Published online Jun 16, 2021. doi: 10.12998/wjcc.v9.i17.4230
Peer-review started: December 10, 2020
First decision: January 7, 2021
Revised: January 20, 2021
Accepted: March 24, 2021
Article in press: March 24, 2021
Published online: June 16, 2021
Processing time: 166 Days and 18.1 Hours
Azathioprine (AZA) and its close analog 6-mercaptopurine are thiopurines widely used in the treatment of patients with cancer, organ transplantation, and autoimmune or inflammatory diseases, including systemic lupus erythematosus. Bone marrow inhibition is a common side effect of AZA, and severe bone marrow inhibition is related to decreased thiopurine S-methyltransferase (TPMT) activity.
We herein report a patient with proliferative lupus nephritis who was using AZA for maintenance therapy, had no common TPMT pathogenic site mutations, and exhibited severe bone marrow inhibition on the 15th day after oral administration.
This report alerts physicians to the fact that even though the TPMT gene has no common pathogenic site mutation, severe myelosuppression may also occur.
Core Tip: Thiopurine S-methyltransferase (TPMT) gene polymorphism testing alone cannot fully predict the occurrence of azathioprine (AZA) adverse reactions such as bone marrow inhibition and alopecia. According to the literature mentioned above, nucleoside diphosphate-linked moiety X motif 15 (NUDT 15) and inosine triphosphate pyrophosphatase (ITPA) gene polymorphism tests should also be performed to predict the occurrence of AZA adverse reactions and further guide initial medication. On the other hand, AZA should be used with caution, and whole blood examination and liver and kidney function should be closely monitored during the entire treatment with AZA regardless of the status of TPMT, NUDT 15, and ITPA single nucleotide polymor
- Citation: Zhou XS, Lu YY, Gao YF, Shao W, Yao J. Bone marrow inhibition induced by azathioprine in a patient without mutation in the thiopurine S-methyltransferase pathogenic site: A case report. World J Clin Cases 2021; 9(17): 4230-4237
- URL: https://www.wjgnet.com/2307-8960/full/v9/i17/4230.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i17.4230
Azathioprine (AZA) and its close analog 6-mercaptopurine (6-MP) are thiopurines widely used in the treatment of patients with autoimmune inner-ear disease[1], inflammatory bowel disease[2], hematological malignancies[3], rheumatoid arthritis [4], autoimmune bullous diseases[5], and systemic lupus erythematosus[6]. Adverse reactions to AZA are mainly reflected as bone marrow inhibition, hepatic function lesions, and rash. Severe bone marrow inhibition is relevant to the patient’s thiopurine S-methyltransferase (TPMT) activity and the pathogenic mutation of TPMT[7]. A case of severe bone marrow suppression in a patient with lupus nephritis induced by AZA but without a common TPMT pathogenic site mutation is reported in this paper.
The patient, a 22-year-old woman of Chinese Han ethnicity, was admitted for severe edema of the facial region and two lower limbs over 3 mo.
The patient developed edema of the facial region and two lower limbs 3 mo ago without obvious cause. Her symptoms were mild in the morning and severe in the afternoon. At the same time, she experienced migratory pain of the small joints, which improved after movement, as well as decreased urinary volume, weakness, and poor appetite.
The patient had a free previous medical history.
The patient’s temperature was 36.6 °C, heart rate was 78 bpm, respiratory rate was 20 breaths per minute, blood pressure was 122/80 mmHg, and oxygen saturation in room air was 98%. The patient was conscious and she complied with the physician's physical examination. Her heart, lungs, and abdomen examinations showed no significant abnormalities, no percussion pain in her kidney area, and severe pitting edema in both lower extremities.
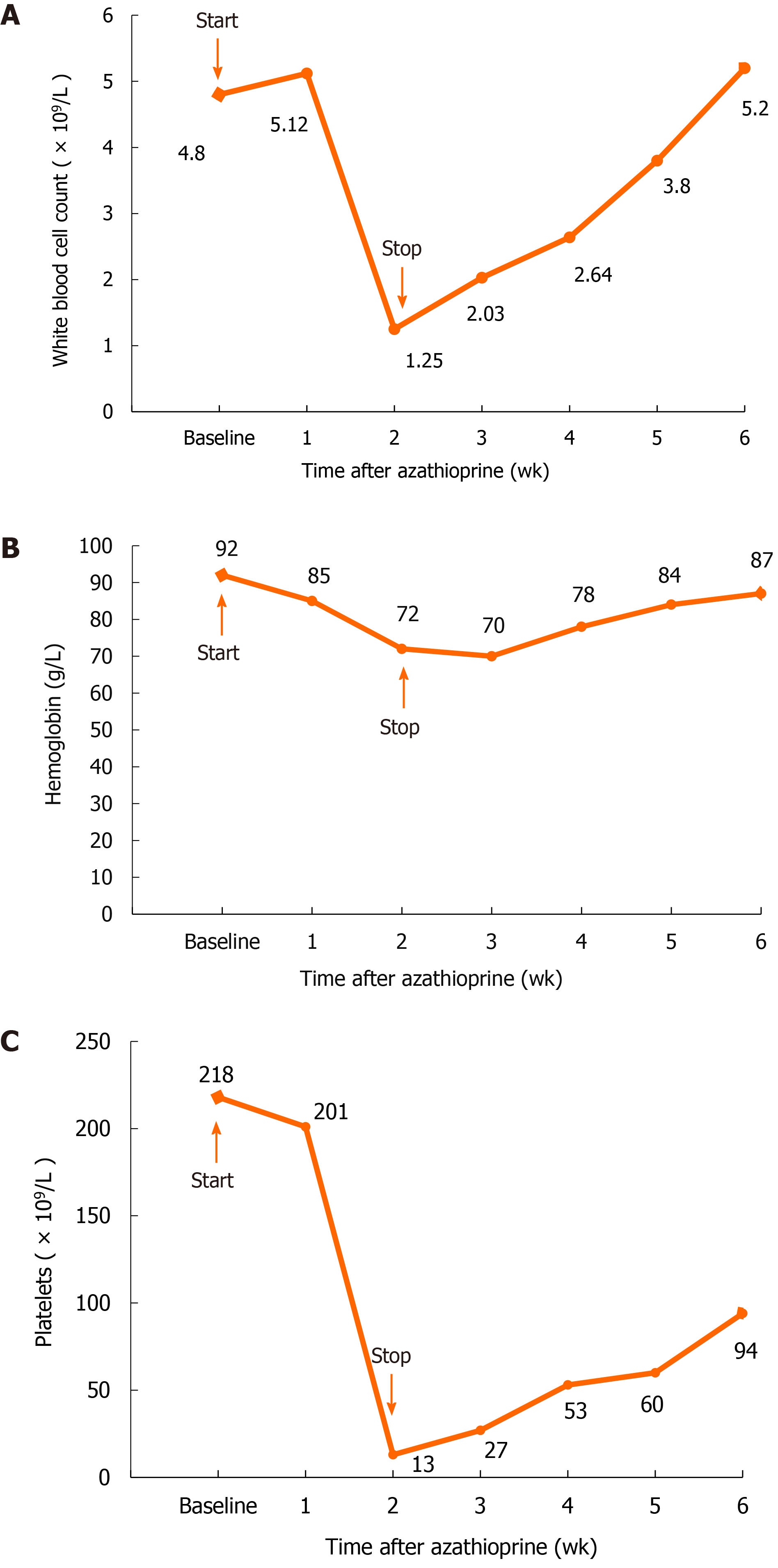
Routine urine examination revealed the following: Proteinuria +++, hematuria ++; phase of urinary red blood cells: Deformed erythrocytosis 70%, urine protein quantitation 4.2 g/24 h; urea nitrogen 15.7 mmol/L, and serum creatinine 141.4 μmol/L. Routine blood test results were as follows: Hemoglobin 92 g/L, white blood cells 4.8 × 109/L, blood platelets 218 × 109/L, antinuclear antibody 1:3200, cytoplasmic granules 1:1000, ds-DNA antibody > 1:3200, C3 0.56 g/L, and C4 0.22 g/L.
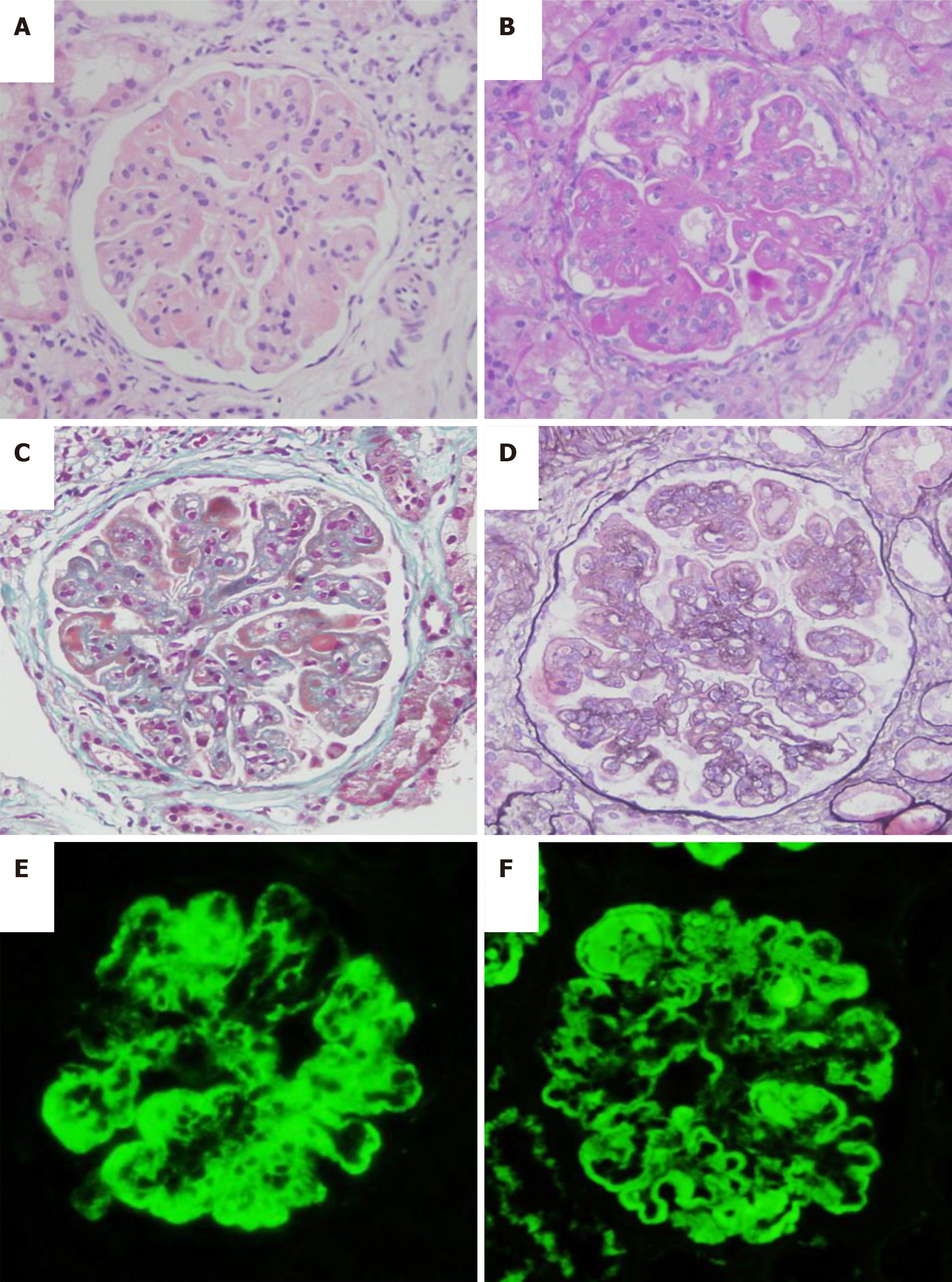
Pathological results showed 35 glomeruli in the punctured renal tissue, including one with global sclerosis, two with ischemic sclerosis, and the remaining glomeruli with diffuse proliferation of mesangial cells and endothelial cells, accompanied by segmental dual-track formation of a thickened basement membrane, segmental Meyer's loop, leukocyte infiltration, and segmental microthrombus formation. Fuchsinophilic protein deposition can be found at the mesangial region and subepithelial region, including fibrin crescent formation of one cell. The kidney tubular epithelium exhibited granular and vacuolar degeneration, as well as multifocal atrophy. The renal interstitium showed multishaped lymphocyte and monocyte infiltration, together with mild thickening of the arteriole wall. Paraffin immunofluorescence revealed: Immunoglobulin (Ig) G (++), IgA (++), IgM (++), C3 (+), fall risk assessment (+), C1q (++), and granular deposition along the mesangial region and capillary wall. Combined with clinical findings, this condition was considered diffuse proliferative lupus nephritis, with IV-G (A), AI = 11, and CI = 5. AI was scored as follows: Cellular proliferation (2 points), leukocyte infiltration (1 point), nuclear fragmentation/fibrinoid necrosis (2 points), cell crescent (0 points), Meyer's loop/thrombus (2 points), and interstitial monocyte infiltration (2 points). CI was scored as follows: Sclerosis (2 points), fibrin crescent (0 points), tubular atrophy (2 points), and interstitial fibrosis (2 points) (Figure 1).
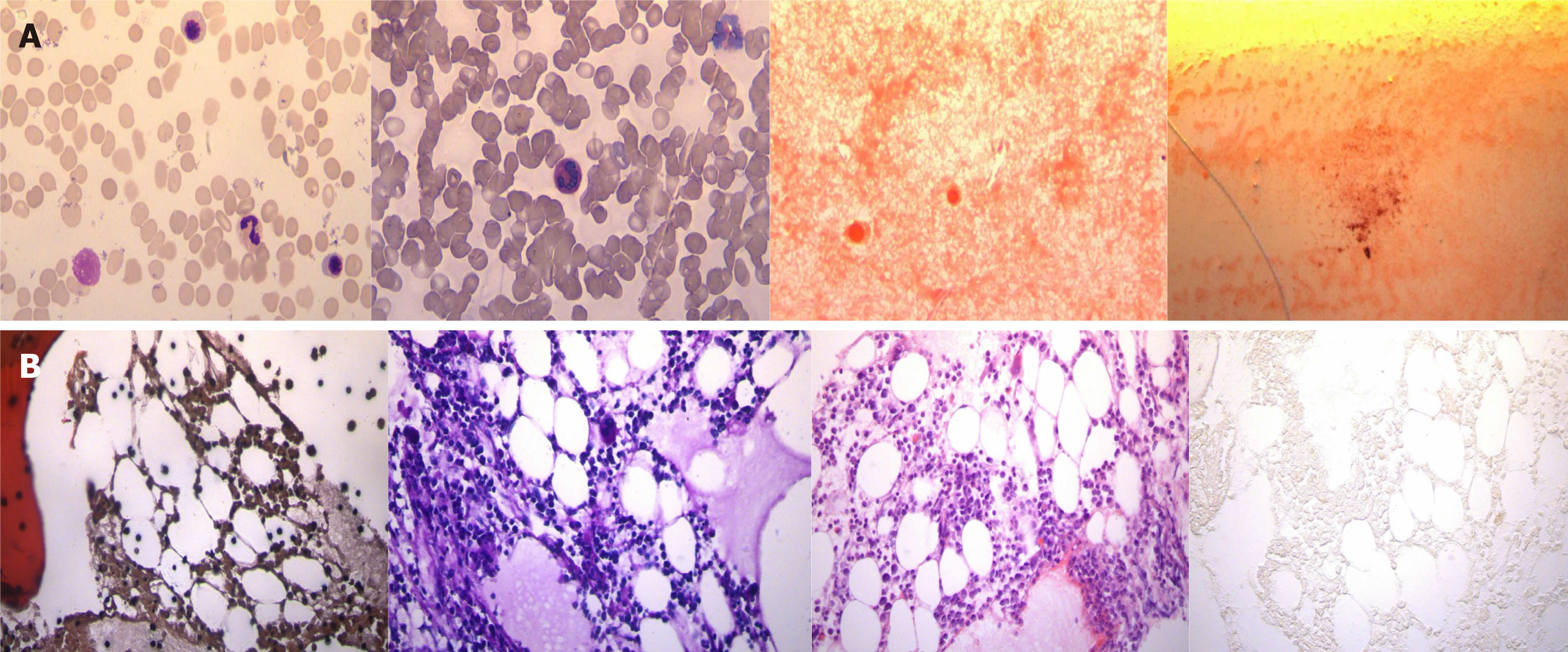
After consideration of the renal puncture results, oral administration of prednisone was begun at 50 mg/d, and meanwhile, cyclophosphamide was applied through intravenous injection at a dosage of 1.0 g monthly, which lasted for a consecutive 6 mo and was stopped after an accumulative use of 6 g. The serum creatinine fluctuated within an approximate range of 120-160 μmol/L, and blood albumin fluctuated from approximately 29-35 g/L. Then, the patient began to take AZA 50 mg/d as maintenance treatment but experienced extensive alopecia and shedding of pubic hair on the 13th day after oral administration. Pharyngalgia appeared on the 14th day, and fever with a body temperature up to 42 ℃ occurred on the 15th day. The patient came to the hospital for the second time. Physical examination revealed the following: Body temperature, 39.4 ℃; pulse, 92 times/min; breath rate, 22 times/min; blood pressure, 182/100 mmHg. The patient exhibited clear consciousness and emotional distress; she presented scattered chromatosis on her skin, and a rash was found on the inner surface of the bilateral thighs. Pharyngeal congestion was noted, and the breath sounds of the two lungs were clear. The heart rate was 92 times/min. The abdomen was flat, soft, and free from tenderness or rebound tenderness, and there was no liver or spleen involvement. Routine blood work showed the following: Hemoglobin 72 g/L, white blood cell count 1.25 × 109/L, blood platelet count 13 × 109/L, lymphocyte ratio 95.7%, neutrophil ratio 1.6%, and neutrophil count 0.01 × 109/L (Figure 2). The results of the bone marrow biopsy were as follows: Myelodysplasia low in the myelogram and focal hyperplasia in bone marrow tissue with active hyperplasia in some areas (Figure 3). Routine urinalysis results were as follows: Protein (+), occult blood 2+, and Epstein-Barr virus (EBV) positivity (3.58 × 104). TPMT genotyping testing (four single nucleotide polymorphisms, single base extension method) revealed the following: TPMT 3C gene polymorphism (719A>G) (this site is the most common gene mutation site in Asians) test result: A/A; TPMT 3B gene polymorphism (460G>A) test result: G/G; TPMT 2 gene polymorphism (238G>C) test result: G/G; no abnormity was found in any of the above results.
Combined with the patient’s medical history, laboratory tests, and examination results, it has been established that the patient has severe bone marrow suppression. The patient had no bone marrow suppression before the medication. She appeared after the medication. Considering the possibility of bone marrow suppression caused by the drug, it is recommended that the patient stops it in time.
The patient currently has systemic lupus erythematosus and lupus nephritis, and suffers from EBV infection. It is recommended that the patient be given oral hormones to control systemic lupus erythematosus activities and receive antiviral treatment.
The patient is currently suffering from chronic renal insufficiency, and nephrotic anemia should be corrected, avoiding the use of nephrotoxic drugs and delaying the progression of kidney disease. It is recommended that the patient be regularly checked and evaluated for renal function.
Systematic lupus erythematosus, lupus nephritis, chronic renal insufficiency, hypertension phase-3, drug-related bone marrow inhibition, granulocytopenia, and EBV infection.
Although the patient had no common TPMT pathogenic site mutation, the relationship of severe bone marrow inhibition and alopecia with the use of AZA was recognized and the treatment stopped in a timely manner. Treatment with ganciclovir, meropenem, cystatin sodium, linezolid, and itraconazole was given, with transfusion of suspended red blood cells, plasma, and blood platelets, as well as injection of filgrastim, recombinant human interleukin-11, and erythropoietin. Moreover, the oral administration of prednisone at 10 mg/d was carried out to control systematic lupus erythematosus.
Three weeks later, the patient’s body temperature decreased to normal, with routine blood parameters recovering to normal values, as well as blood creatine levels at 161 μmol/L. More than 2 mo after AZA was withdrawn, the patient's hair regrew. Serum creatinine was maintained at 160-190 μmol/L.
The enzyme activity of TPMT is key to the safe use of AZA, which strongly inactivates thiopurine metabolites (6-MP and 6-thiguanine nucleotide) to protect the body from thiopurine cytotoxicity[8]. TPMT allelic polymorphism testing shows that the 3/100-14/100 crowd is heterozygous, and its enzyme activity is 50% of that of the normal crowd, while the 1/3736-1/178 crowd is completely defective[9]. Hence, the United States Food and Drug Administration and World Gastroenterology Organization recommend that TPMT levels be tested prior to treatment and hold that those with low TPMT enzyme activity (those with TPMT homozygotes) shall prevent the use of AZA and those with median or normal enzyme activity can be used to an appropriate extent with routine blood tests to avoid severe adverse reactions[10]. Among Chinese Han people, there may be those without TPMT activity or with homozygous TPMT gene mutations. The most common alleles are TPMT*3C[11].
The patient in this paper experienced severe bone marrow inhibition, agranulocytosis, and alopecia 15 d after the use of AZA. The TPMT gene polymorphism test showed no abnormity in TPMT gene polymorphism. Possible reasons for this result are as follows: (1) Over 40 polymorphisms in TPMT have been documented to have an effect on the enzymatic activity of TPMT at present, and we did not test all the sites sufficiently; (2) In addition to TPMT, the activity of other enzymes, such as nucleoside diphosphate-linked moiety X motif 15 (NUDT 15) and inosine triphosphate pyrophosphatase (ITPA), also affects the metabolic process of AZA and is linked with the toxicity of AZA[12-15]. Unfortunately, in this case, the NUDT 15 and ITPA gene polymorphisms were not detected, and we do not know if there is any abnormality of this gene in the patient; (3) There may be other factors related to the toxicity of AZA that have not yet been found or validated, such as fat mass and obesity-associated protein[16]; and (4) In addition, this patient did have hypoproteinemia and decreased renal function, which may cause an increase in plasma concentration and the occurrence of adverse reactions.
This case alerts us that even though the TPMT gene has no common pathogenic site mutation, severe myelosuppression may also occur. The adverse reactions of AZA are caused by polygenes and multiple factors. Only TPMT gene polymorphism testing cannot fully predict the occurrence of AZA adverse reactions such as bone marrow inhibition and alopecia. According to the literature mentioned above, NUDT 15 and ITPA gene polymorphism tests should be performed as well to predict the occurrence of AZA adverse reactions and further guide initial medication. On the other hand, AZA should be used with caution, and whole blood examination and liver and kidney function should be closely monitored during the entire treatment of AZA regardless of the status of TPMT, NUDT 15, and ITPA single nucleotide polymorphisms.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Innocenti T S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Li JH
| 1. | Saraçaydin A, Katircioglu S, Karatay MC. Azathioprine in combination with steroids in the treatment of autoimmune inner-ear disease. J Int Med Res. 1993;21:192-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Gearry RB, Barclay ML. Azathioprine and 6-mercaptopurine pharmacogenetics and metabolite monitoring in inflammatory bowel disease. J Gastroenterol Hepatol. 2005;20:1149-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 174] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 3. | Bhatia S, Landier W, Hageman L, Chen Y, Kim H, Sun CL, Kornegay N, Evans WE, Angiolillo AL, Bostrom B, Casillas J, Lew G, Maloney KW, Mascarenhas L, Ritchey AK, Termuhlen AM, Carroll WL, Wong FL, Relling MV. Systemic Exposure to Thiopurines and Risk of Relapse in Children With Acute Lymphoblastic Leukemia: A Children's Oncology Group Study. JAMA Oncol. 2015;1:287-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 109] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 4. | Ramiro S, Gaujoux-Viala C, Nam JL, Smolen JS, Buch M, Gossec L, van der Heijde D, Winthrop K, Landewé R. Safety of synthetic and biological DMARDs: a systematic literature review informing the 2013 update of the EULAR recommendations for management of rheumatoid arthritis. Ann Rheum Dis. 2014;73:529-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 243] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 5. | Chams-Davatchi C, Mortazavizadeh A, Daneshpazhooh M, Davatchi F, Balighi K, Esmaili N, Akhyani M, Hallaji Z, Seirafi H, Mortazavi H. Randomized double blind trial of prednisolone and azathioprine, vs. prednisolone and placebo, in the treatment of pemphigus vulgaris. J Eur Acad Dermatol Venereol. 2013;27:1285-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Abu-Shakra M, Shoenfeld Y. Azathioprine therapy for patients with systemic lupus erythematosus. Lupus. 2001;10:152-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Meggitt SJ, Gray JC, Reynolds NJ. Azathioprine dosed by thiopurine methyltransferase activity for moderate-to-severe atopic eczema: a double-blind, randomised controlled trial. Lancet. 2006;367:839-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 190] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 8. | Booth RA, Ansari MT, Loit E, Tricco AC, Weeks L, Doucette S, Skidmore B, Sears M, Sy R, Karsh J. Assessment of thiopurine S-methyltransferase activity in patients prescribed thiopurines: a systematic review. Ann Intern Med. 2011;154:814-823, W. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Relling MV, Gardner EE, Sandborn WJ, Schmiegelow K, Pui CH, Yee SW, Stein CM, Carrillo M, Evans WE, Klein TE; Clinical Pharmacogenetics Implementation Consortium. Clinical Pharmacogenetics Implementation Consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing. Clin Pharmacol Ther. 2011;89:387-391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 438] [Cited by in RCA: 399] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 10. | Sanderson JD. TPMT Testing Before Starting Azathioprine or Mercaptopurine: Surely Just Do It? Gastroenterology. 2015;149:850-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Zhang LR, Song DK, Zhang W, Zhao J, Jia LJ, Xing DL. Efficient screening method of the thiopurine methyltransferase polymorphisms for patients considering taking thiopurine drugs in a Chinese Han population in Henan Province (central China). Clin Chim Acta. 2007;376:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Yang J, Wang P, Qin Z, Jia M, Zhang C, Tian X, Zheng Y, Zhang A, Zhang X, Liu S. NUDT15 and TPMT Genetic Polymorphisms Are Related to Azathioprine Intolerance in Chinese Patients with Rheumatic Diseases. Genet Test Mol Biomarkers. 2019;23:751-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Ailing Z, Jing Y, Jingli L, Yun X, Xiaojian Z. Further evidence that a variant of the gene NUDT15 may be an important predictor of azathioprine-induced toxicity in Chinese subjects: a case report. J Clin Pharm Ther. 2016;41:572-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Kishibe M, Nozaki H, Fujii M, Iinuma S, Ohtsubo S, Igawa S, Kanno K, Honma M, Kishibe K, Okamoto K, Ishida-Yamamoto A. Severe thiopurine-induced leukocytopenia and hair loss in Japanese patients with defective NUDT15 variant: Retrospective case-control study. J Dermatol. 2018;45:1160-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Steponaitiene R, Kupcinskas J, Survilaite S, Varkalaite G, Jonaitis L, Kiudelis G, Denapiene G, Valantinas J, Skieceviciene J, Kupcinskas L. TPMT and ITPA genetic variants in Lithuanian inflammatory bowel disease patients: Prevalence and azathioprine-related side effects. Adv Med Sci. 2016;61:135-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Chang JY, Park SJ, Jung ES, Jung SA, Moon CM, Chun J, Park JJ, Kim ES, Park Y, Kim TI, Kim WH, Cheon JH. Genotype-based Treatment With Thiopurine Reduces Incidence of Myelosuppression in Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol 2020; 18: 2010-2018. e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |