Published online Jun 16, 2021. doi: 10.12998/wjcc.v9.i17.4310
Peer-review started: January 10, 2021
First decision: February 11, 2021
Revised: February 21, 2021
Accepted: April 2, 2021
Article in press: April 2, 2021
Published online: June 16, 2021
Processing time: 135 Days and 19.8 Hours
Sodium valproate is widely used in the treatment of epilepsy in clinical practice. Most adverse reactions to sodium valproate are mild and reversible, while serious idiosyncratic side effects are becoming apparent, particularly hepatotoxicity. Herein, we report a case of fatal acute liver failure (ALF) with thrombotic microangiopathy (TMA) caused by treatment with sodium valproate in a patient following surgery for meningioma.
A 42-year-old man who received antiepileptic treatment with sodium valproate after surgery for meningioma exhibited extreme fatigue, severe jaundice accompanied by oliguria, soy sauce-colored urine, and ecchymosis. His postoperative laboratory values indicated a rapid decreased platelet count and hemoglobin level, severe liver and kidney dysfunction, and disturbance of the coagulation system. He was diagnosed with drug-induced liver failure combined with TMA. After plasma exchange combined with hemoperfusion, pulse therapy with high-dose methylprednisolone, and blood transfusion, his liver function deteriorated, and finally, he died.
ALF with TMA is a rare and fatal adverse reaction of sodium valproate which needs to be highly valued.
Core Tip: Sodium valproate is widely used in the treatment of epilepsy in clinical practice although it has potential hepatotoxicity. Herein, we report a case of fatal acute liver failure (ALF) with thrombotic microangiopathy (TMA) caused by sodium valproate treatment. A history of chronic hepatitis B virus infection or combination therapy with sodium valproate and carbapenem may increase the risk of ALF. The combination therapy of plasma exchange, glucocorticoid, and supportive therapy is essential for TMA. Organ transplantation at the early stage of the disease may be the first choice for critically ill patients. Our case report can facilitate further studies on the diagnosis and therapy of ALF with TMA.
- Citation: Mei X, Wu HC, Ruan M, Cai LR. Acute liver failure with thrombotic microangiopathy due to sodium valproate toxicity: A case report. World J Clin Cases 2021; 9(17): 4310-4317
- URL: https://www.wjgnet.com/2307-8960/full/v9/i17/4310.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i17.4310
Sodium valproate is one of the common fatty-acid antiepileptic drugs in the current daily clinical routine[1]. It is effective in the treatment of various types of epilepsy to different extents. With extensive application in the clinic, an increasing number of adverse reactions of sodium valproate have been reported[2,3]. Adverse reactions involve multiple organ systems, such as the hematological system, nervous system, and digestive system[4]. Sodium valproate has been the most common drug to induce liver injury among all antiepileptic drugs in recent years[5]. The proportion of patients with hepatic dysfunction caused by sodium valproate has been reported to be as high as 5%-10%[3]. Symptoms in most patients are temporary and reversible, while a minority can be fatal, and some patients can develop drug-induced thrombotic microangiopathy (DI-TMA)[6]. We report a case of sodium valproate-induced acute liver failure (ALF) with thrombotic microangiopathy (TMA), which has not been reported previously.
A 42-year-old male was admitted to the Department of Hepatobiliary Internal Medicine 10 d following surgery for meningioma with fatigue and abdominal distension for 1 d.
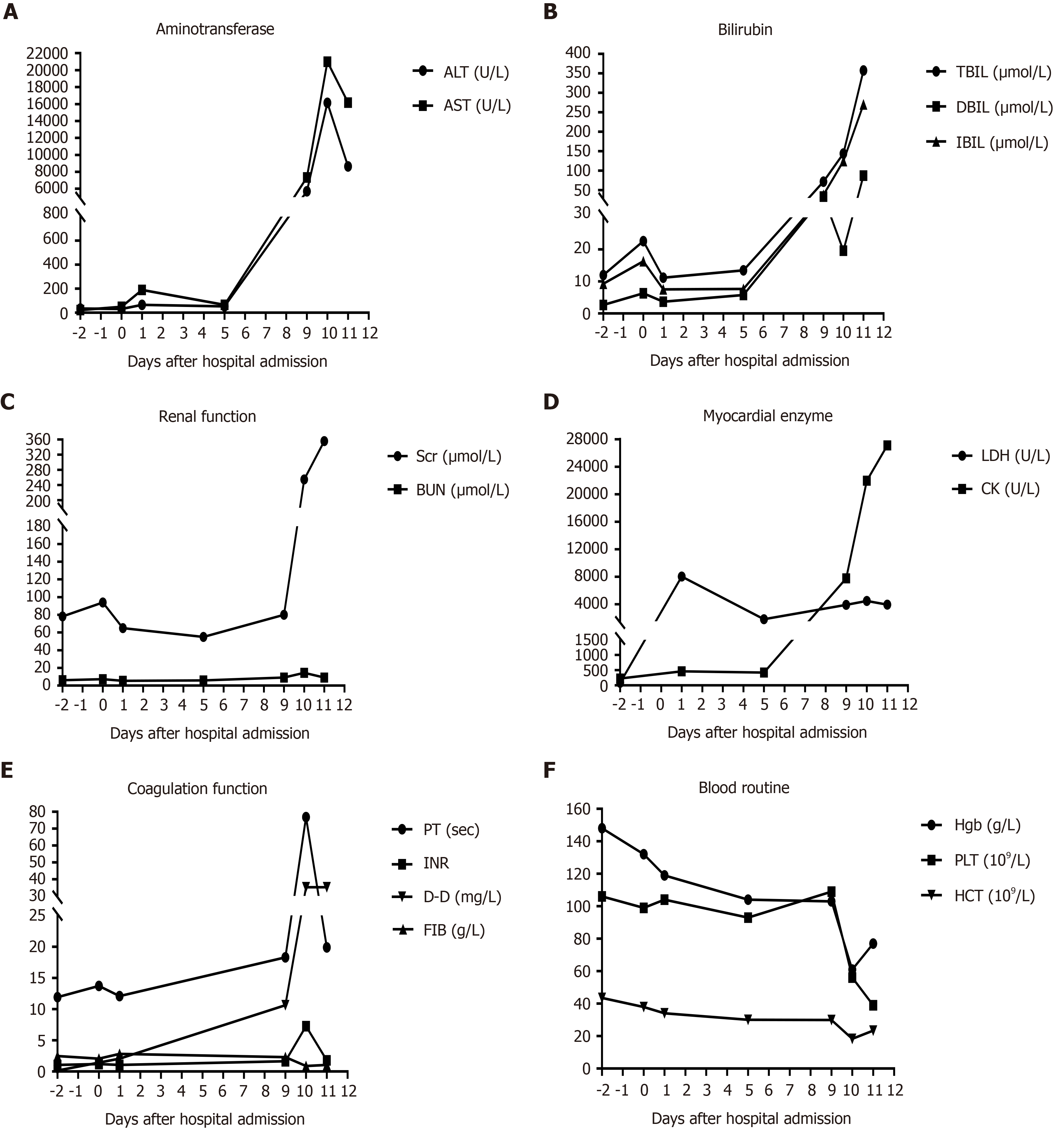
Ten days before admission to the Department of Hepatobiliary Internal Medicine, the patient underwent surgery for atypical meningioma (World Health Organization grade II) resection because of progressive deterioration of vision in the right eye for more than half a year and headache for more than one month. His preoperative hematological parameters were essentially normal: Hemoglobin (Hgb), 132 g/L (130-175 g/L); platelet (PLT) count 99 × 109/L (125-350 × 109/L); alanine aminotransferase (ALT), 34.7 U/L (7-50 U/L); aspartate aminotransferase (AST), 22.5 U/L (13-40 U/L); total bilirubin (TBIL), 12 µmol/L (< 21 mmol/L); direct bilirubin (DBIL), 2.7 µmol/L (< 5 mmol/L); indirect bilirubin (IBIL), 9.3 µmol/L (< 16 mmol/L); creatine kinase (CK), 86 U/L (26-174 U/L); lactate dehydrogenase (LDH), 226 U/L (109-245 U/L); serum creatinine (Scr), 94 µmol/L (53-115 mmol /L); prothrombin time (PT), 11.9 s (9.8-12.1s); international normalized ratio (INR), 1.05 (0.82-1.15); D-dimer (D-D), 0.14 mg/L (< 0.5 mg/L); fibrinogen (FIB), 2.48 g/L (2-4 g/L). Preoperative abdominal color Doppler ultrasound showed that his other organs were normal except a mildly coarse hepatic parenchymal echotexture. Cranial contrast-enhanced magnetic resonance imaging indicated the right sphenoid ridge meningioma (6.6 cm × 5.5 cm). After the surgery, sodium valproate 1.2 g per day was used to treat secondary epilepsy. On postopera
On postoperative day 9, the patient began to experience restlessness and progressive abdominal distension. His hematological parameters were as follows: Hgb, 103 g/L; PLT count, 109 × 109/L; ALT, 5713.8 U/L; AST, 7329.5 U/L; TBIL, 71.7 µmol/L; DBIL, 33.6 µmol/L; IBIL, 38.1 µmol/L; CK, 3912 U/L; LDH, 7744 U/L; Scr, 80 µmol/L; PT, 18.3 s; INR, 1.63; and D-D, 10.63 mg/L. Then, he received treatment for liver protection, gastrointestinal motility promotion, and enema; however, he did not recover from aggravated abdominal distension and gradually developed sleepiness, fatigue, oliguria, soy sauce-colored urine, and jaundice. Therefore, sodium valproate was discontinued. and he was transferred to the Department of Hepatobiliary Internal Medicine for further treatment on postoperative day 10.
The patient was diagnosed with hepatitis B surface antigen (HBsAg) positivity without antiviral treatment for 3 years due to continuous normal hepatic function, and hepatitis B virus (HBV) DNA was < 500 IU/mL (< 500 IU/mL) during periodic re-examinations.
The patient did not have a history of smoking or alcoholism or a remarkable family medical history.
The patient was in a somnolent state with myoclonic jerks in the upper limbs. His skin and sclera were severely yellow. Bilateral petechia and ecchymosis were noted both in the lower limbs and near the injection and puncture sites. His abdomen was distended, with tympanic percussion sounds.
On postoperative day 10, the patient’s laboratory data showed that his Hgb was 61 g/L, hematocrit was 18.3% (40%-50%), PLT count was 56 × 109/L, D-D exceeded 35.2 mg/L, PT was 76.9 s, INR was 7.25, and FIB was 0.88 g/L. His reticulocyte percentage was up to 4.5% (0.5%-1.5%). Although the urinalysis revealed that his urine was positive for occult blood and urobilinogen, there were no fragmented erythrocytes on the peripheral blood smear. His ALT was 16144.0 U/L, AST exceeded 21000.0 U/L, CK was 4471.0 U/L, LDH was 21962.0 U/L, TBIL was 144.1 µmol/L, DBIL was 19.6 µmol/L, IBIL was 124.5 µmol/L, and Scr was 255.0 µmol/L. HBV DNA, ceruloplasmin, and autoimmune antibodies in his serum were all negative. The hematological indices of the patient during hospitalization are shown in Figure 2.
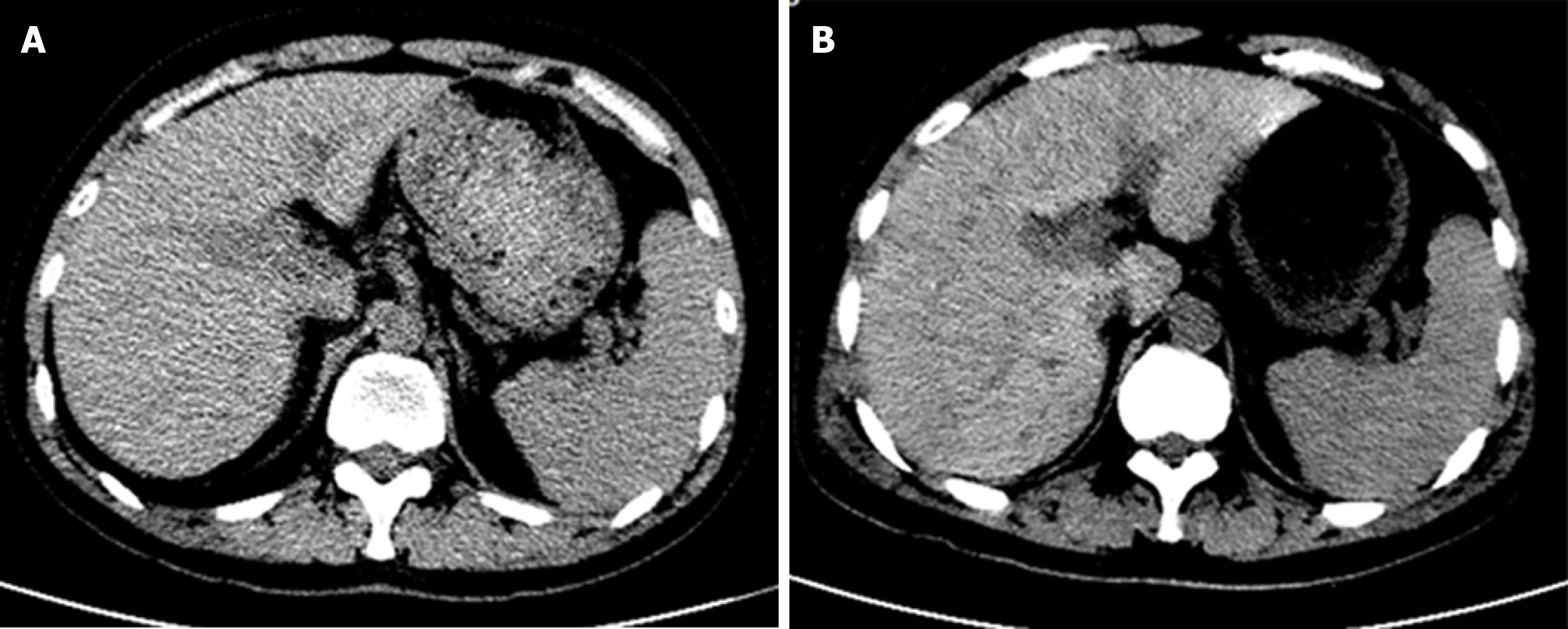
The morphology and density of the liver were normal on preoperative day 5. On postoperative day 9, geographical patterns of low-density shadow in the liver were demonstrated on computed tomography (CT), which suggested extensive hepatic necrosis. No noticeable abnormality was detected in the kidneys and spleen, but a small amount of pelvic and peritoneal effusions. The abdominal CT of the patient during hospitalization is shown in Figure 3.
The patient was diagnosed with drug-induced liver injury (DILI) (hepatocellular type, acute, RUCAM score 6 (probable), level 5 severity of liver injury) complicated with TMA.
The patient was placed on the active waiting list for liver transplantation as soon as the diagnosis was made. Sequential plasma exchange (PE) with fresh frozen plasma and hemoperfusion were initiated immediately after admission. After the above therapy, the patient was still anuric, and continuous renal replacement therapy was performed. He underwent treatment with methylprednisolone 80 mg per day for 2 d on the basis of PE. Meanwhile, he was given human prothrombin complex and washed red blood cells to improve coagulation function and anemia. Polyene phosphatidylcholine, glutathione, ademetionine 1,4-butanedisulfonate, and ornithine aspartate were used to promote hepatic recovery.
After treatment, the patient’s hemolysis was controlled, the color of his separated plasma gradually changed from red-brown to dark yellow, and the hemoglobin level did not decline. However, he was still in a persistent anuric and coma state, and there were no suitable liver or kidney sources for transplantation. Finally, the patient died in the early morning of postoperative day 12.
On the 9th postoperative day for meningioma, the patient developed ALF with extreme fatigue, severe jaundice, prolonged prothrombin time, and acute progressive hepatic coma. Although he was an HBsAg carrier, the HBV DNA in his serum was negative; thus, the possibility of liver injury caused by HBV reactivation can be excluded. In addition, the patient’s history suggested no trauma, exertion, hyperthermia, or infections, which are common causes for liver failure, and his symptoms did not include myalgia, which is typical in rhabdomyolysis. Although he was on several medications during hospital stay, the major adverse effects did not include rhabdomyolysis. Therefore, the liver failure was not considered to be associated with rhabdomyolysis. Because his alcoholism history and hematological indices of autoimmune diseases were negative, we finally considered his ALF to be attributed to the drug.
Sodium valproate, vancomycin, and linezolid are the common drugs associated with DILI[5,7,8]. Among all the adverse reactions of vancomycin, most are rapid onset, and renal injury occurs more frequently than liver injury[9]. Common adverse reactions of linezolid include myelotoxicity and peripheral and optic neuropathy[10]. Studies show that liver injury induced by linezolid usually occurs after two weeks of medication, with a mild elevation of transaminase[11]. The characteristics of our patient who developed severe liver dysfunction after using linezolid for only 5 d were inconsistent with those reported in the literature. Liver injury is one of the most often reported adverse effects of sodium valproate because sodium valproate is metabolized by glucuronidation and mitochondrial beta-oxidation in the liver[5]. ALF due to sodium valproate can still be encountered in the clinic[12]. The RUCAM scoring table is recognized as the primary DILI causality assessment tool[13]. The RUCAM score of this patient was 6 (probable) when we completed the causality assessment for sodium valproate. Thus, we considered that his liver injury was induced by sodium valproate.
Sodium valproate, as one of the most widely used broad-spectrum antiepileptic drugs, has been used as a first-line treatment in clinical practice[1]. It has the characteristics of high bioavailability, good tolerability, and remarkable efficacy except for a narrow therapeutic window[14]. A black box warning of severe hepatotoxicity with sodium valproate was published by the Food and Drug Administration[15]. The pathogenesis of the toxic effects of sodium valproate on the liver has not yet been fully elucidated. The obstacles of cytochrome P450 metabolism and β-oxidation in mitochondria are generally considered the major mechanisms of the hepatotoxicity of sodium valproate, while the former is an important factor for individual differences in liver injury. Studies have shown that risk factors for fatal liver failure caused by sodium valproate include age younger than 2 years, combination therapy with sodium valproate and other antiepileptic drugs, pregnancy, a history of liver diseases, and other neurological diseases[16,17]. It is controversial whether the liver injury induced by sodium valproate is related to the plasma concentration of sodium valproate. The research has shown that patients with high plasma concentrations of sodium valproate are more susceptible to liver injury than those with low plasma concentrations[18]. However, Ghozzi et al[19] supposed that liver injury is independent of the sodium valproate plasma concentration. However, there is currently no research on the correlation between HBV infection and liver injury induced by sodium valproate. Therefore, whether the fatal liver failure induced by sodium valproate in our patient is related to HBV infection needs further research.
Except for ALF, TMA should be considered in diagnosis. Generally, microangiopathic hemolytic anemia is a sine qua non for the diagnosis of TMA[20]. This patient exhibited anuria, hemoglobinuria, progressively decreased levels of hemoglobin and platelets, elevated proportion of reticulocytes, and significantly elevated bilirubin and D-D level during the early stage of his disease, which were consistent with the characteristics of TMA. However, no fragmented erythrocytes were observed on the peripheral blood smear either before or after PE. Cases of TMA without the presence of fragmented erythrocytes on the peripheral blood smear have been reported previously[21,22]. This patient responded to PE and methylprednisolone. Therefore, a diagnosis of TMA was suggested. As far as we are concerned, the presence of fragmented erythrocytes is essential for TMA diagnosis in most cases, but not all.
TMA is defined as a clinical syndrome characterized by thrombocytopenia, hemolytic anemia, and multiple organ dysfunction[21]. The microthrombosis of capillaries and arterioles is the typical pathological feature of TMA. Thrombotic thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS) are the two clinical presentation forms of TMA. TTP mostly involves the nervous system, while there is a preponderance of renal injury in HUS patients. The etiology of TMA is multifactorial, including genetic factors and acquired risk factors. DI-TMA is a type of acquired TMA that is caused by multiple drugs, such as antitumor agents, antiplatelet drugs, and oral contraceptives. Studies show that DI-TMA occurs via two main mechanisms: Immune-mediated reactions or dose-dependent toxicity[23]. TMA mediated by dose-dependent reactions has a slow, progressive onset, while immune-mediated TMA has a rapid onset[21]. Our patient can be classified as having immune-mediated DI-TMA.
For the patient, it was inferred that sodium valproate plus carbapenem antibiotics might increase the risk of hemolysis, which could cause TMA. However, the period of the combination was short. König et al[24] showed that sodium valproate altered the exposure of immunoglobulin receptor and fatty acid content in the erythrocyte membrane. Alteration of the membrane fluidity and receptor proteins on the membrane would facilitate immunoglobulin to destruct erythrocytes. The correlation between destruction of erythrocytes and plasma level of sodium valproate remains unclear. Carbapenem antibiotics can reduce the plasma level of sodium valproate by inhibiting multidrug resistance-associated proteins, which can efflux sodium valproate back to the plasma from erythrocytes and result in an increased erythrocyte distribution of sodium valproate[25]. Therefore, it would be of great significance to study the effects of interaction between carbapenem antibiotics and sodium valproate on hemolysis.
In summary, this is the first case report of ALF with TMA caused by sodium valproate, which is notable. However, there were some limitations to our study. Liver pathology was unavailable. Without results regarding ADAMTS13 activities or anti-ADAMTS13 antibodies, we found it difficult to distinguish between HUS and TTP in this patient. Establishing an animal model can facilitate further studies on pathogenesis and pathophysiological state.
ALF with TMA, which has not been reported before, is a fatal complication caused by sodium valproate. It is a disease with sudden onset and rapid progression, which needs great attention. A history of chronic HBV infection or combination therapy with sodium valproate and carbapenem may increase the risk of ALF. The combination therapy of PE, hemoperfusion, glucocorticoid, and supportive therapy is essential for TMA. However, it is not effective for all. For critically ill patients, organ transplan
The authors would like to thank all researchers and study participants for their contributions.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): E
P-Reviewer: Abe Y, Fahrner R, Maslennikov R, Paramesh AS, Skok P S-Editor: Liu M L-Editor: Wang TQ P-Editor: Li JH
| 1. | Hanaya R, Arita K. The New Antiepileptic Drugs: Their Neuropharmacology and Clinical Indications. Neurol Med Chir (Tokyo). 2016;56:205-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 2. | Yamazaki S, Watanabe T, Sato S, Yoshikawa H. Outcome of renal proximal tubular dysfunction with Fanconi syndrome caused by sodium valproate. Pediatr Int. 2016;58:1023-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Nanau RM, Neuman MG. Adverse drug reactions induced by valproic acid. Clin Biochem. 2013;46:1323-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 214] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 4. | Romoli M, Mazzocchetti P, D'Alonzo R, Siliquini S, Rinaldi VE, Verrotti A, Calabresi P, Costa C. Valproic Acid and Epilepsy: From Molecular Mechanisms to Clinical Evidences. Curr Neuropharmacol. 2019;17:926-946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 221] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 5. | Guo HL, Jing X, Sun JY, Hu YH, Xu ZJ, Ni MM, Chen F, Lu XP, Qiu JC, Wang T. Valproic Acid and the Liver Injury in Patients with Epilepsy: An Update. Curr Pharm Des. 2019;25:343-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 6. | Hebert SA, Bohan TP, Erikson CL, Swinford RD. Thrombotic microangiopathy associated with Valproic acid toxicity. BMC Nephrol. 2017;18:262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Bruniera FR, Ferreira FM, Savioli LR, Bacci MR, Feder D, Pereira EC, Pedreira ML, Peterlini MA, Perazzo FF, Azzalis LA, Rosa PC, Junqueira VB, Sato MA, Fonseca FL. Endothelial, renal and hepatic variables in Wistar rats treated with Vancomycin. An Acad Bras Cienc. 2014;86:1963-1972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | De Bus L, Depuydt P, Libbrecht L, Vandekerckhove L, Nollet J, Benoit D, Vogelaers D, Van Vlierberghe H. Severe drug-induced liver injury associated with prolonged use of linezolid. J Med Toxicol. 2010;6:322-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Peng Y, Li CY, Yang ZL, Shi W. Adverse reactions of vancomycin in humans: A protocol for meta-analysis. Medicine (Baltimore). 2020;99:e22376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Vinh DC, Rubinstein E. Linezolid: a review of safety and tolerability. J Infect. 2009;59 Suppl 1:S59-S74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 161] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 11. | Bayram N, Düzgöl M, Kara A, Özdemir FM, Devrim İ. Linezolid-related adverse effects in clinical practice in children. Arch Argent Pediatr. 2017;115:470-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Bassett JT, Rodriguez B, Mulligan L, Fontana RJ. Acute liver failure in a military recruit treated with valproic acid and harboring a previously unrecognized POLG-1 mutation. Epilepsy Behav Rep. 2019;12:100342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Danan G, Teschke R. RUCAM in Drug and Herb Induced Liver Injury: The Update. Int J Mol Sci. 2015;17:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 480] [Cited by in RCA: 511] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 14. | Trinka E, Höfler J, Zerbs A, Brigo F. Efficacy and safety of intravenous valproate for status epilepticus: a systematic review. CNS Drugs. 2014;28:623-639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | U.S. National Library of Medcine. Valproic acid capsule drug label information, 2015 [cited 8 June 2020]. Datebase: DailyMed [Internet]. Available from: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c628829c-74de-485a-b6cb-42e1da894376. |
| 16. | Koenig SA, Buesing D, Longin E, Oehring R, Häussermann P, Kluger G, Lindmayer F, Hanusch R, Degen I, Kuhn H, Samii K, Jungck A, Brückner R, Seitz R, Boxtermann W, Weber Y, Knapp R, Richard HH, Weidner B, Kasper JM, Haensch CA, Fitzek S, Hartmann M, Borusiak P, Müller-Deile A, Degenhardt V, Korenke GC, Hoppen T, Specht U, Gerstner T. Valproic acid-induced hepatopathy: nine new fatalities in Germany from 1994 to 2003. Epilepsia. 2006;47:2027-2031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Schmid MM, Freudenmann RW, Keller F, Connemann BJ, Hiemke C, Gahr M, Kratzer W, Fuchs M, Schönfeldt-Lecuona C. Non-fatal and fatal liver failure associated with valproic acid. Pharmacopsychiatry. 2013;46:63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Schulpis KH, Karikas GA, Tjamouranis J, Regoutas S, Tsakiris S. Low serum biotinidase activity in children with valproic acid monotherapy. Epilepsia. 2001;42:1359-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Ghozzi H, Hakim A, Sahnoun Z, Ben Mahmoud L, Atheymen R, Hammami S, Zeghal K. [Relationship between plasma concentrations of valproic acid and hepatotoxicity in patients receiving high doses]. Rev Neurol (Paris). 2011;167:600-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371:654-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 777] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 21. | Wirtschafter E, VanBeek C, Linhares Y. Bone marrow transplant-associated thrombotic microangiopathy without peripheral blood schistocytes: a case report and review of the literature. Exp Hematol Oncol. 2018;7:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Daram SR, Philipneri M, Puri N, Bastani B. Thrombotic thrombocytopenic purpura without schistocytes on the peripheral blood smear. South Med J. 2005;98:392-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Chatzikonstantinou T, Gavriilaki M, Anagnostopoulos A, Gavriilaki E. An Update in Drug-Induced Thrombotic Microangiopathy. Front Med (Lausanne). 2020;7:212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 24. | König SA, Knolle J, Friedewald S, Koelfen W, Longin E, Lenz T, Hannak D. Effects of valproic acid, carbamazepine, and phenobarbitone on the fatty acid composition of erythrocyte membranes in children. Epilepsia. 2003;44:708-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Ogawa K, Yumoto R, Hamada N, Nagai J, Takano M. Interaction of valproic acid and carbapenem antibiotics with multidrug resistance-associated proteins in rat erythrocyte membranes. Epilepsy Res. 2006;71:76-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |