, high-grade malignant tumors derived from neural crest cells exhibiting neuroectodermal differentiation. Primary PNET in the pericardium is extremely rare. We present the case of a primary pericardial PNET in a 3-year-old boy, with an emphasis on the computed tomography and magnetic resonance imaging findings. This case adds to the literature on pericardial PNET and provides the first report of the magnetic resonance imaging manifestations of this type of tumor.
- Citation: Xu SM, Bai J, Cai JH. Primary primitive neuroectodermal tumor in the pericardium—a focus on imaging findings: A case report. World J Clin Cases 2021; 9(17): 4336-4341
- URL: https://www.wjgnet.com/2307-8960/full/v9/i17/4336.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i17.4336
Primitive neuroectodermal tumors (PNETs) are rare, high-grade malignant tumors derived from neural crest cells and exhibiting neuroectodermal differentiation. PNETs occur sporadically in the peripheral nervous system, bone, or soft tissues; however, primary PNET in the pericardium is extremely rare. To the best of our knowledge, only three cases of PNET in the pericardium have been reported in the English literature to date[1-3], and their magnetic resonance imaging (MRI) findings have not previously been described. We present the case of a primary pericardial PNET in a 3-year-old boy, with an emphasis on the computed tomography (CT) and MRI findings.
A 3-year-old boy was hospitalized with a 1-wk history of recurrent vomiting and weakness.
A detailed history-taking revealed no evidence of heart disease.
Physical examination showed jugular vein distension and positive hepatojugular reflux.
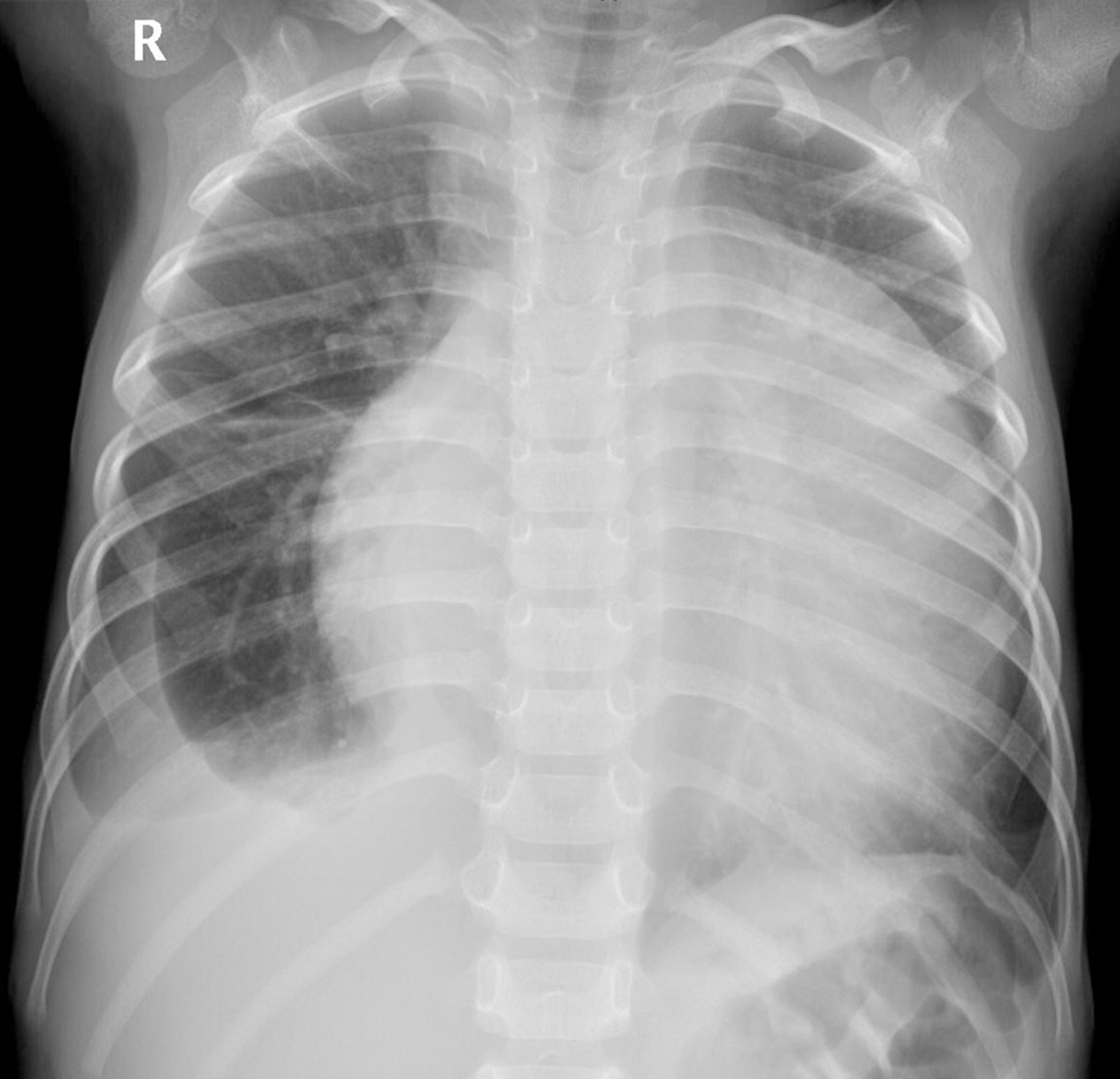
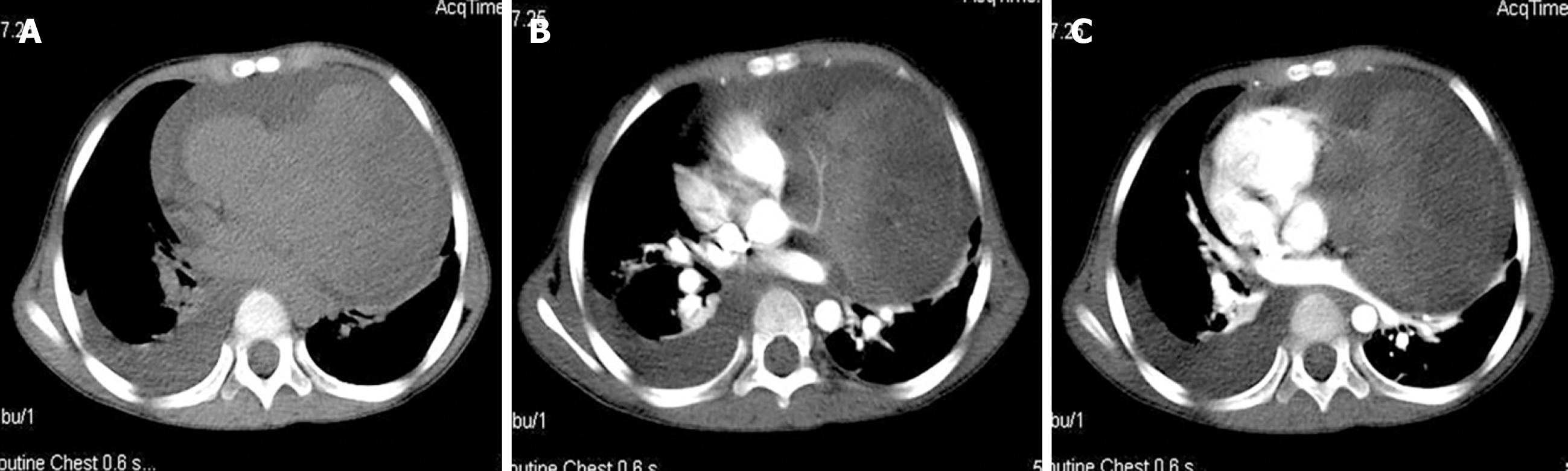
Chest radiography (Figure 1) showed an enlarged, flask-shaped cardiac silhouette, suggesting pericardial effusion. A small amount of effusion was also detected in the right pleural cavity. Plain CT scan showed pericardial effusion and an indefinite soft tissue density mass in the left pericardial cavity (Figure 2A). The mass was heterogeneously enhanced after intravenous administration of contrast medium (Figure 2B and 2C). The border between the mass and the heart was poorly defined. A coronary artery branch was detected passing through the mass (Figure 2B). Other CT findings included right pleural effusion and displacement of the heart to the right side (Figure 2C). CT findings thus indicated a malignant tumor, but no conclusive dia
The patient underwent a thoracotomy with the primary aim of resecting the mass. However, during surgery, the mass was found to be invading the left ventricle and had a rich blood supply, presenting a high risk of bleeding. The mass was therefore considered to be surgically inoperable. Subsequent biopsy was performed and histological examination showed a cellular malignant tumor composed of small, round, uniform cells. These cells were characterized with vesicular nuclei and scanty neoplasm. A few Homer-Wright rosettes with a central core of neuropil were formed. Immunohistochemical results were strongly positive for CD99 and synuclein, but negative for desmin, smooth muscle actin, chromogranin A, leukocyte common antigen, and cytokeratin.
The histological and immunohistochemical findings confirmed the diagnosis of primary PNET of the pericardium.
Following confirmation of the diagnosis, the patient was treated with four cycles of a standard chemotherapy regimen (etoposide d 1-3, cyclophosphamide d 4-5).
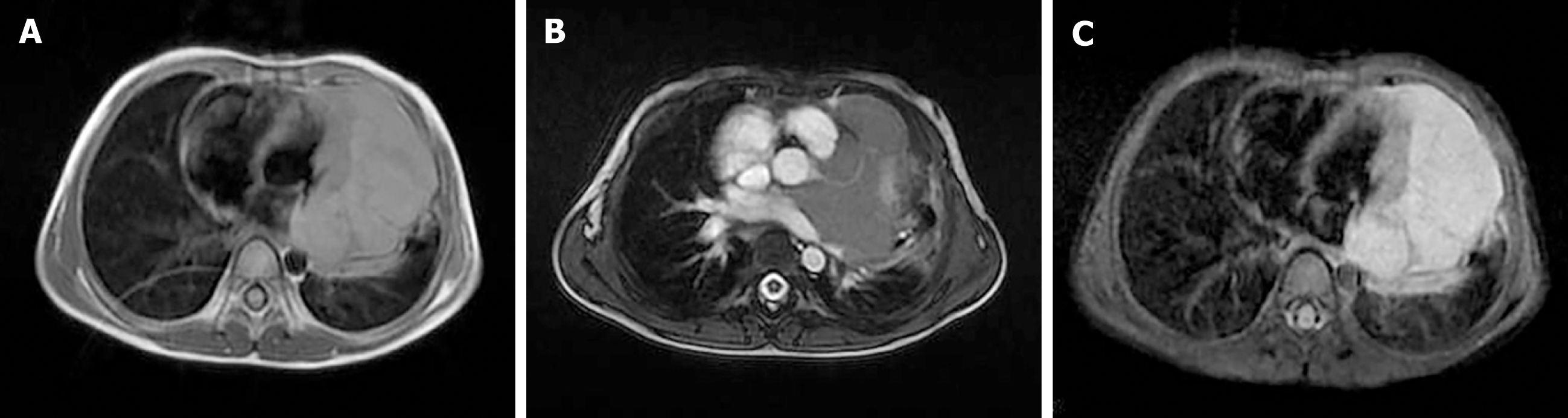
Chest MRI examination (Figure 3) 3 mo after the initiation of chemotherapy revealed that the pleural and pericardial effusions had disappeared. The tumor was still present in the pericardium, but its volume had slightly decreased. The mass showed a heterogeneous signal, mainly slightly hyperintense on T1-weighted images (Figure 3A) and isointense on T2-weighted images (Figure 3B). Intravenous injection of contrast medium showed the mass was inhomogeneous with marked enhancement, with a clear and definite border between the mass and the left ventricle wall (Figure 3C). The left coronary artery branch was shown to be embedded within the mass (Figure 3B), suggesting a pericardial origin of the tumor. The patient was unfortunately lost to follow-up, and his final outcome was therefore unknown.
PNETs are rare, high-grade malignant tumors derived from neural crest cells, exhibiting neuroectodermal differentiation. Primary PNETs have been reported in the peripheral nervous system, thymus, lung, bone, heart, adrenal gland, kidney, pancreas, liver, urogenital tract, and uterus[4-9]. However, primary PNET in the pericardium is extremely rare. To the best of our knowledge, only three cases of primary pericardial PNET have been reported to date[1-3], and none of the reports described MRI findings that might provide useful diagnostic information. In the current report, we presented the case of a 3-year-old boy with a primary pericardial PNET, with an emphasis on the CT and MRI findings. This case provided a significant addition to the literature regarding pericardial PNET, as only the fourth reported case in the English literature and the first to describe the MRI manifestations of the tumor.
The diagnosis of primary pericardial PNET depends on pathological examination and immunohistochemistry. Primary PNET of the pericardium have morphological appearances similar to those arising in other locations[1,9]. PNET belongs to small, round cell malignancies in histomorphology with hyperchromatic nuclei and features of neural differentiation, which typically form either Homer-Wright or Flexner-Winterstein rosettes[1]. It is difficult to differentiate PNET from other small round-cell tumors including malignant lymphoma, neuroendocrine carcinoma, and rhabdomyosarcoma. Immunohistochemical examinations with myogenic, neurogenic, and lymphoid cell markers can rule out many of these tumors[1,2,9]. According to the previous reports, the expression of CD99 (MIC2) and FLI1 are highly specific for PNETs[10]. In our case, the immunohistochemical examination revealed a strong CD99 staining, which is consistent with other reports in the literature.
The imaging findings of PNET arising from cardium or other locations have been described in the previous reports[11,12]. On plain CT images, the majority of PNET lesions showed as ill-defined, irregularly shaped, hypodense masses accompanied with persistent moderate heterogeneous enhancement. The cystic or necrotic changes were frequently observed in larger tumors, which may be due to the rapid growth and lack of blood supply[11]. On MRI images, the tumors generally showed iso- to hypointense T1-weighted signal and intermediate to hyperintense T2-weighted signal. After the administration of intravenous gadolinium, the tumors demonstrated homogeneous to heterogeneous enhancement[12].
Although imaging examinations lack specificity for diagnosing PNET, CT and MRI may reveal the size and location of the tumor and its relationship with the neighboring heart, thus providing valuable information for evaluating and formulating surgical treatment plans. In the current case, CT and MRI both showed a large, solid, heterogeneously enhanced mass circumscribed by a capsule. Compared with CT, MRI was more sensitive for displaying the relationship between the mass and the heart. In addition, the tumor MRI signal was characterized by slight hyperintensity on T1-weighted and iso- to hyperintensity on T2-weighted images. These signal features may relate to the presence of more tumor cells and fewer water molecules in the tumor stroma, indicating the malignant nature of the tumor.
We present the fourth reported case of a primary pericardial PNET and the first to describe the MRI manifestations of this type of tumor. The diagnosis of primary pericardial PNET depends on pathological examination and immunohistochemistry. Medical imaging plays an important role in detecting the mass and understanding its characteristics. MRI can provide more information on the tumor than CT and may thus aid therapeutic planning.
We also thank Dr. Zhu J, from the Department of Pathology, Children’s Hospital of Chongqing Medical University, for interpretation of the pathological and immunohistochemical findings.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Elpek GO, Ho CM S-Editor: Gao CC L-Editor: Filipodia P-Editor: Zhang YL
| 1. | Chen Z, Chen R, Ng C, Shi H. Primary pericardial primitive neuroectodermal tumor: a case report and review of literature. Int J Clin Exp Pathol. 2018;11:2155-2159. [PubMed] |
| 2. | Azribi F, Razak AR, Bough G, Lee D, Rowe D, Bown N, Dildey P, Clark S, Plummer R. Extraosseous pericardial Ewing's sarcoma. J Clin Oncol. 2010;28:e48-e50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Mohandas KM, Chinoy RF, Merchant NH, Lotliker RG, Desai PB. Malignant small cell tumour (Askin-Rosai) of the pericardium. Postgrad Med J. 1992;68:140-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Koufopoulos N, Kokkali S, Manatakis D, Balalis D, Nasi D, Ardavanis A, Korkolis D, Khaldi L. Primary peripheral neuroectodermal tumor (PNET) of the adrenal gland: a rare entity. J BUON. 2019;24:770-778. [PubMed] |
| 5. | Zhong J, Chen N, Chen X, Gong J, Nie L, Xu M, Zhou Q. Peripheral primitive neuroectodermal tumor of the kidney in a 51-year-old female following breast cancer: A case report and review of the literature. Oncol Lett. 2015;9:108-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Nishizawa N, Kumamoto Y, Igarashi K, Nishiyama R, Tajima H, Kawamata H, Kaizu T, Watanabe M. A peripheral primitive neuroectodermal tumor originating from the pancreas: a case report and review of the literature. Surg Case Rep. 2015;1:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Elizalde CR, Yagüe A, Fernandez J, Dieste P, Puente MJ, Hernandez J. Primitive neuroectodermal tumor of the uterus. Gynecol Oncol Rep. 2016;18:25-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Gao L, Zhu Y, Shi X, Gao Z, Chen X. Peripheral primitive neuroectodermal tumors: A retrospective analysis of 89 cases and literature review. Oncol Lett. 2019;18:6885-6890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Vogel H, Fuller GN. Primitive neuroectodermal tumors, embryonal tumors, and other small cell and poorly differentiated malignant neoplasms of the central and peripheral nervous systems. Ann Diagn Pathol. 2003;7:387-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Nutman A, Postovsky S, Zaidman I, Elhasid R, Vlodavsky E, Kreiss Y, Ben Arush MW. Primary intraspinal primitive neuroectodermal tumor treated with autologous stem cell transplantation: case report and review of the literature. Pediatr Hematol Oncol. 2007;24:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Xiao H, Bao F, Tan H, Wang B, Liu W, Gao J, Gao X. CT and clinical findings of peripheral primitive neuroectodermal tumour in children. Br J Radiol. 2016;89:20140450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Restrepo CS, Vargas D, Ocazionez D, Martínez-Jiménez S, Betancourt Cuellar SL, Gutierrez FR. Primary pericardial tumors. Radiographics. 2013;33:1613-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |