Published online Jun 16, 2021. doi: 10.12998/wjcc.v9.i17.4244
Peer-review started: December 16, 2020
First decision: January 7, 2021
Revised: January 10, 2021
Accepted: March 29, 2021
Article in press: March 29, 2021
Published online: June 16, 2021
Processing time: 160 Days and 1.2 Hours
Metastases from pancreas or ampullary malignancies are common, but the spread to testicle and paratesticular tissue is exceedingly rare. To the best of our knowledge, fewer than 30 cases have been reported in the literature. More rarely, metastasis to tunica vaginalis testis occurs without involvement of the testes and epididymis.
A 65-year-old male who complained of painless swelling of the left scrotum for over 1 wk was referred to the Department of Urology. Scrotal ultrasound showed left testicular hydrocele with paratesticular masses. Chest computed tomography revealed lung metastasis and enlarged left supraclavicular lymph node.The blood tumor markersalpha-fetoprotein, human chorionic gonadotropin, and serum lactate dehydrogenase were withinnormal limits.The preoperative diagnosis was left testicular tumor with lung metastasis. Then radical orchidectomy of the left testicle and high ligation of the spermatic cord were performed, and postoperative histopathology suggested metastatic tumors that was confirmed by an abdominal computed tomographic scan. The positive computed tomography findings, in conjunction with the expression of cytokeratin 7 (CK7), CK20, CK5/6, and absence of expression of Wilms’ tumor suppressor gene 1, calretinin, melanocyte, prostate-specific antigen, thyroid transcription factor-1, GATA binding protein 3, caudal type homeobox 2, and napsinA supported the diagnosis of pancreatic adenocarcinoma. The outcome of this patient was unsatisfactory, and he died 3 mo later.
This case suggests that pancreatic metastatic carcinoma must be considered in the differential diagnosis of scrotal enlargement. The advanced age of the patient wassuggestive of a secondary testicular tumor.In addition, careful physical examination and ultrasonography as well as radiological examination have become a standard modality.
Core Tip: Secondary paratesticular tumors are extremely rare, especially when they originate from the pancreas or ampullary malignancies. So it must be considered in the differential diagnosis of scrotal enlargement and careful clinical and radiological examination has become a standard modality.
- Citation: Zhang YR, Ma DK, Gao BS, An W, Guo KM. Tunica vaginalis testis metastasis as the first clinical manifestation of pancreatic adenocarcinoma: A case report. World J Clin Cases 2021; 9(17): 4244-4252
- URL: https://www.wjgnet.com/2307-8960/full/v9/i17/4244.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i17.4244
Metastatic testicular or paratesticular tumors that are derived from another solid tumor origin are relatively uncommon, accounting for only 0.02% to 3.6% of all testicular neoplasms[1]. The most common primary sites are the prostate, kidney, gastrointestinal tract, lung, and breast[2]. Through a literature review, we found fewer than 30 cases of testicular or paratesticular metastases caused by pancreatic tumors.
Here, we present a case of metastatic tunica vaginalis testis from pancreatic adenocarcinoma in a 65-year-old man and conduct a comprehensive literature review aimed at providing valuable information on this malignancy. This is a rare case, showing that paratesticular metastases may also occur in pancreatic adenocarcinoma in elderly patients.
A 65-year-old male was admitted to the department of urology with painless swelling of the left scrotum, which gradually enlarged over 1 wk.
He accidentally found left testicular enlargement 1 mo prior, which did not catch his attention. During the last week before admission, he noticed it growing rapidly. Then he visited a private clinic and was given a scrotal ultrasound, which showed aleft testicular tumor.
He had dyslipidemia, hypertension, and type 2 diabetes and denied anorexia, weight loss, fever, dysuria, history of sexually transmitted infection, and recent sick contacts.
He did not have a history of scrotal trauma, genitourinary tract anomalies, any known asbestos exposure, or surgeries.
Physical examination revealed a 2 cm × 3 cm hard, globular mass with transillumination in the lower part of the left scrotum. His right testis was of normal size with no associated scrotal swelling. Digital rectal examination was negative. There was no palpable inguinal lymphadenopathy or abdominal masses.
Preoperative blood tests including the β-subunit of human chorionic gonadotropin (β-hCG), cancer antigen carbohydrate antigen19-9, α-fetoprotein, prostate specific antigen and lactate dehydrogenase were all in normal range.
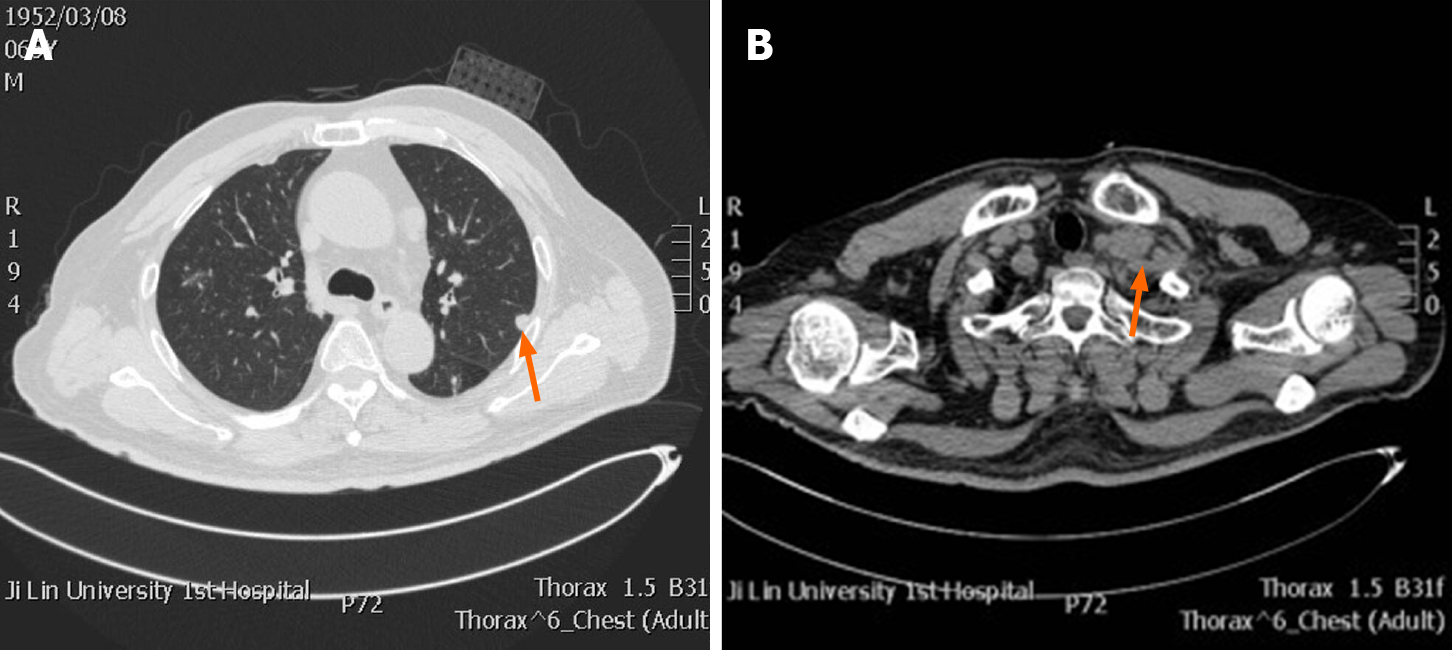
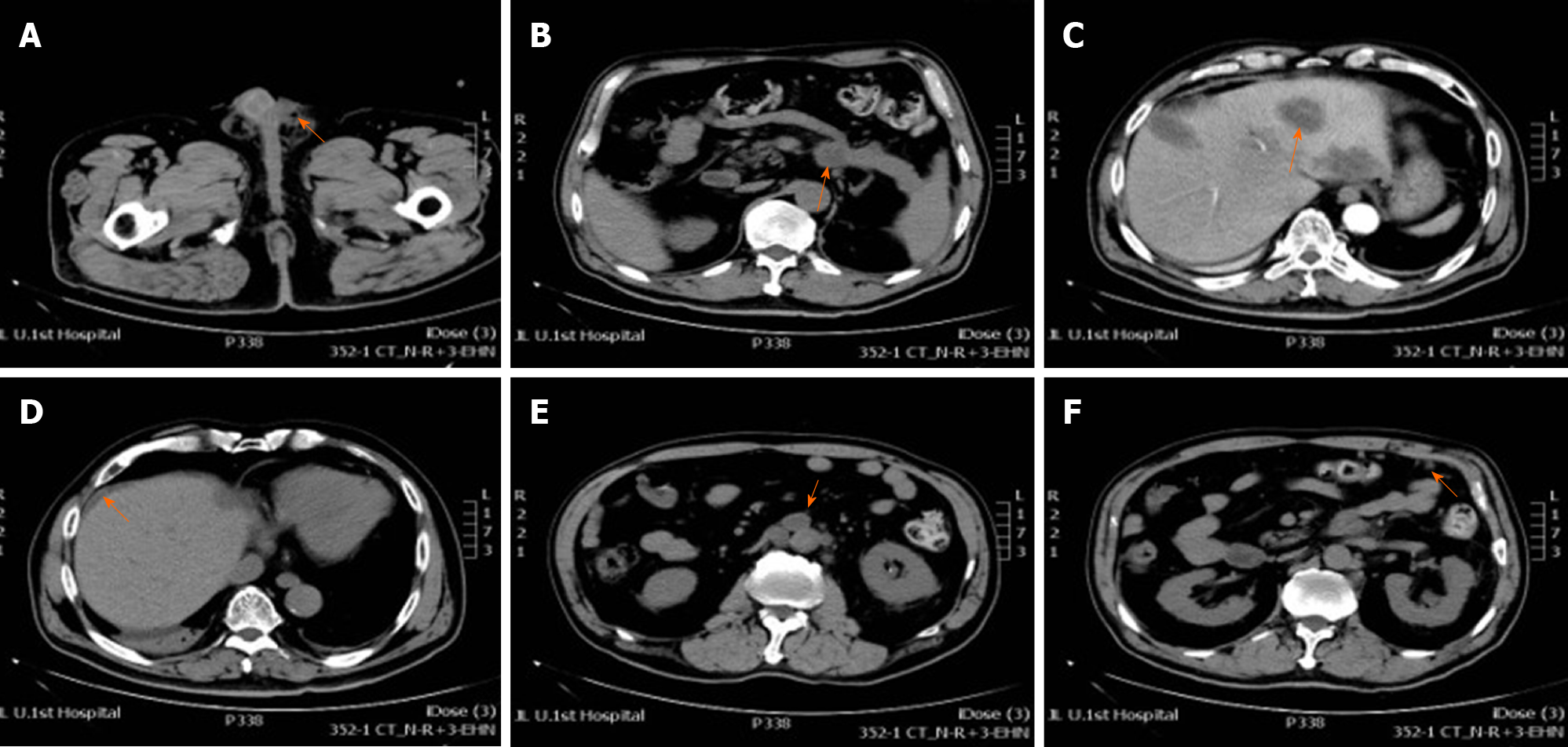
Preoperative scrotal ultrasound showed hydrocele on the left side with paratesticular masses. Chest computed tomography (CT) showed lung metastases and enlarged left supraclavicular lymph node (Figure 1). Postoperative CT of his chest, abdomen, pelvis, and brain was performed as a part of metastatic workup, which showed multiple nodules measuring 0.5-1.7 cm in the tail of the pancreas and many metastatic hypodense masses in the liver with the size of about 0.5-5.8 cm. The pancreatic duct in the tail was not dilated and there was no clear boundary with the ascending duodenum. Retroperitoneal lymphadenopathy, nodular infiltration in the omentum, and ascites were also observed (Figure 2). Brain metastasis was not detected (pictures not list).
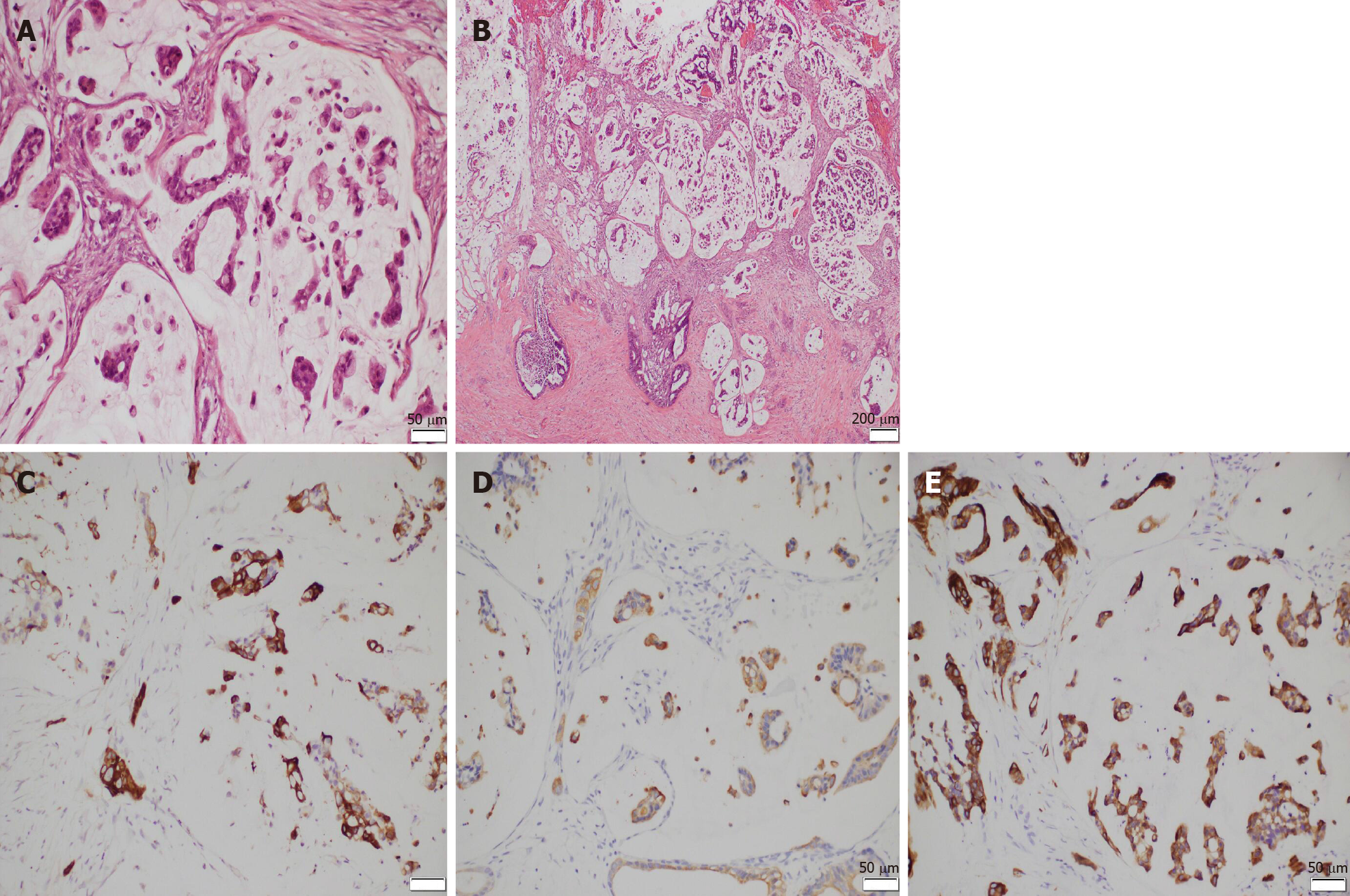
Final pathologic examination showed infiltration of malignant cells into the tunica vaginalis with negative incisional margin, which was highly suggestive of metastatic tumors. The testis, epididymis, and spermatic cord were not involved. Immunohistochemistry showed tumor tissues were diffusely positive for cytokeratin 7 (CK7), CK20, and CK5/6 (Figure 3). Meanwhile, they were negative for Wilms’ tumor suppressor gene 1 (WT-1), calretinin, melanocyte, prostate specific antigen, thyroid transcription factor-1 (TTF-1), GATA binding protein 3 (GATA-3), caudal type homeobox 2 (CDX-2), and napsinA (pictures not listed).
The patient was diagnosed with tunica vaginalis metastasis originating from the pancreas with peritoneal carcinomatosis, retroperitoneal lymphadenopathy, and lung metastasis.
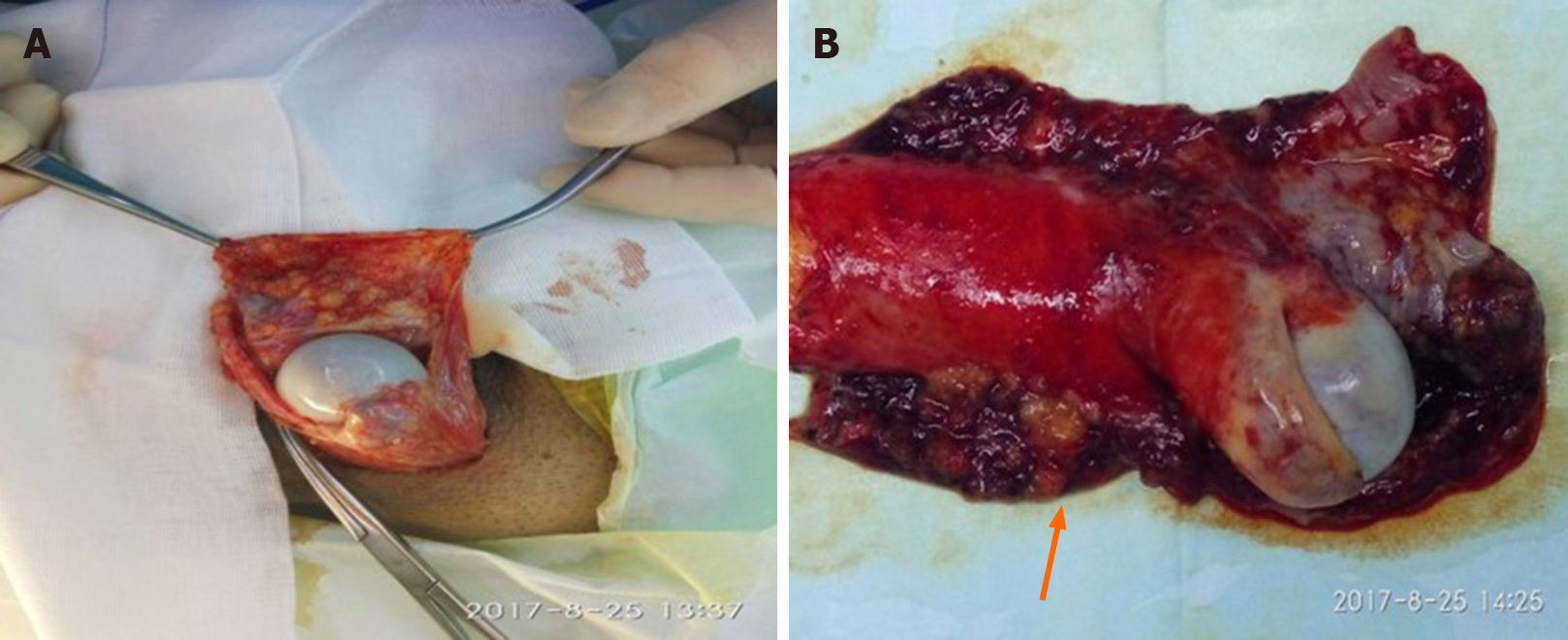
Radical orchidectomy of the left testis and high ligation of the spermatic cord were performed. During the operation, multiple nodules of varying sizes were found on the surface of the tunica vaginalis, while the left testis was not involved (Figure 4). In view of the distant metastasis, we referred to oncologists for further chemotherapy.
He recovered from his surgery without complications,but refused any further medical intervention. The entire course of disease from the first symptom to death was only 3 mo.
Metastatic malignancies of the testis and paratesticular tissue are extremely rare. The most common primary cancers are prostate, lung, kidney, gastrointestinal tract, and breast cancers[2]. In particular, very few cases have been reported about pancreatic cancer metastasizing to testicular and paratesticular tissues. Kiefer[3] first described epididymal spread originating from primary pancreatic cancer, which was incidentally discovered during autopsy in 1927[3]. To date, less than 30 cases have been reported including 1from China[4], 2from South Korea[5,6], 10from Japan[7], 6from the United States[8-12], and the other 5from European countries[13-17]. Scrotal or inguinal metastases occur worldwide, although Japanese males have a much higher incidence, accounting for half of the published cases. For the literature review, we searched for relevant case reports that were available in fulltext. Those case reports that did not have details about treatment and prognosis were excluded. As a result, a total of 15 cases documented in 14 published papers were included in our review (Table 1).
| Ref. | Age in yr | Symptoms | Duration | Site | size in cm | Metastaticorgan | Treatment | Primary location and histopathology | Prognosis after treatment |
| Houet al[4], 2020 | 65 | Painless scrotal swelling | 9 mo | Left | Left testis Right lung | Radical orchiectomy + pancreatic mass resection + right lung tumor biopsy | Pancreatic body adenocarcinoma | Alive, 9 mo | |
| Seoet al[5], 2004 | 67 | Painless scrotal swelling | 3 mo | Left | 7 × 5 | Left paratestis peritoneum, bone | Radical orchiectomy + gemicitabine chemotherapy | Pancreatic tail mucinous cystadenocarcinoma | Deceased, 3 mo |
| Kimet al[6], 2014 | 69 | Painful scrotal swelling | NA | Left | NA | Tunica virginals testis liver, peritoneum Omentum | Hydrocelectomy + gemicitabine chemotherapy | Pancreatic tail adenocarcinoma | NA |
| Tanakaet al[7], 1999 | 58 | Painful scrotal swelling | NA | Left | 3-4 | Epididymis, spermatic cord, stomach, left kidney, spleen | Radical orchiectomy + pancreatic tumor biopsy | Pancreatic tail adenocarcinoma | Deceased, 3 mo |
| Aquinoet al[8], 1989 | 42 | Jaundice, dark urine, pale stool, painful scrotal swelling, weight loss | 3 wk | Left | NA | Omentum, tunica vaginalis testis, porta hepatis | Exploratory laparotomy, left scrotal mass biopsy | Pancreatic tail adenocarcinoma | NA |
| Dookeran et al[9] | 53 | Painful scrotal swelling | NA | Right | 4 | Epididymis liver | Radical orchiectomy + pancreatic tumor biopsy | Pancreatic head, body and uncinate process adenocarcinoma | Deceased, 16 mo |
| Dookeran et al[9], 1997 | 36 | Painless scrotal swelling | 18 mo | Right | NA | Right testis, epididymis spermatic cord | Radical orchiectomy | Ampullary adenocarcinoma | Deceased, 2 mo |
| Rosserand Gerrard[10], 1999 | 58 | Painful scrotal swelling | 1 mo | Left | 7.0 × 4.5 × 3.5 | Left testis, liver | Radical orchiectomy + pancreatic tumor biopsy | Pancreatic tail adenocarcinoma | Alive, 6 mo |
| Laneet al[11], 2014 | 70 | Painless scrotal swelling | 21 mo | Right | NA | Tunica vaginalis testis | Hydrocelectomy pancreaticoduodenectomy capecitabine chemoradiation | Ampullary Adenocarcinoma | Alive, 1 mo |
| Faysalet al[12], 1983 | 41 | Painful scrotal swelling | 4 mo | Right | 1.7, 0.8 | The spermatic cordepididymis | Radical orchidectomy + chemotherapy | Pancreatic head and body adenocarcinoma | Deceased, 12 mo |
| Cormio et al[13], 2015 | 36 | Painful scrotal swelling | NA | Right | NA | Right testis, liver | Radical orchiectomy + chemotherapy | Pancreatic tail. adenocarcinoma | Deceased, 3 mo |
| Sawaet al[14], 2000 | 73 | Painless scrotal swelling, weight loss | NA | Left | 4 × 8 | Left paratestis liver, lung, retroperitoneum, left suprarenal gland | Radical orchiectomy | Pancreatic adenocarcinoma | Deceased, 2 mo |
| Di Francoet al[15], 2018 | 70 | Painless scrotal swelling | NA | Right | 2 | Epididymis spermatic cord, liver | Pancreatic tumor biopsy + orchifunicolectomy + gemicitabine and abraxane chemotherapy | Ductal pancreatic adenocarcinoma | NA |
| Tayloret al[16], 1990 | 77 | Painless scrotal swelling, weight loss, abdominal pain | 1 mo | Right | 3.0 × 2.0 | Right testis | Radical orchiectomy + pancreatic tumor biopsy | Mucinous exocrine pancreatic adenocarcinoma | NA |
| Bandyopadhyay et al[17], 2005 | 67 | Mass in groin, recurrent vomiting | 3 mo | Right | The spermatic cordduodenum | Radical orchidectomy pancreaticoduodenectomy, splenectomy, chemotherapy | Pancreatic body and tail adenocarcinoma | Alive, 4 wk | |
| Our case | 65 | Painless scrotal swelling | 1 wk | Left | 2 × 3 | Tunica vaginalis testis liver, omentum retroperitoneum | Radical orchidectomy | Pancreatic tail adenocarcinoma | Deceased, 3 mo |
Primary testicular tumors are usually diagnosed between the age of 20 and 40. Age-specific diagnosis rates peak in men aged 25 to 29 and 30 to 43 years (14.5 and 13.7 per 100000 men from 2008 to 2012, respectively)[18]. However, secondary testicular tumors peak between the ages of 50and 60.The mean age of onset is 59 years (range: 36 to 77 years), and the peak incidence is between ages of 50 and 70 years, similar to the report by Tanaka et al[7]. The possibility of secondary testicular tumors must be considered in aged male populations with enlarged testis. Because the manifestation of pancreatic cancer is usually insidious and has nonspecific symptoms, such as nausea and anorexia, the diagnosis is often delayed until other more ominous symptoms such as weight loss, abdominal pain, or gastrointestinal symptoms develop. Our review showed that most testicular metastases had a palpable, painless or painful, slowly enlarging mass in the scrotum, which was easily neglected or misdiagnosed as primary testis lesion. Only 3 cases experienced weight loss or other digestive discomfort[8,14,16]. One patient was referred due to severe acute pain in the right testis[13] and may have been diagnosed as orchitis. Kim et al[6] and colleagues reported a case of metastatic testicular tumor from pancreas, whose only initial symptom was hydrocele[6]. In the present case, the scrotal mass was the first clinical manifestation of the underlying malignancy. The average duration from onset ranged from 1 wk to 21 mo. Hirano et al[19] found that the bilateral testes were equally involved in the metastatic tumors of the spermatic cord originated from stomach cancer in 8 cases, colon cancer in 8 cases, liver cancer in 2 cases, and kidney cancer in 2 cases in their review[19]. Tanaka et al[7] showed that the right testis was more likely to be involved in metastatic tumors of the epididymis and spermatic cord from pancreatic cancer in a ratio of 9 to 1[7]. However, in our literature review, we found that scrotal or inguinal metastasis from pancreatic cancer was the same on both sides. Moreover, the average size of the metastatic tumors of the 15 confirmed cases was 3.6 cm in a diameter (range: 1.6 to 6.5 cm),while the primary tumor size ranged from 2.0 to 8.0 cm. A large tumor was reported in 2 cases[5,10]. In addition, we found an important feature that eight cases of carcinoma originated from the pancreatic tail were prone to metastasis to testis or paratestis compared with tumors from pancreatic head or ampulla.
The mechanisms of metastasis to the scrotal and inguinal tissues from primary malignant neoplasms have not been precisely elucidated. But it has been widely recognized that the main routes include arterial embolization, transperitoneal seeding through tunica vaginalis. In our review, pancreatic tumors metastasizing to the testis were found in 5 (31%) of the 16 confirmed cases[4,9,10,13,16], and 1of them invaded the epididymis[9]. Meanwhile, the tumors metastasizing to paratestis were reported in 11 cases[5-8,11,12,14,15,17], of which 4 involved the tunica vaginalis rather than testis[6,8,11], and 6 cases invaded the spermatic cord and/or epididymis [5,7,12,14,15,17]. CT scanning is the primary imaging modality to explore the potential primary and metastatic tumors. The most typical CT finding of pancreatic adenocarcinoma is a solid hypoenhancing mass located in the distal body or tail of the pancreas associated with distal pancreatic atrophy and regional lymphadenopathy. In solid pancreatic pseudopapillary tumor, weak early arterial enhancement and gradual increase in the portal-venous phase was characteristic manifestation[20]. Local invasion to adjacent vasculature causing pseudoaneurysms may also be observed in many pancreatic adenocarcinoma cases[21]. Our case is unique in that there werefew symptoms of the primary tumor, and paratesticular nodules werethe only first sign of metastasis and pancreatic cancer. Due to the evidence of significant retroperitoneal involvement on CT scan, the suspected route of tumor spread in this case waslymph node metastasis or direct transperitoneal seeding from peritoneal carcinomatosis. Since the onset duration was only 1 wk, the right testicle remained clean.
It is not easy to distinguish primary testicular or secondary testicular or para-testicular tumors in clinic, as both may be related to a painful or painless mass or testicular induration. Serum β-hCG level, which is considered atumor marker, cannot help to distinguish primary from secondary testicular tumors. However, one-third of patients with pancreatic exocrine adenocarcinoma have elevated β-hCG levels. Taylor et al[16] described a case of pancreatic adenocarcinoma presenting as a testicular tumor whose serum β-hCG level was 10-fold higher due to extragonadal secretion[16]. Histopathologically, multiple irregular tumors with different sizes werenoted in tunica vaginalis, which was consistent with a metastatic tumor. Another feature that supports metastatic rather than primary tumor is the presence of tumor emboli in the vascular tissue of the parenchyma. Immunohistochemistry is currently considered the most sensitive and specific method to determine the origin of the tumor. Singhet al[22] reported a case of duodenum bleeding due to metastatic endometrial adenocarcinoma which was confirmed by histology and positive cytokeratin 7, vimentin, and paired box gene 8 in immunohistochemistry[21]. In our case, mesothelioma was excluded due to thenegative expression of calretinin, MC, and WT-1. Negative TTF-1 and napsinA helped us rule out the possible origin of lung adenocarcinoma. Meanwhile, the tumor tissue was positive for cytokeratin 7, cytokeratin 20, and cytokeratin 5/6 and negative for CDX2, favoring a gastrointestinal origin of the tumor. In conjunction with CT findings, the diagnosis of pancreatic adenocarcinoma was established.
Due to its highly aggressive behavior, only 20% of patients with pancreaticcancer have surgically resectable disease at time of presentation. At the time of diagnosis, most pancreatic adenocarcinomas have already spread beyond the pancreas. In patients involving the testis, most cases as well as our case underwent radical orchiectomy, while hydrocelectomy with preservation of the testis was found in 2 cases[6,11]. One patient with omental involvement was examined by scrotal mass biopsy[8]. About half of the cases received chemotherapy including gemicitabine or capecitabine. But pancreatic cancer has a very poor prognosis considering all stages with high mortality. In our literature review, two-thirds of patients died within the documented follow-up period. The shortest survival duration recorded was only 2 mo. Compare to other pancreatic origin, ampullary tumorshave a better prognosis (5-year survival 40%-45%vs 10%-20%) due to early symptom onset and received a relative higher rate of resectability and a more favorable prognosis. Our review shows that 1 patient withmetastatic ampullary adenocarcinoma who underwent pancreaticoduodenectomy had a better outcome compared with another patientwho did not receive primary tumor resection.
Pancreatic cancer has a poor prognosis, and metastasis usually occurs when insidious symptoms appear. We report a case of tunica vaginalis testis metastasis from pancreatic adenocarcinoma in a 65-year-old man, who had painless swelling as the initial and unique symptom. Neither similar cases have been previously described in the literature. This case highlights that pancreatic metastatic carcinoma must be considered in the differential diagnosis of scrotal enlargement. The advanced age of patient is suggestive of a secondary testicular tumor. In addition, careful physical examination and ultrasonography as well as radiological examination have become a standard modality.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Machado MCC S-Editor: Zhang L L-Editor: Filipodia P-Editor: Liu JH
| 1. | Dutt N, Bates AW, Baithun SI. Secondary neoplasms of the male genital tract with different patterns of involvement in adults and children. Histopathology. 2000;37:323-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Beccia DJ, Krane RJ, Olsson CA. Clinical management of non-testicular intrascrotal tumors. J Urol. 1976;116:476-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 102] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Kiefer ED. Carcinoma of the pancreas. Arch Intern Med. 1927;40:1-29. |
| 4. | Hou G, Jiang Y, Cheng X. Testicular Metastasis of Pancreatic Carcinoma on FDG-PET/CT. Clin Nucl Med. 2020;45:85-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Seo IY, Kim SG, Han WC, Rim JS. Paratesticular mucinous cystadenocarcinoma: metastasis from pancreatic cancer. Int J Urol. 2004;11:1147-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Kim YW, Kim JW, Kim JH, Lee J, Lee E, Kim MY, Yang HK, Chang H. Metastatic testicular tumor presenting as a scrotal hydrocele: An initial manifestation of pancreatic adenocarcinoma. Oncol Lett. 2014;7:1793-1795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Tanaka H, Yasui T, Watase H. [Metastatic tumor of the epididymis from pancreatic carcinoma: a case report]. Hinyokika Kiyo. 1999;45:649-652. [PubMed] |
| 8. | Aquino NM, Mortan R, Singh H. Carcinoma of pancreas metastasizing to the tunica vaginalis testis. J Clin Ultrasound. 1989;17:287-290. [PubMed] [DOI] [Full Text] |
| 9. | Dookeran KA, Lotze MT, Sikora SS, Rao UN. Pancreatic and ampullary carcinomas with intrascrotal metastases. Br J Surg. 1997;84:198-199. [PubMed] |
| 10. | Rosser CJ, Gerrard E. Metastatic adenocarcinoma of the pancreas to the testicle: a case report. Am J Clin Oncol. 1999;22:619-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Lane WO, Bentley RC, Hurwitz HI, Howard LA, Polascik TJ, Anderson MR, Blazer DG. Metastatic ampullary adenocarcinoma presenting as a hydrocele: a case report. JOP. 2014;15:266-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Faysal MH, Strefling A, Kosek JC. Epididymal neoplasms: a case report and review. J Urol. 1983;129:843-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Cormio L, Sanguedolce F, Massenio P, Di Fino G, Bruno M, Carrieri G. Testicular metastasis as the first clinical manifestation of pancreatic adenocarcinoma: a case report. J Med Case Rep. 2015;9:139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Sawa TE, Duun S, Andersen JT. Paratesticular tumour: a metastasis from primary pancreas cancer. Scand J Urol Nephrol. 2000;34:70-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Di Franco CA, Rovereto B, Porru D, Zoccarato V, Regina C, Cebrelli T, Fiorello N, Viglio A, Galvagno L, Marchetti C, Ringressi A, Barletta D, Giliberto G. Metastasis of the epididymis and spermatic cord from pancreatic adenocarcinoma: A rare entity. Description of a case and revision of literature. Arch Ital Urol Androl. 2018;90:72-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Taylor H, Heaton N, Farrands P, Kirkham N, Fletcher M. Elevated human chorionic gonadotrophin levels in a patient with pancreatic carcinoma presenting with a testicular metastasis. Postgrad Med J. 1990;66:1073-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Bandyopadhyay D, Kapadia CR, Da Costa PE. Pancreatic carcinoma: report of two cases presenting with unusual metastases. Indian J Gastroenterol. 2005;24:75-76. [PubMed] |
| 18. | Filippou P, Ferguson JE 3rd, Nielsen ME. Epidemiology of Prostate and Testicular Cancer. Semin Intervent Radiol. 2016;33:182-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Hirano D, Ohkawa M, Hasegawa R, Okada N, Ishizuka N, Kusumi Y. Metastatic Tumor of the Spermatic Cord in Adults: A Case Report and Review. Case Rep Urol. 2015;2015:747261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Gandhi D, Sharma P, Parashar K, Kochar PS, Ahuja K, Sawhney H, Sharma S. Solid pseudopapillary Tumor of the Pancreas: Radiological and surgical review. Clin Imaging. 2020;67:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Khanna K, Mofakham FA, Gandhi D, Jain N. Desmoid fibromatosis of the pancreas--A case report with radiologic-pathologic correlation. Radiol Case Rep. 2020;15:2324-2328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Singh T, Gandhi D, Arora T, Shapiro J. Upper Gastrointestinal Bleeding due to Metastatic Endometrial Adenocarcinoma. ACG Case Rep J. 2019;6:e00138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |