Published online Jun 16, 2021. doi: 10.12998/wjcc.v9.i17.4327
Peer-review started: January 13, 2021
First decision: February 10, 2021
Revised: February 23, 2021
Accepted: April 6, 2021
Article in press: April 6, 2021
Published online: June 16, 2021
Processing time: 132 Days and 21 Hours
Portal venous thromboembolism caused by malignant pancreatic neuroendocrine tumor metastasis, as the initial presentation of portal hypertension and upper gastrointestinal bleeding, is a rare entity. To our knowledge, there are no reports of this entity in pregnant women. We describe a case of pancreatic neuroendocrine carcinoma during pregnancy with hematemesis and hematochezia as the initial presentation and review the literature to analyze the demographic, clinical, and pathological features to provide a reference for clinical diagnosis and treat
A 40-year-old woman presented with hematemesis and hematochezia at 26-wk gestation; she had no other remarkable medical history. The physical examination revealed normal vital signs, an anemic appearance, and lower abdominal distension. Abdominal color Doppler ultrasonography showed portal vein thrombosis, splenomegaly, intrauterine pregnancy, and intrauterine fetal death. Esophagogastroduodenoscopy revealed esophageal and gastric varicose veins and portal hypertensive gastropathy. Contrast-enhanced computed tomography demonstrated multiple emboli formation in the portal and splenic veins, multiple round shadows in the liver with a slightly lower density, portal vein broadening, varicose veins in the lower esophagus and gastric fundus, splenomegaly, bilateral pleural effusion, ascites and pelvic effusion, broadening of the common bile duct, and increased uterine volume. According to the results of Positron emission tomography-computed tomography and immunohistochemical staining, the final diagnoses were that the primary lesion was a pancreatic neuroendocrine tumor and that there were secondary intrahepatic metastases and venous cancer thro
Upper gastrointestinal bleeding in a pregnant woman may be caused by portal hypertension due to a malignant pancreatic neuroendocrine tumor.
Core Tip: Upper gastrointestinal bleeding caused by portal hypertension is a rare form of advanced pancreatic neuroendocrine tumor. Our patient presented with hematemesis and hematochezia at 26-wk gestation, but no other significant medical history. Positron emission tomography-computed tomography revealed that lesions in the liver were found to be pancreatic neuroendocrine carcinoma during pregnancy. This case demonstrates that upper gastrointestinal bleeding as the initial presentation should be suspected among pregnant patients at high risk for malignancies.
- Citation: Gao LP, Kong GX, Wang X, Ma HM, Ding FF, Li TD. Pancreatic neuroendocrine carcinoma in a pregnant woman: A case report and review of the literature . World J Clin Cases 2021; 9(17): 4327-4335
- URL: https://www.wjgnet.com/2307-8960/full/v9/i17/4327.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i17.4327
Neuroendocrine neoplasms (NENs) are a group of heterogeneous tumors originating from peptide neuroendocrine neurons and neuroendocrine cells, commonly occurring in different parts of the body, including the lung, thymus, pancreas, and gastrointes
A 40-year-old woman at 26-wk gestation was admitted to the hospital for he
Abdominal color Doppler ultrasonography showed portal vein thrombosis, splenomegaly, intrauterine pregnancy, and intrauterine fetal death.
The patient had no significant past medical history.
The patient had no history of smoking or alcohol consumption. She had no relevant family history.
The physical examination revealed that the patient had normal vital signs, a body weight of 56 kg, a height of 1.58 m, a body mass index of 22.4 kg/m2, an anemic appearance, and lower abdominal distension, and had no abdominal shiftiness sound.
The laboratory examination was otherwise unremarkable. The laboratory assessment included an initial blood test of the complete blood count (hemoglobin level, 76 g/L), liver test (albumin level, 29.9 g/L), and tumor markers (alpha-fetoprotein level, 179.60 ng/mL; cancer antigen 125 level, 209.40 U/mL; and cancer antigen 199 level, 168.20 U/mL). Viral hepatitis markers were negative. Glucose and serum insulin levels were normal.
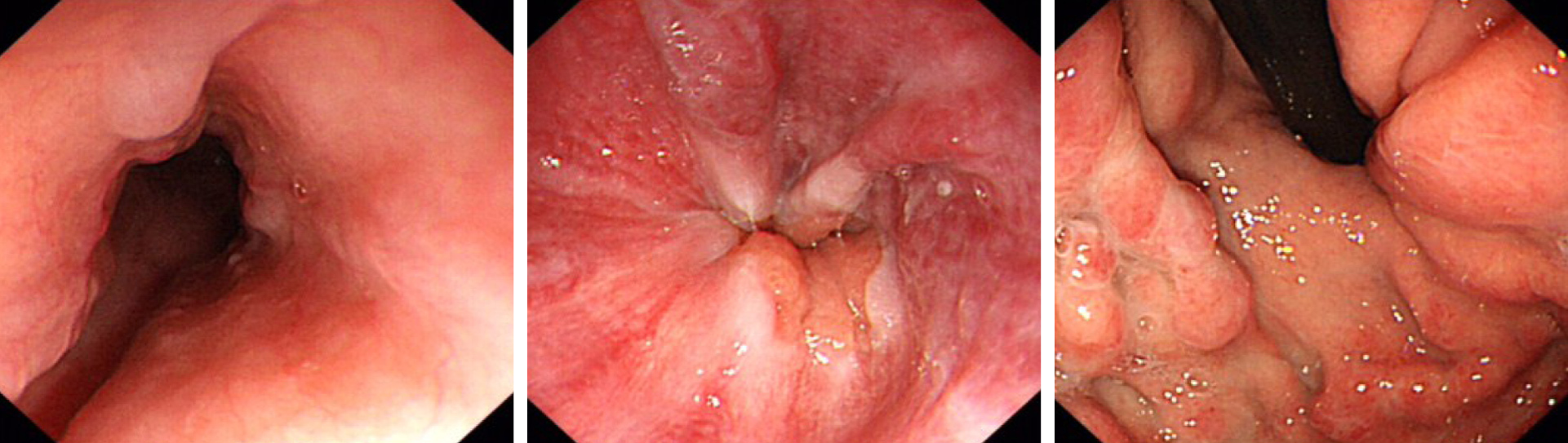
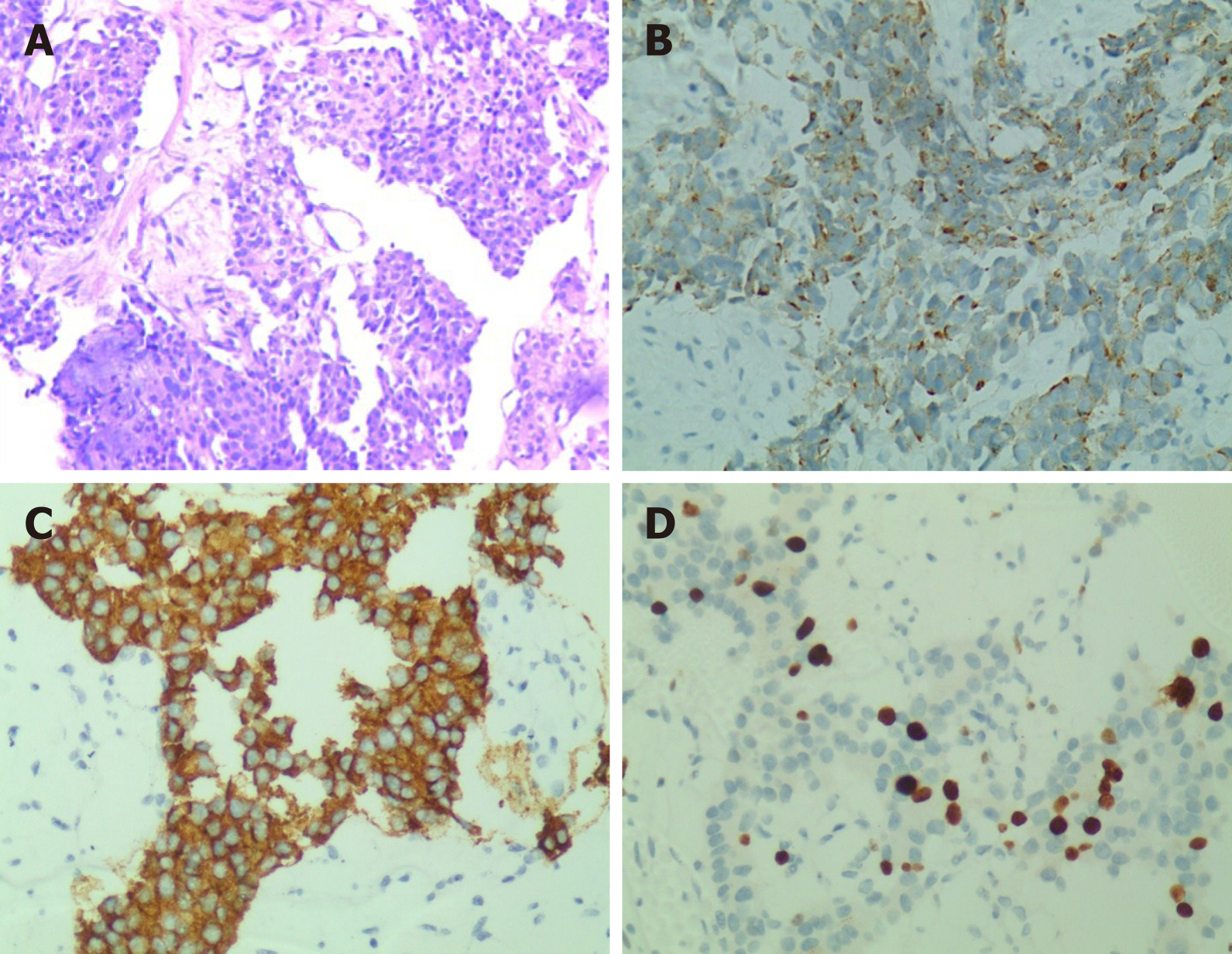
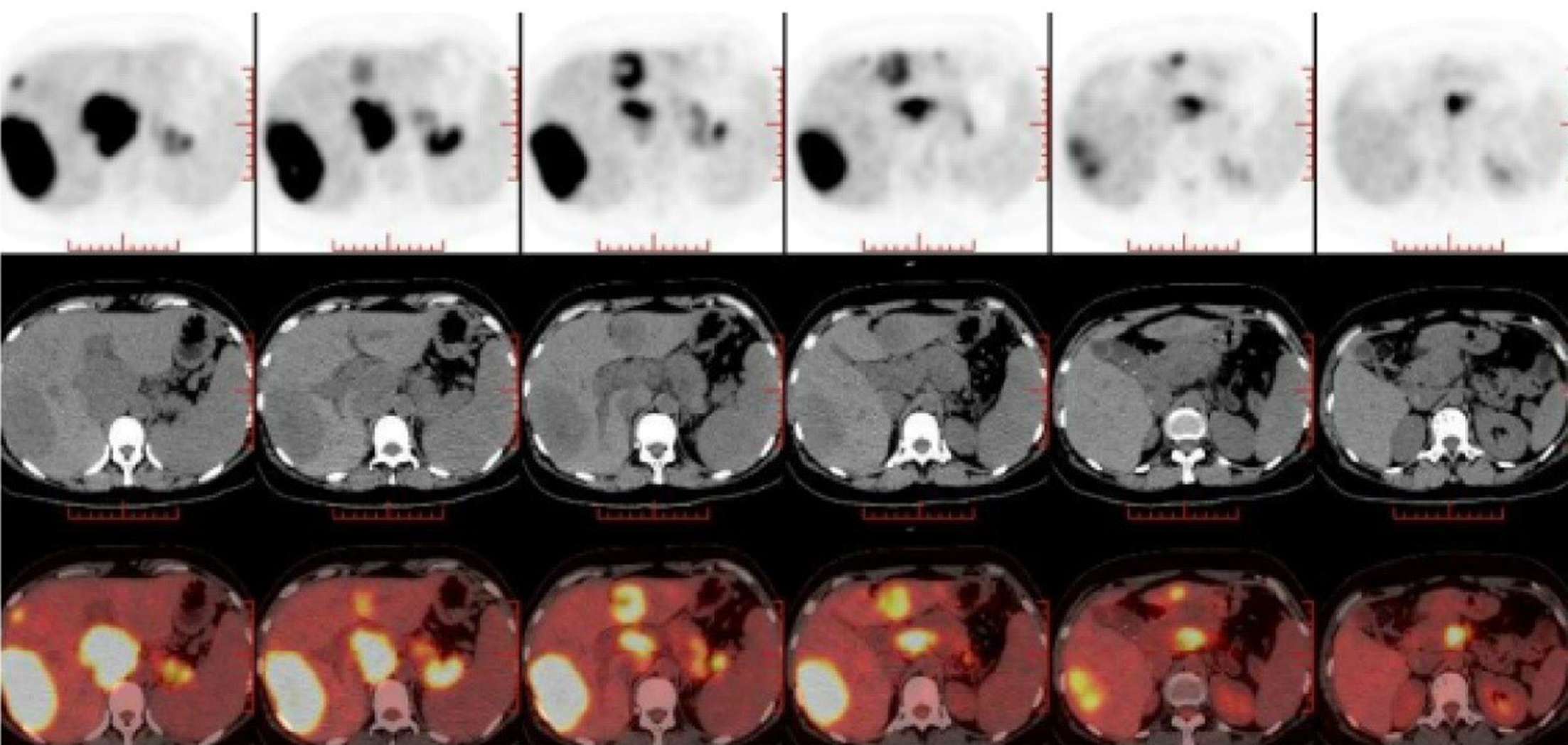
Esophagogastroduodenoscopy revealed esophageal and gastric varicose veins and portal hypertensive gastropathy (Figure 1). Contrast-enhanced computed tomography (CT) demonstrated multiple emboli formation in the portal vein and splenic vein, multiple round shadows in the liver with a slightly lower density (metastatic tumors were mostly considered), portal vein broadening, varicose veins in the lower esophagus and gastric fundus, splenomegaly, bilateral pleural effusion, ascites and pelvic effusion, broadening of the common bile duct, and increased uterine volume. Ultrasound-guided fine-needle aspiration biopsy of the liver tumor was performed. Histologically, the tumor consisted of heterogeneous cells arranged in nests, with a small cell extraction volume, short spindle or polygonal, unclear cell boundary, eosinophilic cytoplasm, increased nucleoplasmic ratio, and varying degrees of nuclear atypia. Immunohistochemical staining revealed that the tumor cells were positive for CKp, synaptophysin (Syn), chromogranin A (CgA), CD10, CD56, CDX-2, CEA, and Ki67 (40%+) (Figure 2). To identify the primary lesion, 18F-fluorodeoxyglucose Positron emission tomography/computed tomography (18F-FDG-PET-CT) was performed and showed multiple metabolic elevations in the pancreatic tail area, intrahepatic portal vein, and adjacent mesenteric and splenic veins. Considering the pathological tendency, it was considered that there was a high possibility of primary lesions of pNEC, secondary intrahepatic metastasis, venous cancer thrombogenesis, and corresponding varicose veins. No other metastatic lesions were found (Figure 3).
These aforementioned findings supported a diagnosis of pNEC (T2N0M2, G3) in pregnancy, secondary malignant tumor of the liver, and multiple venous thrombi.
Subsequently, the patient was transferred to other hospitals for further treatment. The patient underwent transcatheter arterial chemo-embolization three times and radiofrequency ablation one time and was administered Sandostatin (octreotide acetate microsphere, 30 mg) once a month, without systemic chemotherapy or targeted drugs.
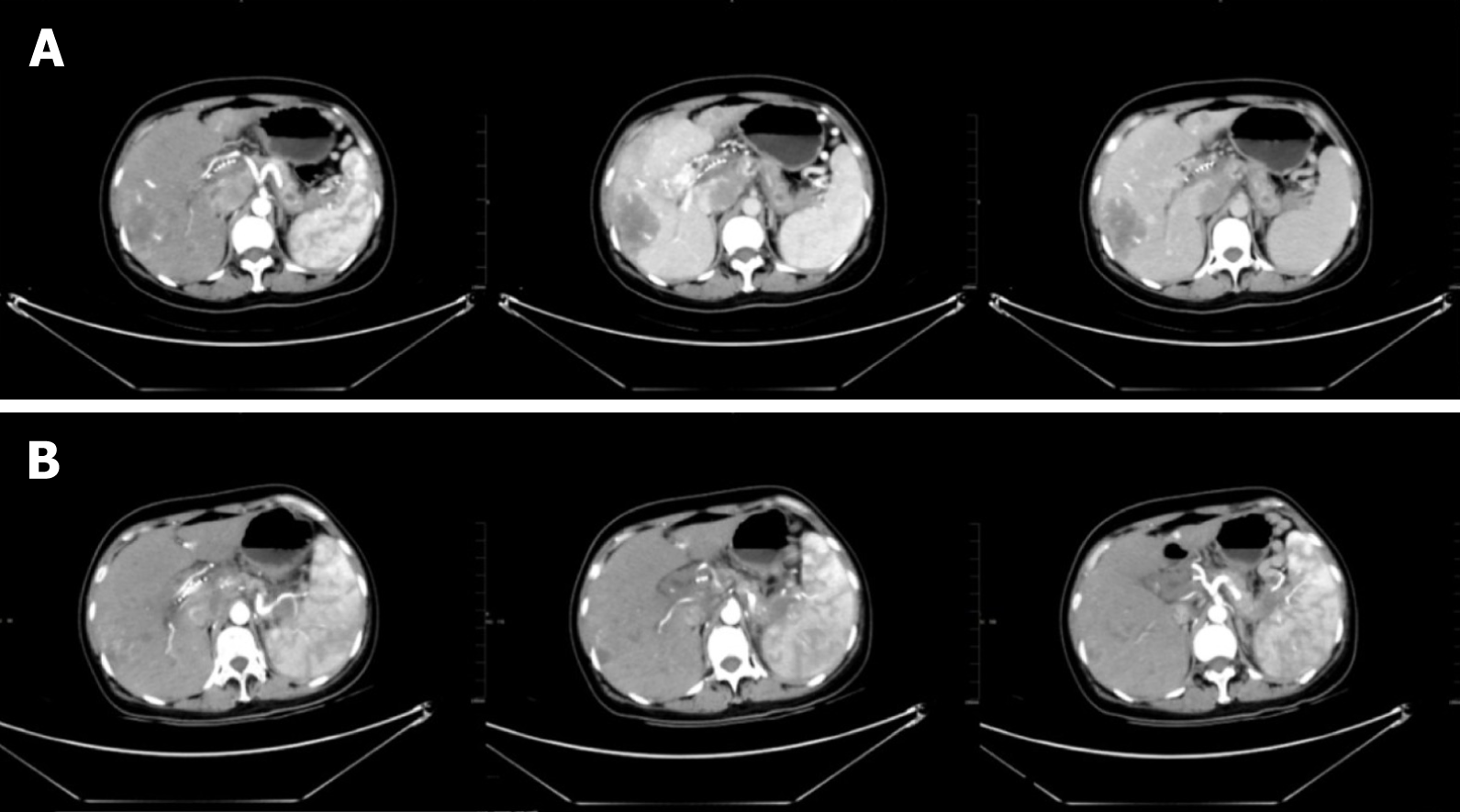
In February 2020, abdominal contrast-enhanced CT demonstrated the following: (1) Multiple intrahepatic tumors were present. Compared with the previous image (November 2019), the lesion volume of the hepatic hilum increased, abdominal exudation and liver injury reduced, necrosis appeared in some lesions, and little change was seen in the rest of the lesions; (2) The volume of emboli in the portal vein and inferior vena cava was increased, and multiple collateral circulations formed around the portal vein. Varicose veins were also present in the lower esophagus and gastric fundus; and (3) Splenomegaly was present (Figure 4A). In June 2020, abdominal contrast-enhanced CT showed the following: (1) Multiple tumors in the liver were accompanied by accumulation of lipiodol, and the accumulation increased in the lesion compared with that in the previous lesion (February 2020). The lesion scope of the pancreatic tail was reduced. Multiple collateral circulations formed around the portal vein. Varicose veins remained present in the lower esophagus and gastric fundus; and (2) Splenomegaly was still present (Figure 4B).
Even though the patient’s weight was about 10 kg less than before, she was physically active and could take care of herself.
In a review of published literature in China and abroad from 1939 to 2019, 41 cases of pNENs during pregnancy were collected, among which 29 were insulinomas in patients with an average age of 29 years[3-28] (Table 1). In this study, the clinical manifestations and pathological characteristics of pNENs during gestation are summarized and analyzed. Pregnancy-associated pNENs, diagnosed during pregnancy and in the first year postpartum, may vary in type and differentiation, leading to atypical symptoms and signs and various clinical manifestations. This is an important reason why physicians and patients ignore and delay diagnosis. Functional pNENs have been observed at various stages of pregnancy, and the most common form of insulinoma is associated with hypoglycemia, including effects on the central nervous system, such as headaches, confusion, visual and behavioral abnormalities, or hypoglycemia that causes excessive catecholamine release, e.g., perspiration, tremor, and palpitations. A functional pNEN is easy to be misdiagnosed clinically because it is confused with pregnancy-associated uncomfortable symptoms[29]. Symptoms of nonfunctional pNENs appear when they have local spread or distant metastases. It is challenging to perform paraclinical tests on patients with nonfunctional pNENs. In particular, invasive exploration (imaging, endoscopy, etc.) may be harmful to the fetus. Clinical diagnosis of pNENs in pregnancy is difficult because of the lack of specific clinical symptoms and CT/magnetic resonance imaging (MRI) findings[30].
| Ref. | Patient age | Onset of symptoms | Symptoms | Management | Maternal outcomes | Fatal outcomes |
| Fredericks et al[19] | 35-yr-old | Three weeks post-partum | Neuroglycopaenic symptoms | Laparotomy the tumor | No symptoms after removal | Live born |
| Takacs et al[20] | 28-yr-old | At 6-wk gestation | Difficult morning arousability | Exploratory laparotomy | No residual symptoms | Cesarean delivery |
| Live born | ||||||
| Lowy and Chisholm[23] | 36-yr-old | Twelve hours post-partum | Severe hypoglycaemia | Excision of the lesion | No symptoms after removal | Live born |
| Diaz et al[24] | 35-yr-old | Three months | Loss of consciousness | Exploratory laparotomy | No residual symptoms | Live born |
| Post-partum | ||||||
| Diaz et al[24] | 35-yr-old | On the 26th postpartum day | Confusion, dysarthria, and quadriplegia | Enucleation of an 8-mm tumor | No symptoms after removal | Live born |
| Diaz et al[24] | 22-yr-old | At 2-mo gestation | Loss of consciousness | Laparoscopic distal pancreatectomy | No residual symptoms | Natural labor |
| Post-partum | Live born | |||||
| Christiansen and Vestergaard[25] | 29-yr-old | At 38-mo gestation | Slurred speech, weakness | Pancreaticoduodenectomy and cholecystectomy | No symptoms after removal | Natural labor |
| Post-partum | Live born | |||||
| Rodrigues | 21-yr-old | Eight days post-partum | Four limbs weahness, diffic-ult walking | Pancreatectomy | No residual symptoms | Live born |
| Mannelli et al[27] | 29-yr-old | At 17 wk gestation | Severe hypoglycemia | Started therapy with everolimus | Died 3 yr after delivery | Cesareandelivery |
| Live born | ||||||
| Tomazic et al[28] | 36-yr-old | In the second trimester of pregnancy | Hypoglycemia associated with neuroglycopenic symptoms | Distal pancreatectomy at 21 wk gestation | No symptoms after removal | Nature labor |
| Live born |
Metastasis of a malignant pNEN to the liver in pregnant women is a rare entity, and little is known about the risk factors for its pathogenesis. The risk of cancer has been reported to increase with age, and the distribution of maternal age affects the incidence of pregnancy-associated cancers. The mechanisms and hormone allocation that lead to changes in maternal cancer risk are not fully understood. According to a wide range of clinical medical data, increasingly more women develop cancer during pregnancy due to delay in the age of conception. According to statistics, the incidence of malignant tumors in pregnant women during pregnancy is about 0.07% to 0.1%. Risk factors specific to pregnant women may include high estrogen levels, long-term fat intake, reduced physical activity, high blood pressure, and pre-existing chronic kidney disease; genetic and environmental sources of pregnancy-related cancers are likely to predate pregnancy. Moreover, hormones and growth factors necessary for fetal growth may accelerate tumor growth, such as estrogen, insulin-like growth factor I, and angiogenic factors, which may lead to maternal cancer and deserve further study. In addition, pregnancy is a pro-angiogenic state, and the placenta and angiogenesis required by pregnancy (placental growth factor and vascular endothelial growth factor, etc.) contribute to the occurrence or growth of tumors[31]. During pregnancy, there is a higher risk of tumor complications, such as thromboembolic events and septicemia; however, physiological changes during pregnancy may delay the diagnosis of tumors and have an adverse effect on pregnancy outcomes.
CT, MRI, PET, transabdominal ultrasonography, gastrointestinal endoscopy, and endoscopic ultrasonography can be used to evaluate tumor staging and establish the diagnosis. The detection of pNENs by CT, MRI[32,33], and other imaging investigations is affected by tumor size, and establishing the diagnosis of pNENs decreases significantly when the tumor is small (< 2 cm). PET-CT seems to be useful in identifying the primary tumor site and assessing the degree of metastases at distant sites. The sensitivity of gallium 68Ga-PET-CT is higher than that of 18F-FDG-PET-CT in determining staging of pNENs[34,35]. However, in pregnant women with pNENs, such tests are considered only after weighing the advantages and disadvantages.
Serological examination of pNENs during pregnancy can be performed, and serum CgA is the most widely used and valuable biomarker for diagnosis and follow-up of NENs[36], which can be used as an adjunctive diagnosis; other tumor biomarkers include neuron-specific enolase (NSE), Syn, and 5-hydroxyindoleacetic acid, and specific immunohistochemical staining markers of CgA, Syn, and NSE are the gold standard for identifying NENs.
Unlike conventional pNENs, cases of pNENs in pregnancy are scarce, and there is no standard therapeutic treatment. However, the therapy for pNENs is primarily surgical. Treatment requires a multidisciplinary approach that results in an ap
Chemotherapy is the first choice for advanced metastatic tumors, and the apparent aim is to prolong the life of the mother until safe delivery[39]. Chemotherapy provides a palliative care option for pregnant women and postpartum patients if the fetus is of appropriate age. The risks to the fetus must be understood before chemotherapy is performed, and a range of moral, religious, ethical, and legal considerations must be taken into account. The extent to which the fetus receives chemotherapy and its effects depend on the progress of the pregnancy; chemotherapy must not be administered at 33 wk before birth or 3 wk after birth. The usual treatment regimen for pancreatic NETs is the use of antimetabolites (such as 5-fluorouracil) and some alkylating agents (such as streptozotocin)[40], with minimal effect on the fetus. The fetal toxicity of multi-drug chemotherapy increased from 17% to 25% compared with mono
In conclusion, the primary lesion of pNEC during pregnancy in this case was relatively hidden, but multiple distant metastases of the liver, portal vein, mesenteric vein, and splenic vein occurred. Additionally, acute upper gastrointestinal hemorrhage was the initial presentation, which is relatively rare. In pregnant women with pNECs, a special patient group, physicians should not only focus on the patient's own disease but also consider the safety of the fetus, family, ethics, law, and other factors. Moreover, there is no unified diagnosis and treatment standard in China and abroad. Such patients require a multidisciplinary team for comprehensive clinical management and individualized treatment based on each patient's condition and the development of the fetus.
We would like to thank the patient and her families for agreeing to publish this case report. We also acknowledge each of the authors for their contributions to this article.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen LW, Dehghanian M, Keikha M S-Editor: Liu M L-Editor: Wang TQ P-Editor: Zhang YL
| 1. | Chinese Society of Clinical Oncology Expert Committee on Neuroendocrine Oncology. The consensus of Chinese gastrointestinal and pancreatic neuroendocrine oncologists. Linchuang Zhongliu Zazhi. 2016;21:927-946. [DOI] [Full Text] |
| 2. | Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, Evans DB. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063-3072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3022] [Cited by in RCA: 3242] [Article Influence: 190.7] [Reference Citation Analysis (0)] |
| 3. | Feldman M, Weinberg T, Feldman MJ. Islet cell tumors of the pancreas. Am J Gastroenterol. 1954;22:320-328. [PubMed] |
| 4. | Serrano-Rios M, Cifuentes I, Prieto JC. Insulinoma in a pregnant woman. Obstet Gynecol. 1976;47:361-364. [PubMed] |
| 5. | Rubens R, Carlier A, Thiery M, Vermeulen A. Pregnancy complicated by insulinoma. Case report. Br J Obstet Gynaecol. 1977;84:543-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Notterman RB, Jovanovic L, Peterson R, Solomon G, Druzin M, Peterson CM. Spontaneous hypoglycemic seizures in pregnancy. A manifestation of panhypopituitarism. Arch Intern Med. 1984;144:189-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Osei K, Kramer DS, Malarkey WB, Falko JM. Pregnancy complicated by insulinoma. Am J Med Sci. 1984;288:133-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Shaw DL, Bernene JL, Williams JW, North CQ, Palestrant AM. Insulinoma in pregnancy. Ariz Med. 1985;42:406-408. [PubMed] |
| 9. | Egley CC, Gutliph J, Bowes WA Jr. Severe hypoglycemia associated with HELLP syndrome. Am J Obstet Gynecol. 1985;152:576-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Friedman E, Moses B, Engelberg S, Levran D, Lieberman P. Malignant insulinoma with hepatic failure complicating pregnancy. South Med J. 1988;81:86-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Galun E, Ben-Yehuda A, Berlatzki J, Ben-Chetrit E, Gross DJ. Insulinoma complicating pregnancy: case report and review of the literature. Am J Obstet Gynecol. 1986;155:64-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Garner PR, Tsang R. Insulinoma complicating pregnancy presenting with hypoglycemic coma after delivery: a case report and review of the literature. Obstet Gynecol. 1989;73:847-849. [PubMed] |
| 13. | Hale PJ, Hale JF, Nattrass M. Insulinoma and pregnancy. Case report. Br J Obstet Gynaecol. 1988;95:514-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Liberman C, Valenzuela MA, Hernández F, Miranda C, Salazar V, Castillo J. [Insulinoma and pregnancy. Clinical case]. Rev Med Chil. 1991;119:564-566. [PubMed] |
| 15. | Akanji AO, George AO, Olasode BJ, Osotimehin BO. Insulinoma in pregnancy presenting as a seizure disorder: a case report. East Afr Med J. 1992;69:117-119. [PubMed] |
| 16. | Atala C, Tapia M. [Insulinoma and pregnancy. A clinical case]. Rev Chil Obstet Ginecol. 1992;57:437-439. [PubMed] |
| 17. | Auinger M, Dudczak R, Fasching W, Leodolter S, Feinböck C, Irsigler K. [Detection of an insulinoma in pregnancy--a rare cause of hypoglycemia]. Wien Klin Wochenschr. 1994;106:426-429. [PubMed] |
| 18. | Bardet S, Mahot P, Deumier B, Le Néel JC, Krempf M, Charbonnel B. [Discovery of an insulinoma during the first trimester of pregnancy]. Presse Med. 1994;23:285-287. [PubMed] |
| 19. | Fredericks B, Entsch G, Lepre F, Nolan G, Davoren P. Pregnancy ameliorates symptoms of insulinoma--a case report. Aust N Z J Obstet Gynaecol. 2002;42:564-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Takacs CA, Krivak TC, Napolitano PG. Insulinoma in pregnancy: a case report and review of the literature. Obstet Gynecol Surv. 2002;57:229-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Gouya H, Vignaux O, Augui J, Dousset B, Palazzo L, Louvel A, Chaussade S, Legmann P. CT, endoscopic sonography, and a combined protocol for preoperative evaluation of pancreatic insulinomas. AJR Am J Roentgenol. 2003;181:987-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 164] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 22. | Hirshberg B, Cochran C, Skarulis MC, Libutti SK, Alexander HR, Wood BJ, Chang R, Kleiner DE, Gorden P. Malignant insulinoma: spectrum of unusual clinical features. Cancer. 2005;104:264-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 23. | Lowy AJ, Chisholm DJ. Insulinoma masked by pregnancy. Intern Med J. 2001;31:128-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Diaz AG, Herrera J, López M, Puchulu FM, Ferraina P, Bruno OD. Insulinoma associated with pregnancy. Fertil Steril 2008; 90: 199.e1-199. e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Christiansen E, Vestergaard H. Insulinoma in a third-trimester pregnant woman combined with pre-eclampsia: a case report and review of the diagnostic strategies. Gynecol Endocrinol. 2008;24:417-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Rodrigues Queiróz AJ, Nazareno LS, Miranda JE, de Azevedo AEB, Teixeira da Cruz CA, Pirani Carneiro F, Florêncio da Costa AC, Lofrano-Porto A. Insulinoma diagnosed in the postpartum: clinical and immunohistochemical features. Gynecol Endocrinol. 2012;28:633-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Mannelli L, Yeh MM, Wang CL. A pregnant patient with hypoglycemia. Gastroenterology. 2012;143:e3-e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Tomazic M, Janez A, Ravnik Oblak M. Hypoglycemia identified by a continuous glucose monitoring system in a second-trimester pregnant woman with insulinoma: a case report. J Med Case Rep. 2017;11:117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Massironi S, Sciola V, Peracchi M, Ciafardini C, Spampatti MP, Conte D. Neuroendocrine tumors of the gastro-entero-pancreatic system. World J Gastroenterol. 2008;14:5377-5384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 121] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 30. | Besemer B, Müssig K. Insulinoma in pregnancy. Exp Clin Endocrinol Diabetes. 2010;118:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Troisi R, Bjørge T, Gissler M, Grotmol T, Kitahara CM, Myrtveit Sæther SM, Ording AG, Sköld C, Sørensen HT, Trabert B, Glimelius I. The Role of Pregnancy, Perinatal Factors and Hormones in Maternal Cancer Risk: a review of the evidence. J Intern Med. 2018;283:430-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 32. | Goldberg-Stein S, Liu B, Hahn PF, Lee SI. Body CT during pregnancy: utilization trends, examination indications, and fetal radiation doses. AJR Am J Roentgenol. 2011;196:146-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Spencer JA, Tomlinson AJ, Weston MJ, Lloyd SN. Early report: comparison of breath-hold MR excretory urography, Doppler ultrasound and isotope renography in evaluation of symptomatic hydronephrosis in pregnancy. Clin Radiol. 2000;55:446-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Ilhan H, Fendler WP, Cyran CC, Spitzweg C, Auernhammer CJ, Gildehaus FJ, Bartenstein P, Angele MK, Haug AR. Impact of (68)Ga-DOTATATE PET/CT on the surgical management of primary neuroendocrine tumors of the pancreas or ileum. Ann Surg Oncol. 2015;22:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Zanotti-Fregonara P, Jan S, Taieb D, Cammilleri S, Trébossen R, Hindié E, Mundler O. Absorbed 18F-FDG dose to the fetus during early pregnancy. J Nucl Med. 2010;51:803-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Panzuto F, Severi C, Cannizzaro R, Falconi M, Angeletti S, Pasquali A, Corleto VD, Annibale B, Buonadonna A, Pederzoli P, Delle Fave G. Utility of combined use of plasma levels of chromogranin A and pancreatic polypeptide in the diagnosis of gastrointestinal and pancreatic endocrine tumors. J Endocrinol Invest. 2004;27:6-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 79] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Predescu D. Pancreatic Neuroendocrine Tumour in Pregnancy - Diagnosis and Treatment Management. Chirurgia (Bucur). 2019;114:550-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Turaga KK, Kvols LK. Recent progress in the understanding, diagnosis, and treatment of gastroenteropancreatic neuroendocrine tumors. CA Cancer J Clin. 2011;61:113-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 39. | Starke A, Saddig C, Mansfeld L, Koester R, Tschahargane C, Czygan P, Goretzki P. Malignant metastatic insulinoma-postoperative treatment and follow-up. World J Surg. 2005;29:789-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Fjallskog ML, Janson ET, Falkmer UG, Vatn MH, Oberg KE, Eriksson BK. Treatment with combined streptozotocin and liposomal doxorubicin in metastatic endocrine pancreatic tumors. Neuroendocrinology. 2008;88:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 41. | Leslie KK, Koil C, Rayburn WF. Chemotherapeutic drugs in pregnancy. Obstet Gynecol Clin North Am. 2005;32:627-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 42. | Vogl TJ, Naguib NN, Zangos S, Eichler K, Hedayati A, Nour-Eldin NE. Liver metastases of neuroendocrine carcinomas: interventional treatment via transarterial embolization, chemoembolization and thermal ablation. Eur J Radiol. 2009;72:517-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 118] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 43. | Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A, Chen JS, Hörsch D, Hammel P, Wiedenmann B, Van Cutsem E, Patyna S, Lu DR, Blanckmeister C, Chao R, Ruszniewski P. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2032] [Cited by in RCA: 1828] [Article Influence: 130.6] [Reference Citation Analysis (0)] |
| 44. | Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG, Tomassetti P, Pavel ME, Hoosen S, Haas T, Lincy J, Lebwohl D, Öberg K; RAD001 in Advanced Neuroendocrine Tumors; Third Trial (RADIANT-3) Study Group. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2039] [Cited by in RCA: 2115] [Article Influence: 151.1] [Reference Citation Analysis (0)] |
| 45. | Pavel ME, Baudin E, Öberg KE, Hainsworth JD, Voi M, Rouyrre N, Peeters M, Gross DJ, Yao JC. Efficacy of everolimus plus octreotide LAR in patients with advanced neuroendocrine tumor and carcinoid syndrome: final overall survival from the randomized, placebo-controlled phase 3 RADIANT-2 study. Ann Oncol. 2017;28:1569-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |