Published online Jun 16, 2021. doi: 10.12998/wjcc.v9.i17.4221
Peer-review started: October 21, 2020
First decision: January 17, 2021
Revised: January 26, 2021
Accepted: March 24, 2021
Article in press: March 24, 2021
Published online: June 16, 2021
Processing time: 217 Days and 9.2 Hours
Radical resection of gastric cancer liver metastases (GCLM) can increase the 5-year survival rate of GCLM patients. However, patients may lose the theoretical feasibility of surgery due to the critical location of liver metastasis in some cases.
A 29-year-old woman had a chief complaint of chronic abdominal pain for 1 year. Abdominal computed tomography and magnetic resonance imaging examinations suggested a mass of unknown pathological nature located between the first and second hila and the margin of the lower segment of the right lobe of the liver. The anterior wall of the gastric antrum was unevenly thickened. The diagnosis of (gastric antrum) intramucosal well-differentiated adenocarcinoma was histopathologically confirmed by puncture biopsy with gastroscopy guidance. She underwent radical resection (excision of both gastric tumors and ex vivo liver resection followed by autotransplantation simultaneously) followed by XELOX adjuvant chemotherapy. Without serious postoperative complications, the patient was successfully discharged on the 20th day after the operation. Pathological examination of the excised specimen indicated that gastrectomy with D2 lymph node dissection for primary gastric tumors and R0 resection for liver metastases were achieved. The resected mass was confirmed to be poorly differentiated gastric carcinoma (hepatoid adenocarcinoma with neuroendocrine differentiation) with liver metastases in segments VIII. No recurrence or metastasis within the liver was found during a 7.5-year follow-up review that began 1 mo after surgery.
Application of ex vivo liver resection followed by autotransplantation in radical resection for GCLM can help selected patients with intrahepatic metastases located in complex sites obtain a favorable clinical outcome.
Core Tip: Application of ex vivo liver resection followed by autotransplantation in radical resection for gastric cancer liver metastases can help selected patients with intrahepatic metastases located in complex sites obtain a favorable clinical outcome.
- Citation: Wang H, Zhang CC, Ou YJ, Zhang LD. Ex vivo liver resection followed by autotransplantation in radical resection of gastric cancer liver metastases: A case report. World J Clin Cases 2021; 9(17): 4221-4229
- URL: https://www.wjgnet.com/2307-8960/full/v9/i17/4221.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i17.4221
The incidence of gastric cancer liver metastases (GCLM) ranges from 9.9% to 18.7% among gastric cancer patients[1,2], and GCLM patients have a median survival time of 11 mo and a 5-year survival rate < 20%[3]. Some retrospective studies have declared that radical resection (excision of both gastric tumors and liver metastases simultaneously) can increase the 5-year survival rate of GCLM patients, but the timing and ideal surgical type remain controversial. Based on the likelihood of a surgical treatment being successful, Chinese type for-GCLM has been proposed by the Chinese consensus on the diagnosis and treatment of GCLM[4]. While we acknowledge that a tumor-free margin is the predictor of a better outcome for GCLM patients, an unavoidable question may emerge: What if liver metastases meet all the surgical qualifications judged by size and scope according to the aforementioned classification criteria, but their location is so critical that the technical risk of surgery significantly increases? It would be regretful to give up the chance of radical resection because of the limited number of eligible patients. The application of ex vivo liver resection followed by autotransplantation (ELRA) may provide an alternative solution to this problem. Here, we present a case pertaining to the treatment procedures and prognosis of a young female patient with GCLM, which showed that the application of ELRA in radical resection for GCLM can help patients with intrahepatic metastases located in complex sites obtain a favorable clinical outcome.
A 29-year-old woman had a chief complaint of chronic abdominal pain for one year and she experienced continuous weight loss of 5 kg over 2 mo prior to her admission.
The patient had chronic abdominal pain for 1 year and she experienced continuous weight loss of 5 kg over 2 mo prior to her admission.
The patient had undergone ovarian cystectomy, appendectomy, and subtotal hysterectomy for uncontrollable hemorrhage according to her past medical history.
No special family history was provided except that her mother was diagnosed with squamous cell carcinoma of the vulvar epithelium and the disease was cured according to her description.
Upon physical examination, a round mass approximately 7 cm in diameter could be palpated in the right upper abdomen and the mass was smooth, pushable, hard, and indistinguishable from adjacent tissue. Other detailed basic information is displayed in Table 1.
| Item | Value |
| Height | 158 cm |
| Weight | 48 kg |
| BMI | 19.2 kg/m2 |
| Obstructive jaundice | No |
| Zubrod-ECOG-WHO | 1 |
| Hypertension | No |
| Diabetes | No |
| Heart disease | No |
| Kidney disease | No |
| Liver disease | No |
| Breast disease | No |
| Genitourinary diseases | No |
| Infectious disease | No |
| Respiratory function | Good |
| Mental state | Good |
| Neoadjuvant chemotherapy | No |
Laboratory data demonstrated normal liver function and routine blood test results (Table 2). The tumor marker results showed that the level of alpha-fetoprotein was 9850 ng/mL and that of carcinoma embryonic antigen was 0.55 ng/mL.
| Item | Value |
| WBC | 6.77 × 109/L |
| RBC | 3.62 × 1012/L |
| Hgb | 111 g/L |
| PLT | 168 × 109/L |
| Neu | 4.88 × 109/L |
| Neu% | (Neu%) 72.1%↑ |
| ALT | 34 IU/L |
| AST | 32 IU/L |
| ALP | 37 IU/L |
| GGT | 12 IU/L |
| ALB | 38 g/L |
| PALB | 0.15 g/L |
| TBA | 16.2 umol/L |
| DBIL | 6.1 umol/L |
| IDBIL | 10.1 umol/L |
| PT | 12.50 s |
| INR | 0.95 |
| AFP | 9850 ng/mL |
| CEA | 1.46 ng/mL |
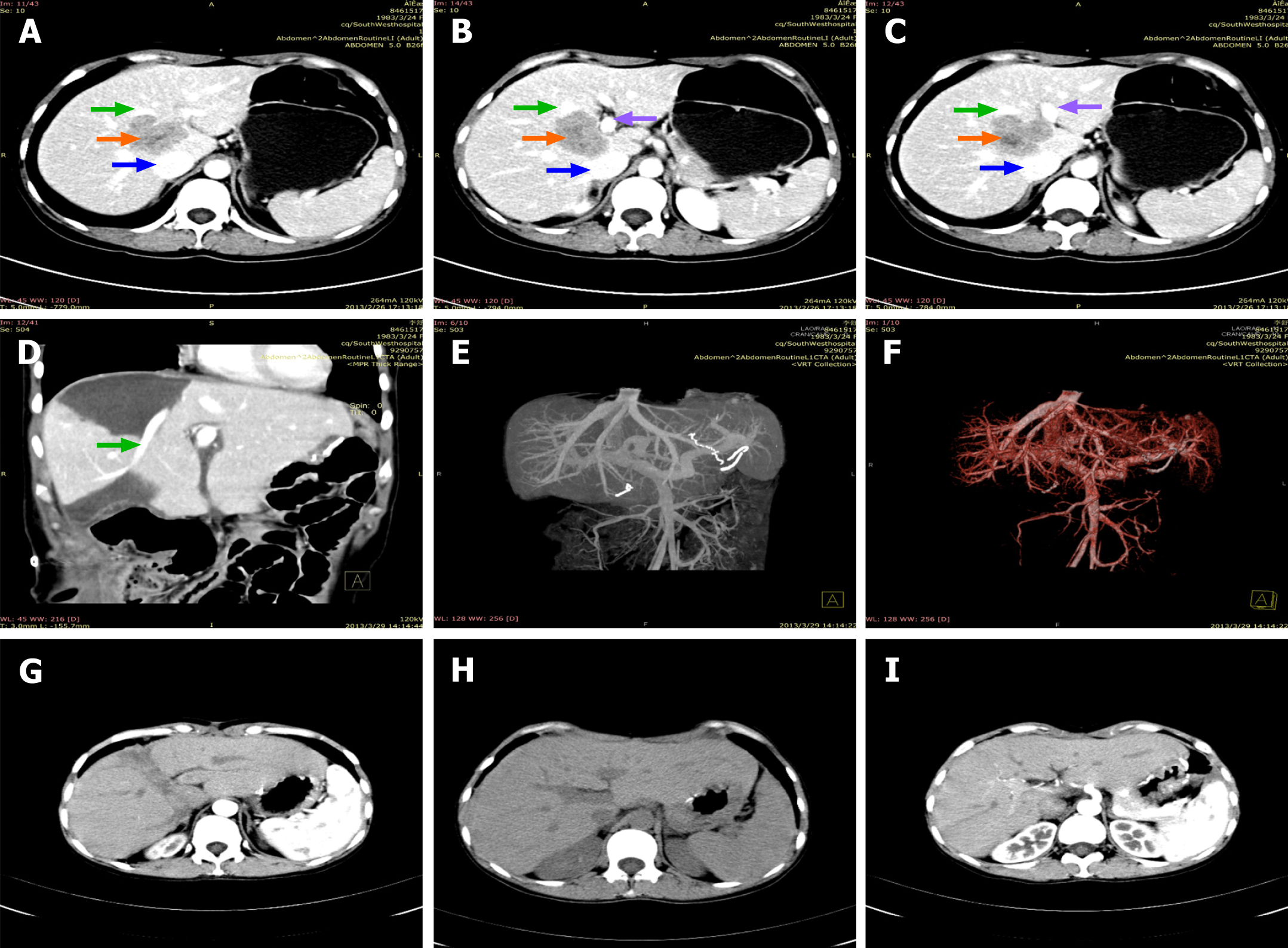
Abdominal computed tomography (CT) and magnetic resonance imaging examinations suggested a mass of unknown pathological nature located in segment VIII and it was between the first and second hila and the margin of the lower segment of the right liver lobe (Figure 1). The anterior wall of the gastric antrum was unevenly thickened. Positron emission tomography/CT (whole body) examination showed two main points: (1) The anterior wall of the gastric antrum was unevenly thickened, and fluorodeoxyglucose uptake was increased, which was consistent with the manifestations of gastric cancer; and (2) A mass shadow was located at the first and second hepatic hila and the lower right lobe of the liver. As increased fluorodeoxyglucose uptake is a sign of malignancy, whether the mass was liver metastasis or primary liver cancer remained to be identified. Electrocardiograph, chest digital radiography, and CT of the lower abdomen and pelvis showed no obvious abnormalities.
Due to the unknown nature and special location of the mass in the liver, it was considered to be a liver metastatic focus of gastric cancer after consultation by general and hepatobiliary surgeons.
The results of puncture biopsy with gastroscopy guidance supported the pathological diagnosis of gastric antrum intramucosal well-differentiated adenocarcinoma.
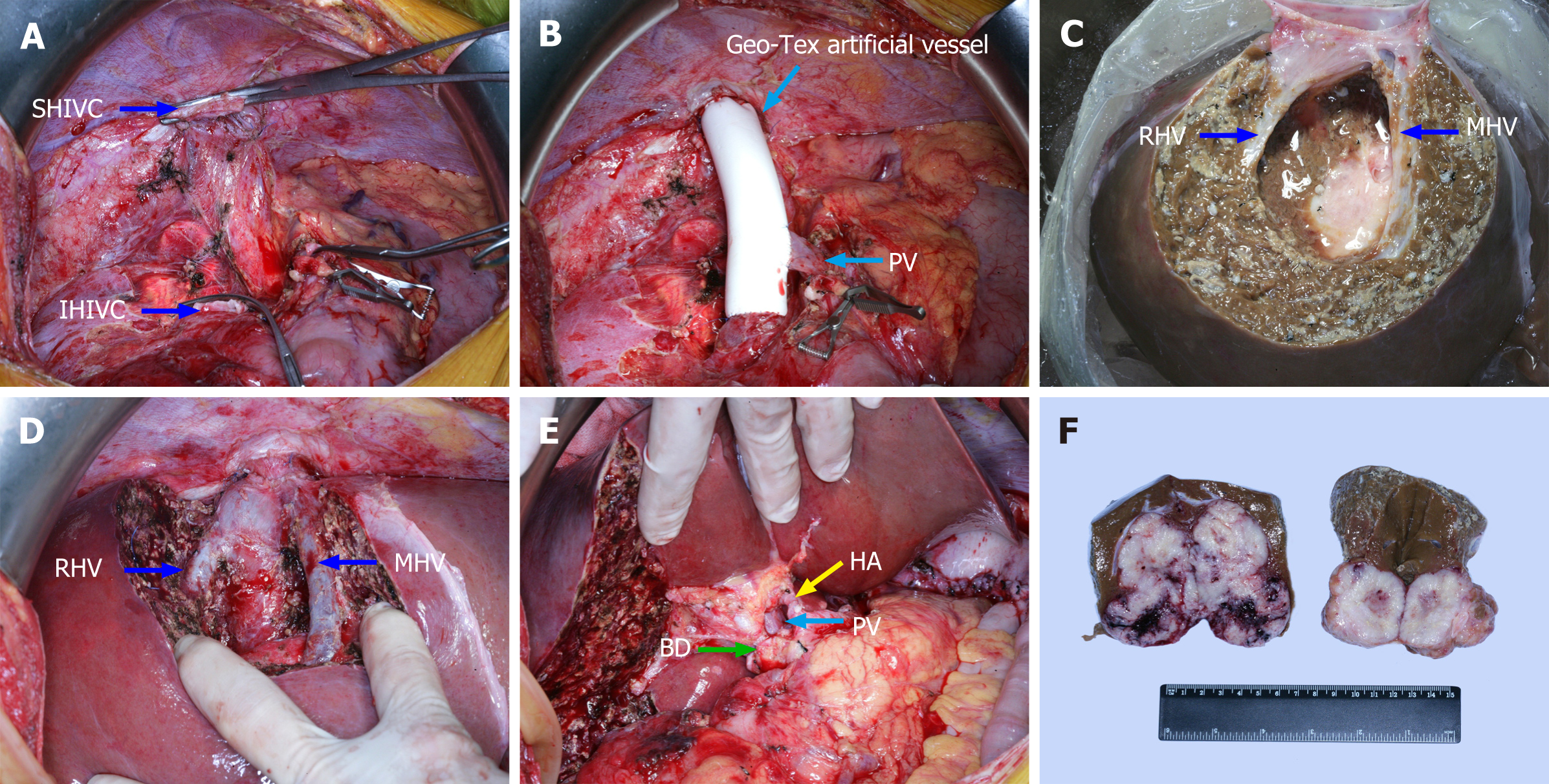
A tumor protruding through the liver capsule with unclear borders and an uneven surface was found in the right liver during abdominal exploration. It was hard and pale and was tightly adhered to the surrounding omentum. Consistent with the preoperative imaging findings, the tumor extended to the junction of the left and right liver lobes and was adjacent to the vena cava. Intraoperative frozen biopsy of the liver mass was performed and the results supported a poorly differentiated malignant tumor. The presence of intrahepatic microlesions was ruled out by intraoperative ultrasound. The results of intraoperative exploration led us to perform ELRA combined with radical resection for gastric cancer as the original plan.
Hepatectomy was accomplished by using standard technique[5] with extensive lymph node dissection around the superior mesenteric artery and celiac trunk. The common bile duct was completely removed up to the head of the pancreas. Preservation and perfusion of the removed isolated liver were performed with ice-cold solution at 4 ℃ and UW solution via the intact portal vein (PV). At the same time, a temporary channel was established between the superior hepatic inferior vena cava and inferior hepatic inferior vena cava with an internal diameter of 2 cm and Geo-Tex artificial vessel. The PV was also anastomosed to the vessel to construct a portacaval shunt. After the intrahepatic tumor and target liver segment (VIII) were completely resected and the repairment of the middle and right hepatic vein was done, the aforementioned temporary venous channel was removed. The remnant of the liver was implanted similar to orthotopic liver transplantation, which was followed by anastomosis of the superior hepatic inferior vena cava, inferior hepatic inferior vena cava, PV, hepatic artery, and bile duct. The total anhepatic period was 245 min, including 180 min of cold ischemia time (CIT) for extracorporeal tumor resection and vein repair, 40 min of vena cava anastomosis, and 25 min of PV anastomosis. Observation of the normal liver surface color after reperfusion was commonly performed, and the surgeons usually confirmed bile outflow before bile duct anastomosis. Some of intraoperative photos are presented in Figure 2. As we performed ex vivo liver resection on the back table, radical surgery for gastric cancer was accomplished simultaneously by general surgeons on the operating table. The stomach and duodenum were respectively transected at 15 cm from the distal end of the cardia and 2 cm from the distal end of the pylorus with a disposable linear cutting closure. After the lymph nodes around the PV were resected, the digestive tract was reconstructed by gastrojejunal anastomosis and a gastric tube was inserted into the jejunum for input loop.
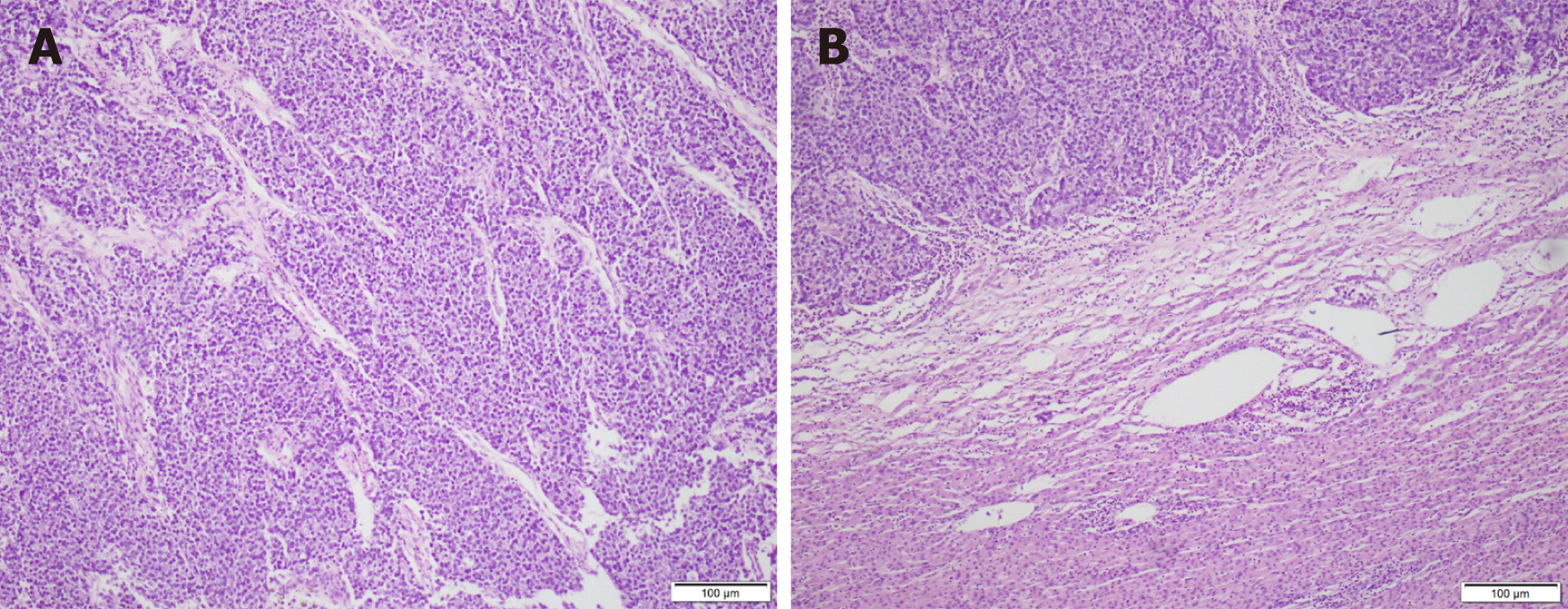
Our medical team provided the patient with symptomatic treatment after surgery such as gastrointestinal decompression, abdominal drainage, anti-infection medication, and nutritional support. She underwent bile leakage drainage on the fifth day after the operation. Fluid accumulated in the liver section and caused symptoms of gastric compression. The effusion was completely drained out after CT-guided puncture and catheter drainage 1 wk later. No other postoperative complications such as small-for-size syndrome, acute liver failure, vascular embolism, intra-abdominal infection, or hemorrhage occurred. She was successfully discharged on the 20th day after the operation. Postoperative pathological examination of the excised specimen indicated that gastrectomy with D2 lymph node dissection for primary gastric tumors and R0 resection for liver metastases were achieved. The resected mass was confirmed to be poorly differentiated gastric carcinoma (hepatoid adenocarcinoma with neuroendocrine differentiation) with liver metastases in segments of 6 and 8 (Figure 3). The patient began her first follow-up 1 mo after the operation, and received six courses of XELOX chemotherapy every 21 d since then, during which no serious adverse reactions or treatment discontinuation occurred. The patient also insisted on oral Tegafur Gimeracil and Oteracil Potassium Capsules for 1 mo after intravenous chemotherapy. She went back to the hospital on time for follow-up every 3 mo after the end of chemotherapy. Routine blood, liver and kidney function, and tumor markers tests, as well as abdominal CT are routine examination items. No obvious signs of tumor recurrence or metastasis within the abdomen or liver were recorded during a 7.5-year follow-up period. The patient had a good overall condition and did not undergo a second operation. Selected pre- and post-operative abdominal CT images of the patient are displayed in Figure 1. Postoperative laboratory data of the patient are displayed in Table 3.
| Item | Value |
| WBC | 5.66 × 109/L |
| RBC | 4.07 × 1012/L |
| Hgb | 121 g/L |
| PLT | 154 × 109/L |
| Neu | 4.00 × 109/L |
| Neu% | (Neu%) 70.6%↑ |
| ALT | 53.3 IU/L↑ |
| AST | 53.5 IU/L↑ |
| ALP | 113 IU/L |
| GGT | 17 IU/L |
| ALB | 42 g/L |
| PALB | 0.18 g/L |
| TBA | 17.3 umol/L |
| DBIL | 4.32 umol/L |
| IDBIL | 12.98 umol/L |
| PT | 11.40 s |
| PT-INR | 0.85 |
| AFP | 2.8 ng/mL |
| CEA | 3.56 ng/mL |
GCLM is usually transglobally, multifocally, or even diffusely spread. It is always complicated by peritoneal, extensive lymph node and organ metastases due to its highly malignant and rapidly invasive oncologic nature. Poor prognosis and deadly surgical complications make the resection rate so low that there is no multicenter clinical trial with large samples on the significance of liver resection for GCLM. No well-established criteria exist except for a few retrospective studies with a limited number of cases[4]. What is worth noting is that the results of these limited studies almost indicate that patients with GCLM can benefit from radical resection[6-9].
According to the Chinese type for-GCLM, the definitions of both type I and II include the same criterion: “Technological resectability of liver metastases judged by a hepatobiliary surgeon”. It emphasizes the principle that surgery should be performed only when R0 resection is anticipated[4]. Then the aforementioned question arises: What if the size and scope of liver metastases comply with this standard, but the anatomical complexity puts the hepatobiliary surgeon into a dilemma? The most common situation is that the tumor is located deeply within the liver and has extensive involvement of the main hepatic veins or retrohepatic vena cava. These types of tumors are usually deemed unresectable by conventional surgery because it is extremely hazardous due to the potential risk of uncontrollable hemorrhage and long ischemia time[10,11].
We can infer that ELRA may overcome this issue from our case report[4,12]. It effectively alleviated the technical bottleneck in traditional hepatectomy and allowed the hepatobiliary surgeons to perform precise liver tumor resection and effective vascular repairment by enabling them to operate with bloodless vision and access to critical structures easily since the first report of ex vivo hepatectomy by Pichlmayr et al[13] in 1988. What is more, full exposure of the surgical area can help reduce the risk due to vascular or bile duct variation that may not be detected before the operation.
ELRA is a challenging and time-consuming surgery with cumbersome procedures and complex operations. The theoretical basis and detailed surgical procedures that have been reported systematically in many studies will not be repeated here[14]. The most valuable experience that our team has summarized from this case is that accurate preoperative evaluation for specific patients based on detailed medical examination is of great importance. Strict patient selection and precise assessment of the size and quality of the remnant liver are pivotal to the decision-making process[14]. Although experiences gained from previous studies have indicated that after extended hepatectomy involving 70% to 75% of the liver, the remnant liver can still function well in non-cirrhosis patients[15,16], the strategy may need to be adjusted in the application of ERLA. A major feature of endoscopic retrograde appendicitis treatment (ERAT) is the longer CIT compared with ordinary liver transplantation (LT)[17]. In addition, procedures of ERAT are more complex than common LT and hepatectomy so a longer operative time and more intraoperative blood loss seem inevitable[10]. These are two poor prognostic indicators for LT recipients and hepatectomy patients because they may lead to graft loss due to the high incidence of postoperative biliary and arterial complications or severe hepatic dysfunction[18]. Based on the above theory, the liver function, liver quality, and estimated Rauscher leukemia virus of ELRA patients need to meet higher requirements. Livers with poor quality are less tolerant to cold ischemia-reperfusion injury. In addition, sufficient preoperative preparations, such as biliary drainage in patients with obstructive jaundice and nutritional support in malnourished patients, are indispensable once the doctor and patient reach a consensus to perform the surgery. Without exaggeration, it can be considered that half the success will be achieved if suitable GCLM patients are screened out, but inaccurate preoperative assessments will force surgeons to discontinue the operation or will even cause fatal postoperative complications such as small-for-size syndrome or acute liver failure[19]. Aji et al[14] described the largest case series of 69 patients with end stage hepatic alveolar echinococcosis who underwent ELRA. The detailed methods and procedures applied to select patients preoperatively are explicitly described in the literature. We also adopted the same issues to evaluate the patient in this case report.
A longer anhepatic period can lead to circulatory and metabolic disorders. Hepatobiliary surgeons must pursue more accurate and rapid surgical techniques to shorten the CIT and anhepatic phase. This is also a major test for the monitoring and intraoperative management of the anesthesiology department. So launching this type of major surgery can help improve the overall medical level of institutions and optimize the multidisciplinary diagnosis and treatment model. The lack of consensus and guidance on the application of ELRA drives us to accumulate more successful cases and precious experiences. Once successful, it will bring great significance in two aspects. For patients, the painful clinical symptoms caused by the tumor will be controlled, and the quality of their lives will be dramatically improved. It is our responsibility and obligation to create an opportunity for patients who never give up on themselves to extend their lives to fulfill their unfinished wishes. From a medical point of view, an increased survival rate may provide a longer time for more postoperative treatment options and promote the progress of clinical research. For instance, Qiu et al[20] proposed their vascular infiltration-based classification as a tool to improve anatomic comprehension and facilitate surgical planning for ELRA.
In conclusion, the application of ELRA in radical resection of GCLM could provide an alternative for selected patients. Strict patient selection and careful perioperative management guaranteed by an experienced multidisciplinary team could contribute to favorable clinical outcomes[21,22].
All images were taken and post-processed by a professional photographer in our center.
Manuscript source: Unsolicited manuscript
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Morelli L S-Editor: Zhang L L-Editor: Wang TQ P-Editor: Zhang YL
| 1. | Riihimäki M, Hemminki A, Sundquist K, Sundquist J, Hemminki K. Metastatic spread in patients with gastric cancer. Oncotarget. 2016;7:52307-52316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 298] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 2. | Cheon SH, Rha SY, Jeung HC, Im CK, Kim SH, Kim HR, Ahn JB, Roh JK, Noh SH, Chung HC. Survival benefit of combined curative resection of the stomach (D2 resection) and liver in gastric cancer patients with liver metastases. Ann Oncol. 2008;19:1146-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 140] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 3. | Xiao Y, Zhang B, Wu Y. Prognostic analysis and liver metastases relevant factors after gastric and hepatic surgical treatment in gastric cancer patients with metachronous liver metastases: a population-based study. Ir J Med Sci. 2019;188:415-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Zhang K, Chen L. Chinese consensus on the diagnosis and treatment of gastric cancer with liver metastases. Ther Adv Med Oncol. 2020;12:1758835920904803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Kaishan T, Dong C, Zhaoxu Y, Xiao L, Ying T. Chinese Code of practice for liver Transplantation. Zhonghua Qiguan Yizhi Zazhi. 2019;13:171-176. [DOI] [Full Text] |
| 6. | Kunieda K, Saji S, Sugiyama Y, Osada S, Sano J, Nagao N, Takahashi T, Takagi Y, Arai Y. Evaluation of treatment for synchronous hepatic metastases from gastric cancer with special reference to long-term survivors. Surg Today. 2002;32:587-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Ueda K, Iwahashi M, Nakamori M, Nakamura M, Naka T, Ishida K, Ojima T, Yamaue H. Analysis of the prognostic factors and evaluation of surgical treatment for synchronous liver metastases from gastric cancer. Langenbecks Arch Surg. 2009;394:647-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Thelen A, Jonas S, Benckert C, Lopez-Hänninen E, Neumann U, Rudolph B, Schumacher G, Neuhaus P. Liver resection for metastatic gastric cancer. Eur J Surg Oncol. 2008;34:1328-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Petrelli F, Coinu A, Cabiddu M, Ghilardi M, Borgonovo K, Lonati V, Barni S. Hepatic resection for gastric cancer liver metastases: A systematic review and meta-analysis. J Surg Oncol. 2015;111:1021-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Shen S, Qiu Y, Yang X, Wang W. Remnant Liver-to-Standard Liver Volume Ratio Below 40% is Safe in Ex Vivo Liver Resection and Autotransplantation. J Gastrointest Surg. 2019;23:1964-1972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Moris D, Tsilimigras DI, Chakedis J, Beal EW, Felekouras E, Vernadakis S, Schizas D, Fung JJ, Pawlik TM. Liver transplantation for unresectable colorectal liver metastases: A systematic review. J Surg Oncol. 2017;116:288-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Zhanyu Y, Qian L, Xiangde L, Zhiqing Y, Tengqian T, Ping B. Ex-vivo liver resection combined partial liver autotransplantation for hepatocellular carcinoma located at critical site. Zhonghua Xiaohua Waike Zazhi. 2010;18-20. [DOI] [Full Text] |
| 13. | Pichlmayr R, Bretschneider HJ, Kirchner E, Ringe B, Lamesch P, Gubernatis G, Hauss J, Niehaus KJ, Kaukemüller J. [Ex situ operation on the liver. A new possibility in liver surgery]. Langenbecks Arch Chir. 1988;373:122-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 95] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Aji T, Dong JH, Shao YM, Zhao JM, Li T, Tuxun T, Shalayiadang P, Ran B, Jiang TM, Zhang RQ, He YB, Huang JF, Wen H. Ex vivo liver resection and autotransplantation as alternative to allotransplantation for end-stage hepatic alveolar echinococcosis. J Hepatol. 2018;69:1037-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 15. | Pak LM, Chakraborty J, Gonen M, Chapman WC, Do RKG, Groot Koerkamp B, Verhoef K, Lee SY, Massani M, van der Stok EP, Simpson AL; Memorial Sloan Kettering Cancer Center Hepatopancreatobiliary Service. Quantitative Imaging Features and Postoperative Hepatic Insufficiency: A Multi-Institutional Expanded Cohort. J Am Coll Surg. 2018;226:835-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Markar SR, Mikhail S, Malietzis G, Athanasiou T, Mariette C, Sasako M, Hanna GB. Influence of Surgical Resection of Hepatic Metastases From Gastric Adenocarcinoma on Long-term Survival: Systematic Review and Pooled Analysis. Ann Surg. 2016;263:1092-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 17. | Rammohan A, Govil S, Vargese J, Kota V, Reddy MS, Rela M. Changing pattern of biliary complications in an evolving liver transplant unit. Liver Transpl. 2017;23:478-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Ikegami T, Yoshizumi T, Sakata K, Uchiyama H, Harimoto N, Harada N, Itoh S, Nagatsu A, Soejima Y, Maehara Y. Left lobe living donor liver transplantation in adults: What is the safety limit? Liver Transpl. 2016;22:1666-1675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 19. | Haiwen Y, Ying Z, Qian L, Lingqiang Z, Yong D, Xiaolei X. Small-for-size syndrome in hepatic alveolar echinococcosis treated by ex vivo liver resection and autotransplantation: A case report. Shiyong Gandanbing Zazhi. 2019;35:858-860. [DOI] [Full Text] |
| 20. | Qiu Y, Yang X, Shen S, Huang B, Wang W. Vascular infiltration-based surgical planning in treating end-stage hepatic alveolar echinococcosis with ex vivo liver resection and autotransplantation. Surgery. 2019;165:889-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 21. | Kato T, Hwang R, Liou P, Weiner J, Griesemer A, Samstein B, Halazun K, Mathur A, Schwartz G, Cherqui D, Emond J. Ex Vivo Resection and Autotransplantation for Conventionally Unresectable Tumors - An 11-year Single Center Experience. Ann Surg. 2020;272:766-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Zawistowski M, Nowaczyk J, Jakubczyk M, Domagała P. Outcomes of ex vivo liver resection and autotransplantation: A systematic review and meta-analysis. Surgery. 2020;168:631-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |