Published online Jun 16, 2021. doi: 10.12998/wjcc.v9.i17.4268
Peer-review started: December 31, 2020
First decision: February 25, 2021
Revised: March 15, 2021
Accepted: March 29, 2021
Article in press: March 29, 2021
Published online: June 16, 2021
Processing time: 145 Days and 17.6 Hours
Anaplastic lymphoma kinase-positive (ALK+) large B-cell lymphoma (LBCL) is a rare type of lymphoma with high invasiveness and rapid progression. It occurs in all age groups, but is extremely rare in children. The lesions mainly involve the lymph nodes and may present with extra-nodal involvement. Response to conventional chemotherapies and local radiotherapy is poor, with a 5-year overall survival of less than 40%. Recently, the use of ALK inhibitors for the treatment of this disease has been reported.
We present a case of a 12-year-old boy diagnosed with ALK+LBCL. The patient had a 2-mo medical history of a calvarial mass, extensive systemic involvement, and positive bone marrow clathrin heavy chain (CLTC)-ALK fusion gene. Complete remission 1 (CR1) was achieved using the modified LMB89 Group C regimen followed by autologous stem cell transplantation. The patient relapsed 3 mo later. He then achieved CR2 with three short courses of chemotherapy (COP, reduced-dose ICE, low-dose Ara-c+VP16) and continuous alectinib targeted therapy. Afterward, allogeneic hematopoietic stem cell transplantation (allo-HSCT) was performed. At 16 mo after the allo-HSCT, the patient was still in CR2.
The modified LMB89 Group C regimen and ALK inhibitors are effective. Allo-HSCT should be performed after remission.
Core Tip: Anaplastic lymphoma kinase-positive (ALK+) large B-cell lymphoma (LBCL) is a rare subtype of diffuse LBCL and is particularly rare in pediatric non-Hodgkin lymphoma patients. The modified LMB89 Group C regimen is effective for ALK+LBCL. Since bone marrow failure occurs after relapse, the patient cannot tolerate intensive chemotherapy; thus, ALK inhibitors combined with low-dose chemotherapy could be considered, and there is still hope for complete remission 2. It is advised that allogeneic hematopoietic stem cell transplantation be performed as soon as possible after remission.
- Citation: Zhang M, Jin L, Duan YL, Yang J, Huang S, Jin M, Zhu GH, Gao C, Liu Y, Zhang N, Zhou CJ, Gao ZF, Zheng QL, Chen D, Zhang YH. Diagnosis and treatment of pediatric anaplastic lymphoma kinase-positive large B-cell lymphoma: A case report. World J Clin Cases 2021; 9(17): 4268-4278
- URL: https://www.wjgnet.com/2307-8960/full/v9/i17/4268.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i17.4268
Anaplastic lymphoma kinase-positive (ALK+) large B-cell lymphoma (LBCL) was first described by Delsol et al[1] in 1997. Since then, nearly 140 cases have been reported[2-11]. In the 2016 World Health Organization Classification of Lymphoid Neoplasms, ALK+LBCL was considered to be a distinct entity of mature B-cell neoplasms[12]. They are exceptionally rare and account for less than 1% of diffuse LBCL (DLBCL)[2-6]. The disease has been reported in both children and adults, with a male to female prevalence ratio of 3.5:1[2]. It commonly presents with high-stage nodal disease, but extra-nodal sites can also be involved[2-6]. Typically, neoplastic cells show a t(2;17)(p23;q23) resulting in a clathrin heavy chain (CLTC)-ALK fusion product. Rare cases have been associated with t(2;5)(p23;q35) or nucleophosmin/ALK, as described in ALK-positive anaplastic large cell lymphoma (ALCL)[4,13,14]. Unfortunately, the tumor frequently demonstrates an aggressive clinical behavior, with a high relapse rate and poor response to CHOP and CHOP-like regimens[2,5,15]. Recent studies have suggested that ALK inhibitors may be effective for the treatment of ALK + LBCL [11,16-20].
Here, we report the diagnosis and treatment of a rare case of pediatric ALK+LBCL, with bone marrow failure at the time of relapse. The patient re-achieved complete remission (CR) after treatment with low-dose chemotherapy and alectinib targeted therapy, followed by allogeneic stem cell transplantation.
This report was approved by Beijing Children’s Hospital Institutional Ethics Committee (IEC-C-006-A03-V.05).
A 12-year-old boy was admitted to our hospital on September 10, 2018 with a chief complaint of calvarial mass for 2 mo and multiple masses around the whole body for more than 1 mo.
At 2 mo before hospital admission, a mass with a diameter of 2 cm on the left parietal was found in the patient, without local swelling, heat, or pain. The mass was considered a “scalp cyst” by the local hospital and was surgically resected without a pathological diagnosis. The masses resurfaced on the scalp on day 7 after surgery and quickly involved the whole body within 4 wk. The masses were hard and in progressive enlargement. The patient suffered fever, low back pain, sensory and motor dysfunction in the lower limbs, left eyeball protrusion, poor appetite and weight loss of 4 kg (13.3%). Ceftizoxime (1.3 g, intravenous infusion every 12 h [Q12H]) for anti-infection and mannitol (100 mL, intravenous infusion Q8H) for decreasing intracranial pressure were not effective.
According to the past medical history, the patient was in good health.
He had no family history of hematological diseases or tumors.
Physical examination on admission was as follows: clear mind, weak reaction, appearance of malnutrition, painful expression, and passive position. The superficial lymph nodes around the whole body appeared a multiple, enlarged, and qualitative hard, with a diameter of 1.5-5 cm. Multiple subcutaneous nodules and masses were observed throughout the body, with sizes of 1-3 cm in diameter and tough/hard in texture. The left maxillofacial area was swollen with exophthalmos of the left eyeball. Cardiopulmonary and abdominal examinations showed no abnormalities. Muscle tension in both lower limbs was reduced with a muscle strength grade of 0. Abdominal reflexes and cremasteric reflexes still exist, but bilateral patellar tendon and Achilles tendon reflex are absent. Tests results were positive for Kernig sign, bilateral Babinski sign and Chaddock sign; and negative for Brudzinski’s sign.
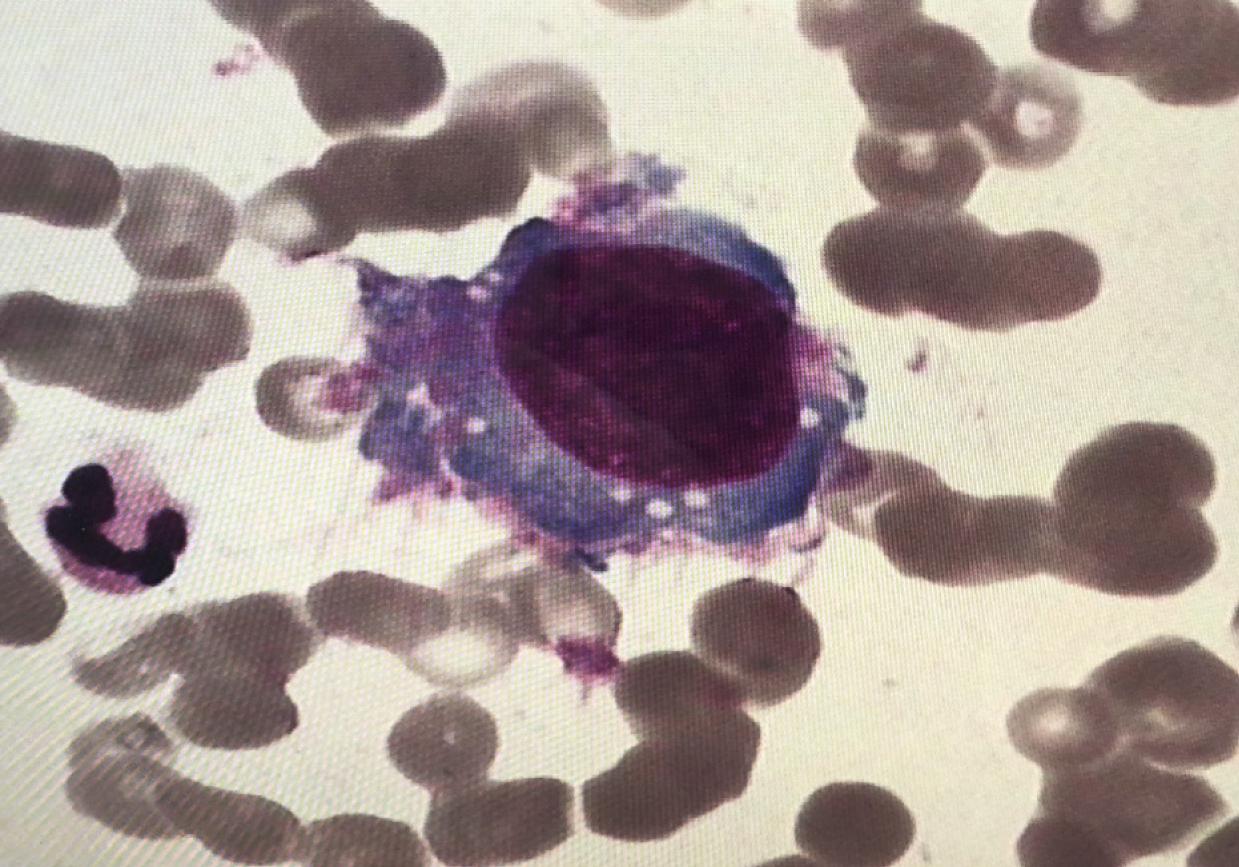
There were normal peripheral white blood cell counts and hemoglobin levels, elevated platelet 822 × 109/L (normal reference range 100-300 × 109/L), significantly elevated lactic dehydrogenase 1345 U/L (normal reference range 110-295 U/L), normal blood coagulation, and test results for hepatitis B, hepatitis C, hepatitis E, Epstein-Barr virus, cytomegalovirus, human immunodeficiency virus were negative. The cerebrospinal fluid was pale yellow and transparent with normal white blood cells, glucose, chloride, and protein levels, and no tumor cells were found. Bone marrow smears indicated 7%-47.5% of tumor cells, with large cell bodies, large amount of gray-blue cytoplasm, and fine nuclear chromatin (Figure 1). Flow cytometry analysis of bone marrow demonstrated that 4.48% of the cells expressed CD4dim but not CD45, CD56, CD8, CD3, CD2, CD7, CD30, cCK, GD2, CD15, cCD3, CD20, cCD79, Ki67, and so on, malignant hematopoietic system cells should be considered.
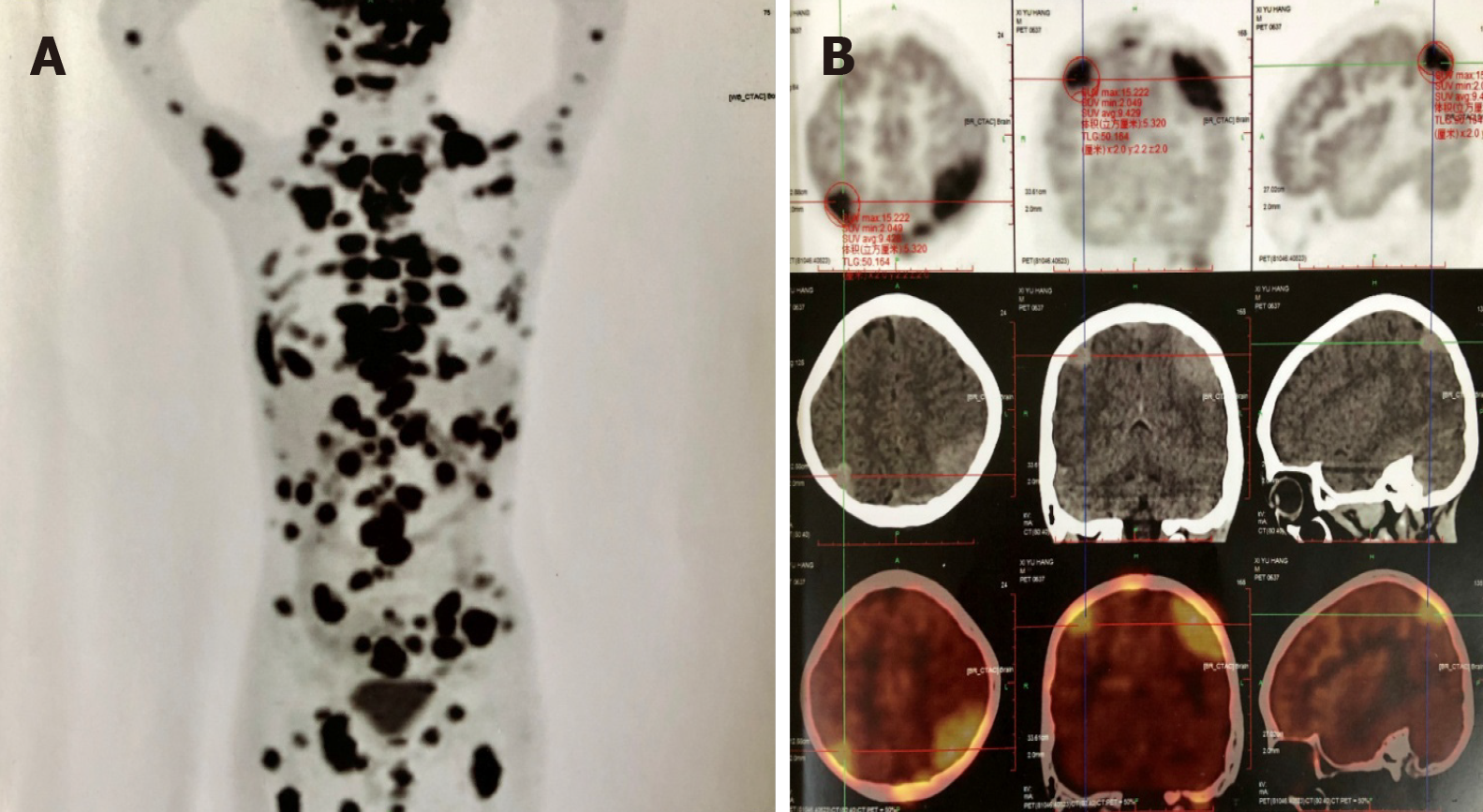
Positron emission tomography/computed tomography revealed multiple enlarged lymph nodes in the neck, mediastinum, abdomen, pelvis, and inguinal region; and nodular lesions with increased level of 18-fluorodeoxyglucose metabolism in the encephalon, left posterior pharyngeal wall, left external rectus muscle, left lateral femur muscles and left kidney (Figure 2A and B).
Bone marrow biopsy revealed the absence of hematopoietic cells, infiltration of diffuse tumor cells, large volume of tumor cells, few lightly stained cytoplasm, some of which were vacuolar, presenting immunoblast-like appearance; round or irregular cell nuclei, light staining, prominent nucleoli; mononuclear and multinuclear tumor cells. Immu
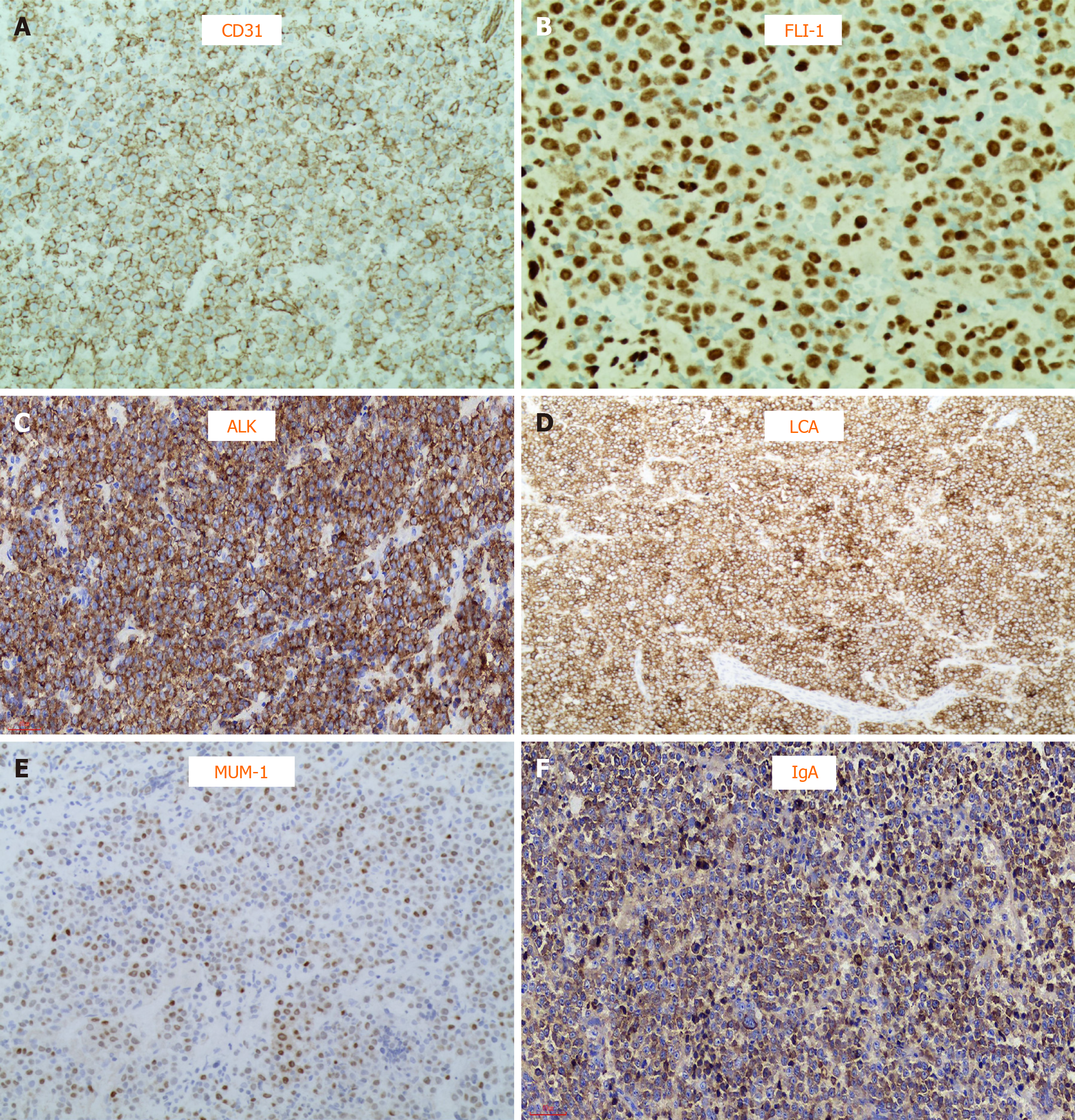
To obtain a definite diagnosis, a neck lymph node biopsy was performed. Microscopic analysis showed the structure of the lymph node was destroyed, and it consisted of single large immunoblast-like cells, with round and light nuclei, large nucleoli and abundant cytoplasm; plasmoblast differentiation appeared; atypical multinucleated tumor giant cells were observed occasionally; presented as intrasinusoidal growth patterns. Immunohistochemistry analysis indicated as follows: T/natural killer cell markers: negative for CD2, CD3, CD43, CD4, CD8, TIA-1, and CD56; B/plasmocyte markers: positive for OCT2, BOB1, CD38 (focal weak), and MUM1; and negative for CD20, CD79a, PAX5, CD138; other immunomarkers: positive for LCA (leukocyte common antigen), ALK (cytoplasm, granular), immunoglobulin A, Ki-67 (> 90%), EMA, CD31, and FLI-1 (Figure 3A-F); negative for CD30, CD163, S-100, CD34, and TDT.
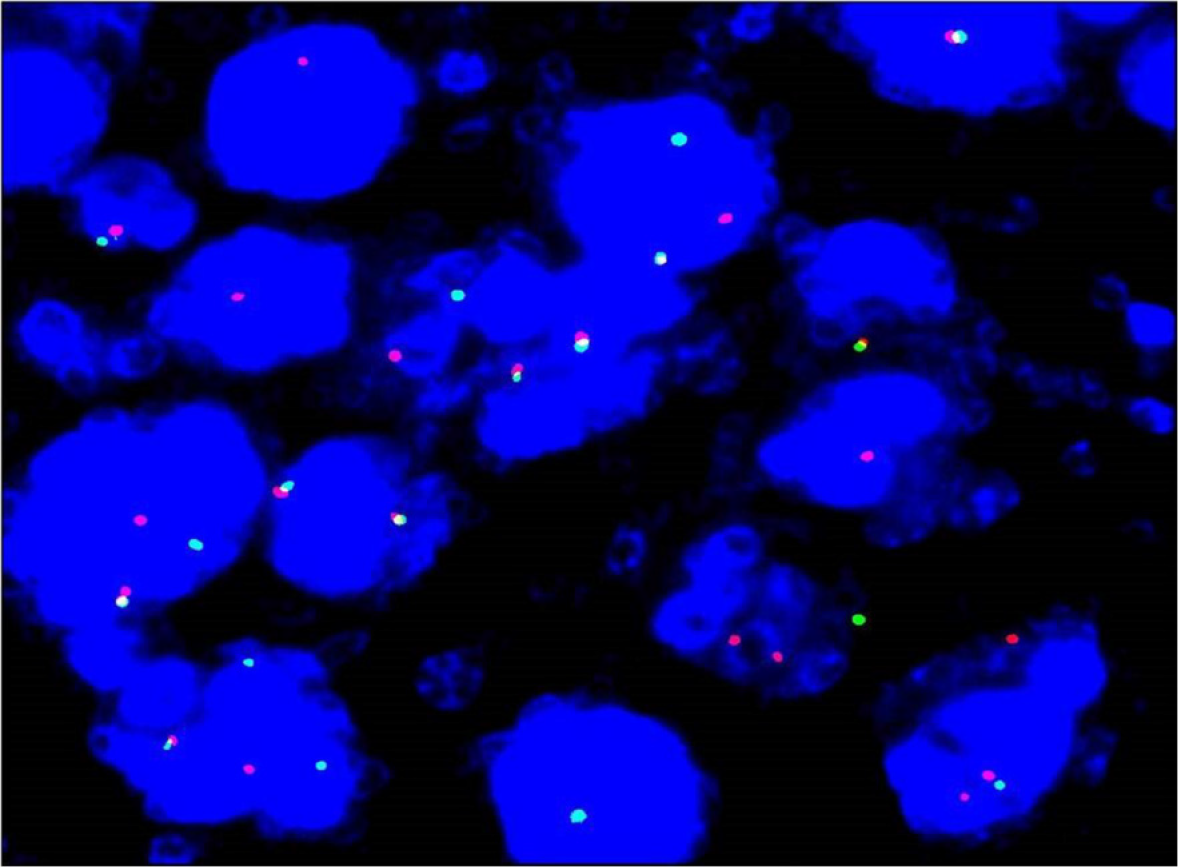
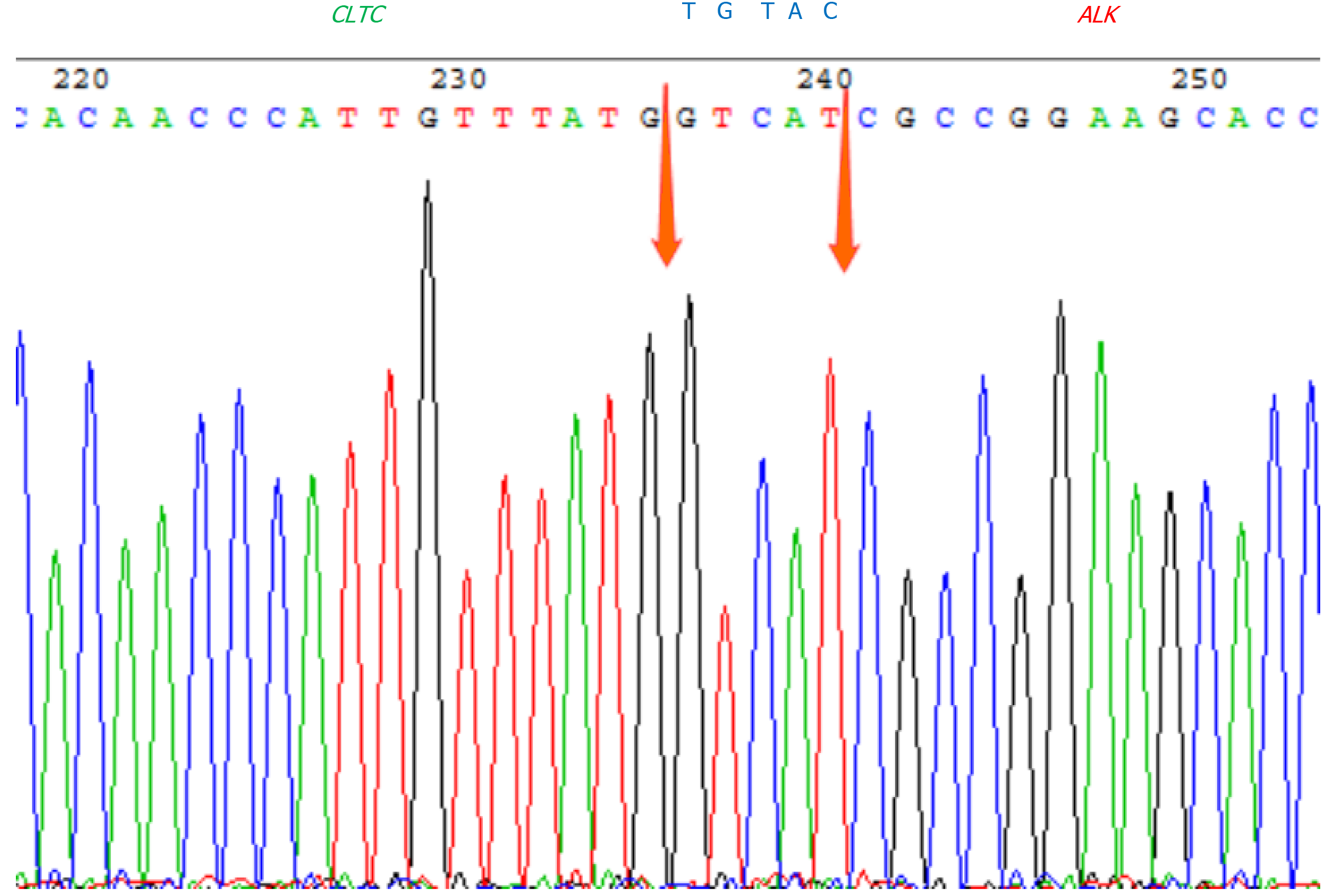
Fluorescence in situ hybridization (FISH) study with ALK break apart probe (Wuhan HealthCare Biotechnology Co., Ltd., Wuhan, China) showed ALK gene disruption (Figure 4) in the cervical lymph node. B-cell clonality assays revealed monoclonal IGH rearrangement. Next-generation sequencing (NGS) high-throughput RNA sequencing was performed for genetic testing of tumor cells in bone marrow, and in combination with Sanger first-generation sequencing, the CLTC-ALK fusion gene in the bone marrow of the patient was observed positive (Figure 5), in addition to the PGS1-CLTC fusion gene.
ALK+LBCL, according to the St Jude Children’s Research Hospital staging system, the disease was stage IV.
Modified LMB89 Group C regimen chemotherapy was used in this patient. COP, COPADM1, COPADM2, CYVE1+HD-MTX, CYVE2, M1, M2 and M3 regimens were administered sequentially. An early evaluation which performed on the 7th d of the COP regimen indicated a 60% of reduction in the main tumor focus, and imaging tests in the mid-term evaluation (prior to CYVE2 regimen) suggested residual lesions in the left testis (0.6 cm × 0.6 cm), the greater omentum of the left iliac fossa (0.6 cm × 0.6 cm), the proximal abdominal wall near the bladder (0.7 cm × 0.3 cm), and the pleura of the left posterior chest wall (1.4 cm × 0.7 cm). Later evaluation (prior to M2 regimen) indicated CR. Autologous stem cell transplantation was successfully performed after the M3 regimen. However, 3 mo after transplantation, the patient suffered weakness and scalp numbness. Physical examinations indicated anemia, scattered bleeding spots on the skin throughout the body, a nodule of 1 cm in diameter in the right occipital region, and 2 nodules of 0.5 cm in diameter in the abdominal wall. The 80.5% of tumor cells were observed in bone marrow smears with morphology consistent with the initial tumor. And 13.17% of abnormal cells were observed in bone marrow samples using flow cytometry. Both forward scatter and side scatter were large, the expression of CD45 showed strong positive, CD31 and CD10 expression were observed, and cCD79a was expressed in some cells. The expression rate of Ki-67 was 59.42%. Immunohistochemistry performed in bone marrow biopsy indicated positive for CD38, BOB1, ALK (serosa), CD138, MUM-1, c-MYC, EMA, LCA, and 80% positive for Ki-67, while negative for CD20, CD3, CD30, MPO, CD79a, PAX-5, CD34, CD43, CD5, CD7, CK (AE1/AE3). In addition, 42.77% of peripheral blood and 293.06% of bone marrow cells were quantitatively positive for the CLTC-ALK fusion gene. The diagnosis of ALK+LBCL recurrence was made. Combined with imaging examinations, the sites of recurrence were the left mandibular branch, bilateral pleura, T11-L3 vertebral body and appendix, intervertebral foramen, epidural soft tissue, abdominal wall, right occipital scalp, pancreas, left testis, and bone marrow. Because of bone marrow failure and concurrent infection, the patient was in poor general condition after recurrence and chemotherapy was difficult, therefore, COP regimen induction therapy was re-administered, combined with ALK targeted drug alectinib (150 mg, oral, Q12H) simultaneously. Evaluation on day 7 of COP regimen showed that the size of the abdominal wall nodules was reduced greater than 50%, while the remaining tumor foci disappeared. Quantitative detection of the CLTC-ALK fusion gene was 0.091% in peripheral blood and 0.32% in the bone marrow. A reduced dose of ICE regimen (ifosfamide 2 g/m2 d1-3, etoposide 100 mg/m2 d1-3, carboplatin 375 mg/m2 d1, cytarabine 30 mg + dexamethasone 4 mg + methotrexate 15 mg intrathecal injection d1, 15) was administered sequentially. CR2 was achieved after this regimen and quantitative detection of the CLTC-ALK fusion gene in peripheral blood and bone marrow turned negative. Low-dose etoposide and cytarabine were administered later and allogeneic hematopoietic stem cell transplantation (allo-HSCT) was then performed.
As of December 31, 2020, the patient was still in CR after allo-HSCT. At present, the patient is being followed up every 3 mo in our specialist lymphoma clinic.
ALK+LBCL is a rare subtype of DLBCL and is particularly rare in pediatric non-Hodgkin lymphoma patients. Tumor cells show immunoblast-like or plasmablast-like morphology and do not express conventional B cell immunohistochemical markers (i.e. CD20, CD79a, and PAX5). Instead, they mostly express two B cell transcription factors, BOB1 and OCT2, with characteristic ALK rearrangements. t(2;17)(p23;q23) chromosomal translocation generates a CLTC-ALK fusion gene resulting in mostly granular staining in the cytoplasm, with several showing patterns in the nucleus and cytoplasm or membrane staining. This indicates the possible existence of new unknown ALK chromosomal translocation patterns[2-6,12]. This disease is extremely rare and has unique pathological morphology and unusual immunophenotypic characteristics. In addition, its characteristics significantly overlap with other hematological and non-hematological tumor characteristics. The large volume of tumor cells may lead to false-negative results in flow cytometry. Hence, it is difficult to diagnose, and there is a necessity with differentiate it from ALK+ALCL, plasmablastic lymphoma, plasmablastic plasmacytoma, primary effusion lymphoma, human herpesvirus 8-positive LBCL, and immunoblastic DLBCL. The patient’s clinical manifestation was atypical, with pathological diagnosis being very complicated. However, it was the complicated process that helped us to figure out the key factors for diagnosis and differential diagnosis. Immunohistochemistry initially performed on the bone marrow biopsy showed only positive expression for the hematopoietic marker CD45, weakly positive for CD10 and CD163, and the absence of common T and B cell marker expression, which indicated the possibility of a myeloid tumor. Although a large number of tumor cells were observed in bone marrow smears, only 4.48% of cells expressed CD4dim were detected by flow cytometry, which may indicate a soft tissue tumor. To further confirm the diagnosis, a neck lymph node biopsy was performed. Conventional T and B cell markers were still absent, however, CD31 and FLI-1 were expressed. Sinusoidal structures could be observed microscopically, hence, angiosarcoma was also considered pathologically. Meanwhile, pathologist consultation considered that ALCL should not be ruled out. ALK staining was indeed positive, hence, the diagnosis of ALK+ALCL was considered. However, the patient’s CD30 expression was negative, and several pathologists disagreed with the diagnosis of ALK+ALCL. Additional immunohistochemical staining demonstrated that LCA was positive, suggesting neoplastic diseases of the lymph system. According to the above analysis, ALK+ lymphoid system tumors accompanied by large tumor cell volume may indicate ALK+LBCL. Based on this, OCT2, BOB1, CD138, and CD38 immunohistochemical staining was performed and indicated positive expression for all these markers. Simultaneously, FISH assays indicated ALK gene disruption, while B-cell clonality assay revealed monoclonal IGH rearrangement. NGS high-throughput RNA sequencing in combination with Sanger 1st generation sequencing determined the presence of CLTC-ALK and PGS1-CLTC fusion genes in the bone marrow. Hence, the final diagnosis was ALK+LBCL. It should be noted that the presence of the PGS1-CLTC fusion gene in this disease was observed for the first time, however, the clinical significance is yet to be deciphered.
Based on the previous literatures, ALK+LBCL can occur in all age groups (9-72-years-old, median age 35-years-old), with a male-to-female prevalence ratio of about 3.5:1. The main manifestation of ALK+LBCL is a painless enlargement of the cervical and mediastinal lymph nodes and possibly accompanied by fever, abdominal pain, low back pain, and so on. The lesions may involve extra-nodal organs, such as the nasopharynx, digestive tract, liver, spleen, pancreas, ovaries, bone, and soft tissues, and in a few cases, it may also involve the bone marrow and central nervous system. Approximately 57% of the patients are in stage III/IV at diagnosis[2-6]. By literature review, the clinical characteristics of 15 children with ALK+LBCL are summarized in Table 1. Compared with previous reports, the clinical manifestations of our patient were atypical. The patient mainly manifested multiple subcutaneous nodules and masses throughout the body, with rapid metastasis to the testicles, bone marrow, and central nervous system. We are the first to demonstrate the PGS1-CLTC fusion gene in this disease, which suggested that the patient may have a specific clinical subtype of ALK+LBCL. The PGS1-CLTC fusion gene may be involved in the rapid and extensive metastasis of tumor cells. However, more clinical cases are needed to demonstrate its clinical significance.
| Case | Ref. | Gender/age in yr | Primary location | Other locations | BM | Stage | Treatment | Follow up in mo | Outcome |
| 1 | Beltran et al[23] (2009) | Female/13 | Left cervical LN | Axillary and mediastinal LN, costal bone | No | IIB | LNH96-2002 | 62 | AWD |
| 2 | De Paepe et al[24] (2003) | Male/10 | Cervical Mass | No | Ⅱ | SFOP-LMB 96 (group B) | 6 | CR | |
| 3 | De Paepe et al[24] (2003) | Female/13 | Cervical LN | Mediastinal mass, spleen, liver | No | Ⅱ | NHL-BFM ALCL99 with ALCL relapse, autologous BMT | 3 | PR.DOD |
| 4 | Delsol et al[1] (1997) | Male/15 | Peripheral LN | No | I | COPAD-Ara-C | 156 | AWD | |
| 5 | Laurent et al[19] (2009) | Male/14 | Nasal tumor | No | IE | CHOP-derived regimens | 5 | AWD | |
| 6 | Laurent et al[19] (2009) | Male/14 | Cervical LN | No | I | CHOP-derived regimens | 13 | AWD | |
| 7 | Laurent et al[19] (2009) | Male/15 | Cervical LN | No | I | CHOP-derived regimens | 180 | AWD | |
| 8 | Gesk et al[25] (2005) | Male/13 | Cervical LN | No | Ⅱ | ALCL99 protocol (standard-risk group) | NA | PR | |
| 9 | Gesk et al[25] (2005) | Female/12 | Mediastinal mass | Cervical LN | No | Ⅱ | Multiagent chemotherapy | greater than 48 | CR |
| 10 | Gesk et al[25] (2005) | Male/16 | Mediastinal mass | Cervical LN, left pleura, chest wall | Yes | IV | Multiagent chemotherapy, BMT | 12 | DOD |
| 11 | Isimbaldi et al[26] (2006) | Female/9 | Left cervical mass | Multiple LNs at diagnosis; Relapsed at the initial site with BM, scalp, liver, spleen, and kidney involvement | NA at diagnosis; Yes, at relapse | NA at diagnosis; IV at relapse | AIEOP LNH 97 protocol; ICE, PVDA | 9 | CR. relapsed; DOD |
| 12 | Bubała et al[27] (2006) | Male/9 | Cervical, axillary, and supraclavicular LN and supraclavicular lymph gland | Mediastinal mass, LN along the aorta and in the left iliac fossa, bone | No | Ⅲ | LMB 89, RT | 5 | DOD |
| 13 | Onciu et al[14] (2003) | Male/16 | Scalp and parietal bone | Cervical, axillary, and inguinal LN, multiple lytic skeletal lesions | Yes | IV | LMB 89 | 24 | CR. relapsed; DOD |
| 14 | Onciu et al[14] (2003) | Male/10 | Laryngeal supraglottic mass | Cervical and submandibular LN | No | Ⅱ | POG8719, RT, 3 DAHP courses | 156 | CR |
| 15 | Pan et al[2] (2017) | Male/18 | Axillary LN | NA | NA | NA | 11 | DOD |
The prognosis for this disease is poor, and there is a lack of effective standard treatment options for both adults and children. Pan et al[2] retrospectively analyzed 134 cases (26 in this study and 108 in literatures) of ALK+LBCL. The majority of patients received chemotherapy, including CHOP, CHOEP, EPOCH, CVAD and so on, with some patients receiving a combination of local radiotherapy and hematopoietic stem cell transplantation. The 5-year overall survival (OS) is only 34%, while the median survival time is only 1.83 years. Clinical stage and age of onset are risk factors for poor prognosis. The 2-year OS (76%) and 5-year OS (66%) of patients in stage I/II are significantly higher compared to patients in stage III/IV (2-year OS 27% and 5-year OS 8%). The patient in this report has already had extensive metastasis throughout the body, involving the bone marrow and central nervous system at the initial visit. CR1 was achieved using high-dose and short-course of modified LMB89 Group C regimen, which was effective against pediatric B-cell lymphoma. Autologous stem cell transplantation was performed after completion of all chemotherapy regimens to reduce the risk of recurrence due to residual lesions at mid-term evaluation. However, our patient rapidly relapsed 3 mo later. Is the mechanism of relapse related to the mutations of TP53, NRAS, KRAS, and other genes in the tumor? Further investigation is necessary to fully understand its mechanism.
Recent studies have suggested that ALK inhibitors may be effective for the treatment of ALK+LBCL. The selective ALK inhibitor NVP-TAE684 could inhibit the growth of the Ba/F3 cell line expressing SEC31A-ALK[16]. Mossé et al[17] demon-strated that NVP-TAE684 could inhibit the growth of cells expressing CLTC-ALK fusion in vitro and result in the regression of murine tumor xenografts in vivo. Crizotinib has been shown to have therapeutic activity in relapsed and resistant ALK+ lymphomas in adult patients, particularly in ALK+LBCL[11,16-20]. Studies have shown that alectinib is effective for ALK+ALCL, and has been successfully used in our center to treat a female patient with CLTC-ALK fusion gene-positive ALCL after central nervous system relapse[21]. However, no relevant reports have been published on the use of alectinib, a second-generation ALK inhibitor, for ALK+LBCL patients. When our patient relapsed, he was unable to tolerate intensive chemotherapy due to bone marrow failure. Hence, we administered low-dose chemotherapy and alectinib in combination, and he achieved CR2 rapidly, which suggested that chemotherapy in combination with alectinib was still effective in our patient with relapsed ALK+LBCL. Thus, ALK inhibitors such as crizotinib, alectinib, and seretinib should be considered as new treatment options. Allo-HSCT was performed after CR2. Our patient has been in CR2 for 16 mo after allo-HSCT, which showed that allo-HSCT may be more effective compared to autologous stem cell transplantation. In addition, several reports have been published on the success of Chimeric antigen receptor T-cell therapy (CAR-T) treatment in children with refractory and relapsed Burkitt's lymphoma and DLBCL[22]. If a patient with ALK+LBCL is unable to tolerate intensive chemotherapy after relapse, CAR-T treatment followed by bridging allo-HSCT may also be an effective option.
In summary, we present a rare case of ALK+LBCL with PGS1-CLTC fusion gene that has not been reported previously in the literature. The patient was given a modified LMB89 Group C regimen chemotherapy and autologous stem cell transplantation and achieved CR1, but soon relapsed. The patient was then administered low-dose chemotherapy in combination with alectinib targeted therapy, and achieved CR2 soon afterward, and then subsequently he underwent allo-HSCT. The patient was still in CR2 16 mo after transplantation. To our knowledge, this is the first published pediatric case report describing the use of alectinib for the treatment of relapsed ALK+LBCL. However, our findings are based on one patient, and more cases are needed to prove the efficacy and safety in pediatric patients.
The authors would like to thank the patient and his family.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lim AST S-Editor: Gao CC L-Editor: Filipodia P-Editor: Wang LL
| 1. | Delsol G, Lamant L, Mariamé B, Pulford K, Dastugue N, Brousset P, Rigal-Huguet F, al Saati T, Cerretti DP, Morris SW, Mason DY. A new subtype of large B-cell lymphoma expressing the ALK kinase and lacking the 2; 5 translocation. Blood. 1997;89:1483-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 210] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 2. | Pan Z, Hu S, Li M, Zhou Y, Kim YS, Reddy V, Sanmann JN, Smith LM, Chen M, Gao Z, Wang HY, Yuan J. ALK-positive Large B-cell Lymphoma: A Clinicopathologic Study of 26 Cases With Review of Additional 108 Cases in the Literature. Am J Surg Pathol. 2017;41:25-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 3. | Quesada AE, Huh YO, Wang W, Medeiros LJ, Thakral B. Anaplastic lymphoma kinase (ALK)-positive large B-cell lymphoma in a patient treated with azathioprine for ulcerative colitis. Pathology. 2016;48:513-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Zanelli M, Valli R, Capodanno I, Ragazzi M, Ascani S. Anaplastic lymphoma kinase-positive large B-cell lymphoma: description of a case with an unexpected clinical outcome. Int J Surg Pathol. 2015;23:78-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Corean J, Li KD. A Rare Case of ALK-Positive Large B-Cell Lymphoma with CD33 Expression. Case Rep Hematol. 2018;2018:5320590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Sakr H, Cruise M, Chahal P, Cotta C, Cook J, Chalikonda S, Rosenblatt S, Hamadeh F, Al-Nourhji O, Sturgis CD. Anaplastic lymphoma kinase positive large B-cell lymphoma: Literature review and report of an endoscopic fine needle aspiration case with tigroid backgrounds mimicking seminoma. Diagn Cytopathol. 2017;45:148-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Chen J, Feng X, Dong M. Anaplastic lymphoma kinase-positive diffuse large B-cell lymphoma presenting in nasal cavity: a case report and review of literature. Int J Clin Exp Pathol. 2015;8:2123-2130. [PubMed] |
| 8. | Lin SY, Chuang SS, Jhuang JY, Sakamoto K, Takeuchi K, Bahrami A, Tsai CC. ALK positive large B-cell lymphoma with a massive neutrophilic infiltrate: report of a case mimicking epithelioid inflammatory myofibroblastic tumour. J Clin Pathol. 2015;68:496-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Xing X, Lin D, Ran W, Liu H. ALK-positive diffuse large B-cell lymphoma of the duodenum: A case report and review of the literature. Exp Ther Med. 2014;8:409-412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Sachdev R, Goel S, Gupta S, Sood N. Anaplastic lymphoma kinase (ALK) positive diffuse large B cell lymphoma in a 20 year old: a rare entity. Indian J Pathol Microbiol. 2014;57:157-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Wass M, Behlendorf T, Schädlich B, Mottok A, Rosenwald A, Schmoll HJ, Jordan K. Crizotinib in refractory ALK-positive diffuse large B-cell lymphoma: a case report with a short-term response. Eur J Haematol. 2014;92:268-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Swerdlow SH, Campo E, Harris NL. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues (Revised 4th edition). IARC: Lyon, 2016. |
| 13. | Ebert TJ, Muzi M, Berens R, Goff D, Kampine JP. Sympathetic responses to induction of anesthesia in humans with propofol or etomidate. Anesthesiology. 1992;76:725-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 292] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 14. | Onciu M, Behm FG, Downing JR, Shurtleff SA, Raimondi SC, Ma Z, Morris SW, Kennedy W, Jones SC, Sandlund JT. ALK-positive plasmablastic B-cell lymphoma with expression of the NPM-ALK fusion transcript: report of 2 cases. Blood. 2003;102:2642-2644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Gambacorti-Passerini C, Messa C, Pogliani EM. Crizotinib in anaplastic large-cell lymphoma. N Engl J Med. 2011;364:775-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 212] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 16. | Cerchietti L, Damm-Welk C, Vater I, Klapper W, Harder L, Pott C, Yang SN, Reiter A, Siebert R, Melnick A, Woessmann W. Inhibition of anaplastic lymphoma kinase (ALK) activity provides a therapeutic approach for CLTC-ALK-positive human diffuse large B cell lymphomas. PLoS One. 2011;6:e18436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Mossé YP, Lim MS, Voss SD, Wilner K, Ruffner K, Laliberte J, Rolland D, Balis FM, Maris JM, Weigel BJ, Ingle AM, Ahern C, Adamson PC, Blaney SM. Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: a Children's Oncology Group phase 1 consortium study. Lancet Oncol. 2013;14:472-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 521] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 18. | Cleary JM, Rodig S, Barr PM, Shinagare AB, Clark JW, Shapiro GI, Armand P. Crizotinib as salvage and maintenance with allogeneic stem cell transplantation for refractory anaplastic large cell lymphoma. J Natl Compr Canc Netw. 2014;12:323-6; quiz 326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Laurent C, Do C, Gascoyne RD, Lamant L, Ysebaert L, Laurent G, Delsol G, Brousset P. Anaplastic lymphoma kinase-positive diffuse large B-cell lymphoma: a rare clinicopathologic entity with poor prognosis. J Clin Oncol. 2009;27:4211-4216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Galkin AV, Melnick JS, Kim S, Hood TL, Li N, Li L, Xia G, Steensma R, Chopiuk G, Jiang J, Wan Y, Ding P, Liu Y, Sun F, Schultz PG, Gray NS, Warmuth M. Identification of NVP-TAE684, a potent, selective, and efficacious inhibitor of NPM-ALK. Proc Natl Acad Sci U S A. 2007;104:270-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 310] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 21. | Yang J, Li J, Gu WY, Jin L, Duan YL, Huang S, Zhang M, Wang XS, Liu Y, Zhou CJ, Gao C, Zheng HY, Zhang YH. Central nervous system relapse in a pediatric anaplastic large cell lymphoma patient with CLTC/ALK translocation treated with alectinib: A case report. World J Clin Cases. 2020;8:1685-1692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Zhang W, Hu B, Jing L, Yang J, Zhang Y. Early Response Observed in Pediatric Patients with Refractory/Relapsed B-Cell Non-Hodgkin Lymphoma Treated with Sequential Chimeric Antigen Receptor T Cells. Blood. 2019;134:1945-1945. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Beltran B, Castillo J, Salas R, Quiñones P, Morales D, Hurtado F, Riva L, Winer E. ALK-positive diffuse large B-cell lymphoma: report of four cases and review of the literature. J Hematol Oncol. 2009;2:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | De Paepe P, Baens M, van Krieken H, Verhasselt B, Stul M, Simons A, Poppe B, Laureys G, Brons P, Vandenberghe P, Speleman F, Praet M, De Wolf-Peeters C, Marynen P, Wlodarska I. ALK activation by the CLTC-ALK fusion is a recurrent event in large B-cell lymphoma. Blood. 2003;102:2638-2641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 130] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 25. | Gesk S, Gascoyne RD, Schnitzer B, Bakshi N, Janssen D, Klapper W, Martín-Subero JI, Parwaresch R, Siebert R. ALK-positive diffuse large B-cell lymphoma with ALK-Clathrin fusion belongs to the spectrum of pediatric lymphomas. Leukemia. 2005;19:1839-1840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Isimbaldi G, Bandiera L, d'Amore ES, Conter V, Milani M, Mussolin L, Rosolen A. ALK-positive plasmablastic B-cell lymphoma with the clathrin-ALK gene rearrangement. Pediatr Blood Cancer. 2006;46:390-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Bubała H, Małdyk J, Włodarska I, Sońta-Jakimczyk D, Szczepański T. ALK-positive diffuse large B-cell lymphoma. Pediatr Blood Cancer. 2006;46:649-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |