Published online Jun 16, 2021. doi: 10.12998/wjcc.v9.i17.4210
Peer-review started: December 24, 2020
First decision: January 7, 2021
Revised: February 24, 2021
Accepted: March 29, 2021
Article in press: March 29, 2021
Published online: June 16, 2021
Processing time: 152 Days and 23.7 Hours
Tranexamic acid (TXA) has been used as an anti-fibrinolytic drug for over half a century and has received much attention in recent decades.
To evaluate the efficacy of topical vs intravenous TXA in reducing blood loss and promoting wound healing in bone surgery.
From the electronic resources, PubMed, Cochrane Library, Embase, ISI, and Scopus were used to perform a literature search over the last 10 years between 2010 and 2020. EndNote™ X8 was used for managing the electronic resource. Searches were performed with mesh terms. The data were retracted blindly by two independent reviewers. Random effects were used to deal with potential heterogeneity and I2 showed heterogeneity. Chi-square (I2) tests were used to quantify the extent of heterogeneity (P < 0.01 was considered statistically significant). The efficacy of topical TXA in reducing blood loss and promoting wound healing in bone surgery was compared with intravenous TXA and placebo.
According to the research design, 1360 potentially important research abstracts and titles were discovered in our electronic searches, and 18 papers remained in agreement with our inclusion criteria. It was found that TXA reduced 277.51 mL of blood loss compared to placebo, and there was no significant difference between topical TXA and IV TXA in reducing blood loss in bone surgery. Our analyses also showed that TXA significantly reduced blood transfusion compared to placebo and there was no significant difference between topical TXA and IV TXA.
The use of both topical and intravenous TXA are equally effective in reducing blood loss in bone surgery, which might be beneficial for wound healing after surgery.
Core Tip: Although tranexamic acid (TXA) is regularly used by surgeons, a comprehensive guideline on safe topical doses and methods for TXA administration has remained controversial. This study showed that both topical and intravenous TXA are equally effective in reducing blood loss in bone surgery, which is thus beneficial for wound healing after surgery.
- Citation: Xu JW, Qiang H, Li TL, Wang Y, Wei XX, Li F. Efficacy of topical vs intravenous tranexamic acid in reducing blood loss and promoting wound healing in bone surgery: A systematic review and meta-analysis. World J Clin Cases 2021; 9(17): 4210-4220
- URL: https://www.wjgnet.com/2307-8960/full/v9/i17/4210.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i17.4210
Wound healing is a natural biological process, in which all four stages, including homeostasis (stop bleeding), inflammation, proliferation, and maturation, must occur within a time frame for successful wound healing[1,2]. The use of tranexamic acid (TXA) as an anti-fibrinolytic drug has been available for over half a century and has received much attention in recent decades[3]. By binding to plasminogen, TXA prevents the conversion of plasminogen to plasmin, thus preventing fibrinolysis[4]. The use of TXA reduces blood loss and blood transfusion in major orthopedic surgery, and the safety is also well recognized[5-8]. Previous studies have not confirmed any increased risk of thromboembolism after the use of TXA in various surgeries[9-11]. Topical use of TXA is increasingly popular today, but surgeons do not have a comprehensive guideline on safe topical doses and methods of administration, as topical use is still off-label[12]. There have been two meta-analysis studies discussing efficacy of topical vs intravenous TXA in total hip arthroplasty and total knee arthroplasty, respectively[13,14]. However, the efficacy of topical vs intravenous TXA in reducing blood loss and promoting wound healing in bone surgery remains to be systemically reviewed.
Therefore, the aim of this systematic review and meta-analysis was to evaluate the efficacy of topical vs intravenous TXA in reducing blood loss and promoting wound healing in bone surgery.
From the electronic resources, PubMed, Cochrane Library, Embase, ISI, and Scopus were used to perform a literature search over the last 10 years between 2010 and 2020. EndNote™ X8 was used for managing the electronic resources. Searches were performed with mesh terms: (“Tranexamic Acid/administration and dosage”[Mesh] OR “Tranexamic Acid/adverse effects”[Mesh] OR “Tranexamic Acid/blood”[Mesh] OR “Tranexamic Acid/standards”[Mesh] OR “Tranexamic Acid/toxicity”[Mesh])) AND (“Wound Healing/blood”[Mesh] OR “Wound Healing/blood supply”[Mesh] OR “Wound Healing/complications”[Mesh] OR “Wound Healing/drug effects”[Me
The present systematic review and meta-analysis protocol was prepared by PRISMA checklist[15], and Population/Patient, Exposure/Intervention, Comparison, and Outcome strategy (Table 1).
| PICO or PECO strategy | Description |
| P | Population/Patient: Adult patients |
| E | Exposure/Intervention: Tranexamic acid |
| C | Comparison: Placebo or standard care |
| O | Outcome: Blood loss |
Inclusion criteria: Randomized controlled trials, controlled clinical trials, and prospective and retrospective cohort studies; human; topical TXA or intravenously administered TXA; adults; bone surgery trials; and in English.
Exclusion criteria: In vitro studies, case studies, case reports, and reviews; animal studies; oral TXA; and studies without a control group.
Data extraction and method of analysis: The data were extracted from the related studies including years, study design, number of patients, mean/range of age, interventions group, control group, and clinical endpoints. The quality of studies included was assessed using the Cochrane Collaboration’s tool[16]. The scale score for low risk was 1 and that for high and unclear risk was 0. Scale scores ranged from 0 to 6. A higher score indicated higher quality.
Two reviewers blindly and independently extracted the data. Odds ratio (OR) with 95% confidence interval (CI), fixed effects model and Mantel-Haenszel method and mean difference with 95%CI, random effect model and restricted maximum likelihood method were calculated. Random effects were used to deal with potential heterogeneity and I2 showed heterogeneity. Chi-square (I2) tests were performed to quantify the extent of heterogeneity (P value < 0.01 was considered statistically significant). I2 values > 50% indicated moderate-to-high heterogeneity. Software Stata/MP v.16 (fastest version of Stata) was used for statistical analysis.
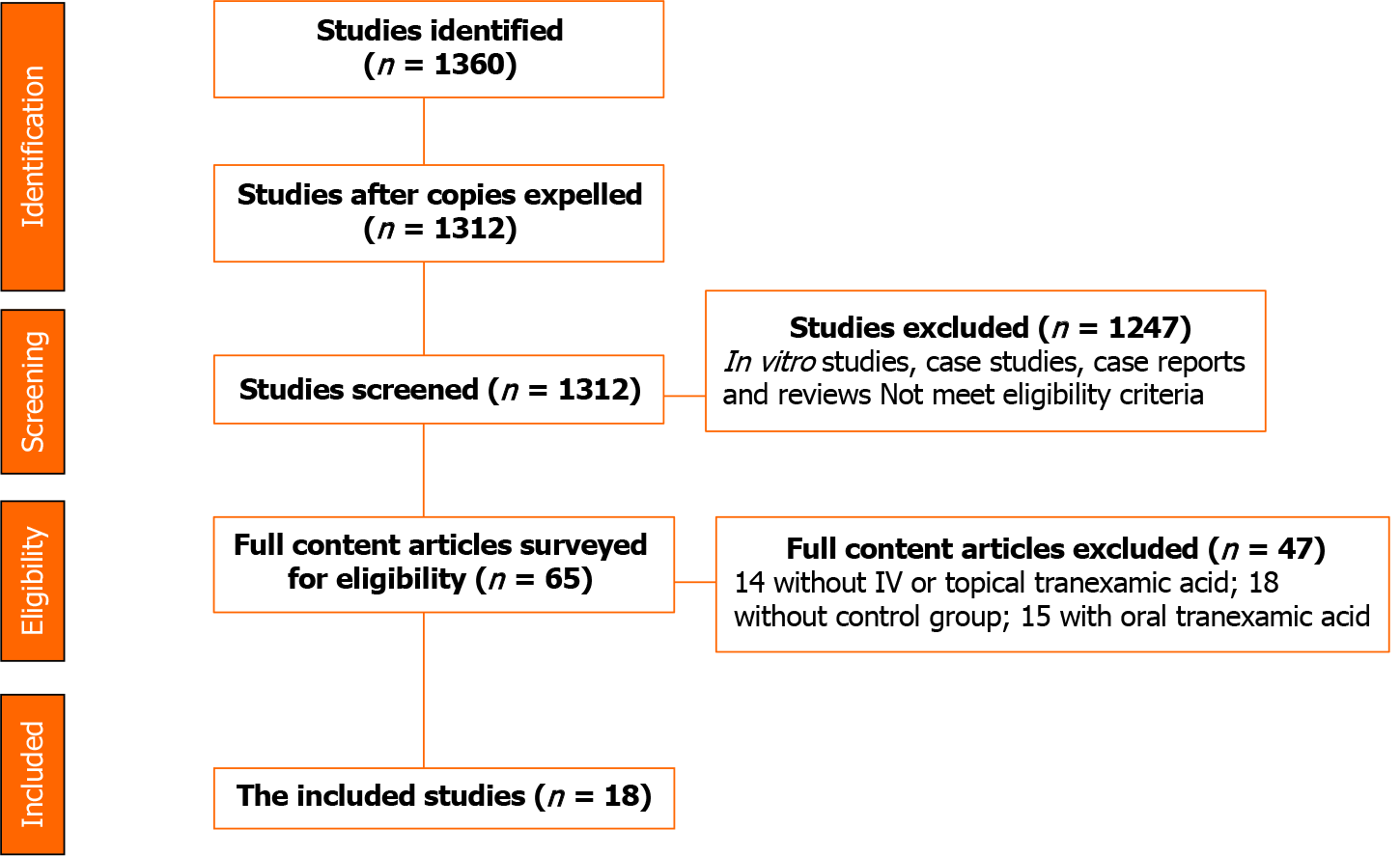
According to the research design, 1360 potentially important research abstracts and titles were discovered in our electronic searches. In the first phase of the study selection, 1312 studies were left after removing copies. Then 1247 in vitro studies, case studies, case reports, and reviews or those that did not meet the eligibility criteria were excluded. Therefore, we fully assessed the complete full-text papers of the remaining 65 studies in the second stage, and 47 publications were excluded due to the lack of the defined inclusion criteria. Finally, 18 papers remained in agreement with our inclusion criteria required (Figure 1).
Eighteen studies (randomized controlled trials) were included. The total sample size was 1994. All of the studies evaluated the efficacy of TXA in bone surgical patients. In detail, nine studies evaluated the efficacy of TXA in total knee arthroplasty, two evaluated the efficacy of TXA in trochanteric fracture surgery, one evaluated the efficacy of TXA in intertrochanteric fractures, two evaluated the efficacy of TXA in total shoulder arthroplasty, two evaluated the efficacy of TXA in total hip replacement and one evaluated the efficacy of TXA in orthognathic surgery (Table 2)[17-34].
| Ref. | Study design | Sample size | Procedure | Intervention group and control group | |
| 1 | Lei et al[17], 2020 | RCT | 132 | Total knee arthroplasty | IV TXA, placebo |
| 2 | Luo et al[18], 2019 | RCT | 90 | Trochanteric fracture surgery | IV TXA, placebo |
| 3 | Chen et al[19], 2019 | RCT | 166 | Trochanteric fracture surgery | IV TXA, placebo |
| 4 | Zhang et al[20], 2019 | RCT | 50 | Total knee arthroplasty | Topical TXA, IV TXA |
| 5 | Zhou et al[21], 2019 | RCT | 100 | Intertrochanteric fractures | Topical TXA (1 g), placebo |
| 6 | Cvetanovich et al[22], 2018 | RCT | 110 | Total shoulder arthroplasty | TXA, placebo |
| 7 | Huang et al[23], 2017 | RCT | 150 | Total knee arthroplasty | Topical TXA (1 g), IV TXA, placebo |
| 8 | Vara et al[24], 2017 | RCT | 102 | Total shoulder arthroplasty | Topical TXA, placebo |
| 9 | Goyal et al[25], 2017 | RCT | 168 | Total knee arthroplasty | TXA, IV TXA |
| 10 | Chen et al[26], 2016 | RCT | 100 | Total knee arthroplasty | Topical TXA, IV TXA |
| 11 | Drosos et al[27], 2016 | RCT | 90 | Total knee arthroplasty | Topical TXA: 1 g, placebo, IV TXA |
| 12 | Keyhan et al[28], 2016 | RCT | 120 | Total knee arthroplasty | Topical TXA: 3 g, placebo, IV TXA (500 g) |
| 13 | North et al[29], 2016 | RCT | 139 | Total hip replacement | Topical TXA: 2 g, IV TXA (2 g) |
| 14 | Aguilera et al[30], 2015 | RCT | 150 | Total knee arthroplasty | Topical TXA: 1 g, IV TXA (2 g), placebo |
| 15 | Eftekharian et al[31], 2015 | RCT | 56 | Orthognathic surgery | Topical TXA: 1 g, placebo |
| 16 | Gillespie et al[32], 2015 | RCT | 111 | Total shoulder arthroplasty | Topical TXA: 2 g, placebo |
| 17 | Taheriazam et al[33], 2015 | RCT | 80 | Total hip replacement | Topical TXA, IV TXA |
| 18 | Yang et al[34], 2015 | RCT | 80 | Total knee arthroplasty | Topical TXA, placebo |
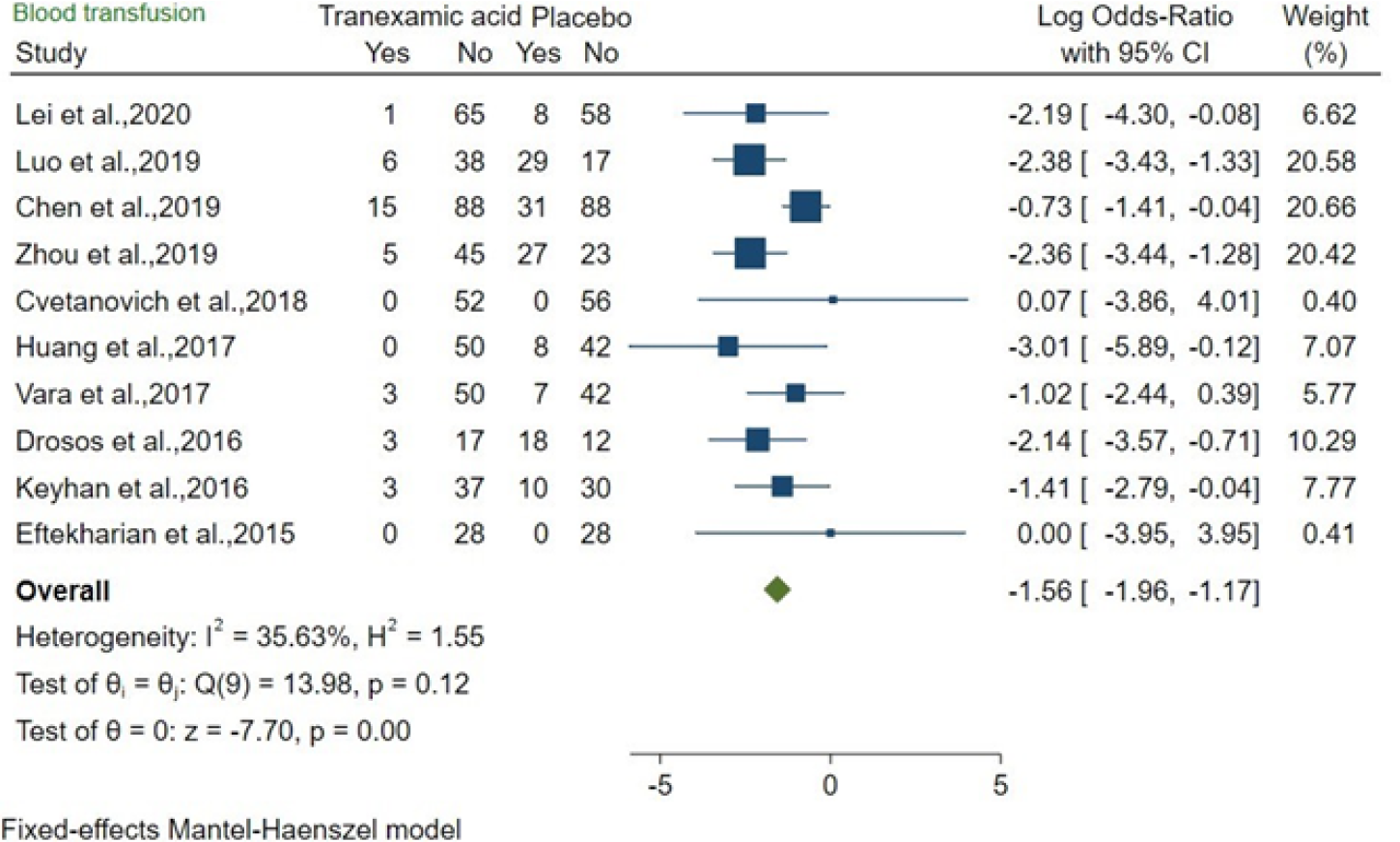
The effects of TXA and placebo were compared in 10 studies about bone surgery. The OR was -1.56 (95%CI: -1.96 to -1.17; P = 0.00), and moderate heterogeneity was found
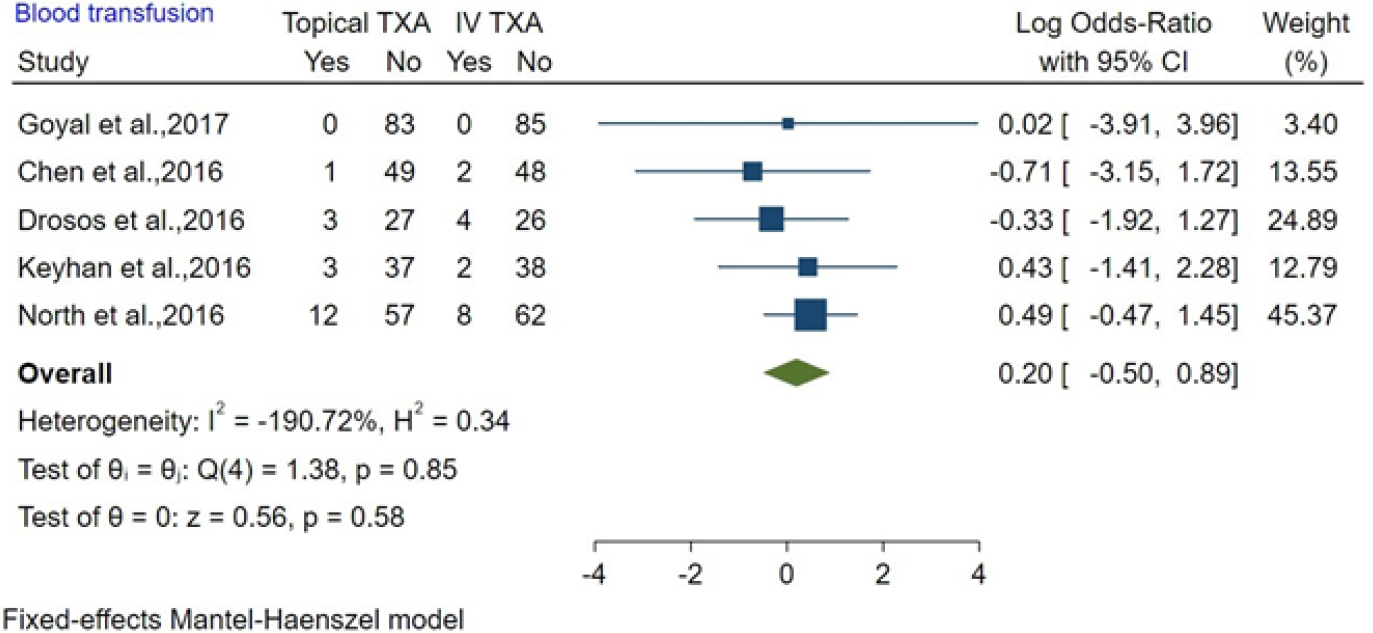
The effects of topical TXA and IV TXA were compared in five studies about bone surgery. The OR was 0.20 (95%CI: -0.50 to 0.89; P = 0.58), and there was mild heterogeneity (I2 < 0%). Our results showed there was no significant difference between topical TXA and IV TXA in reducing blood transfusion in bone surgery (Figure 3).
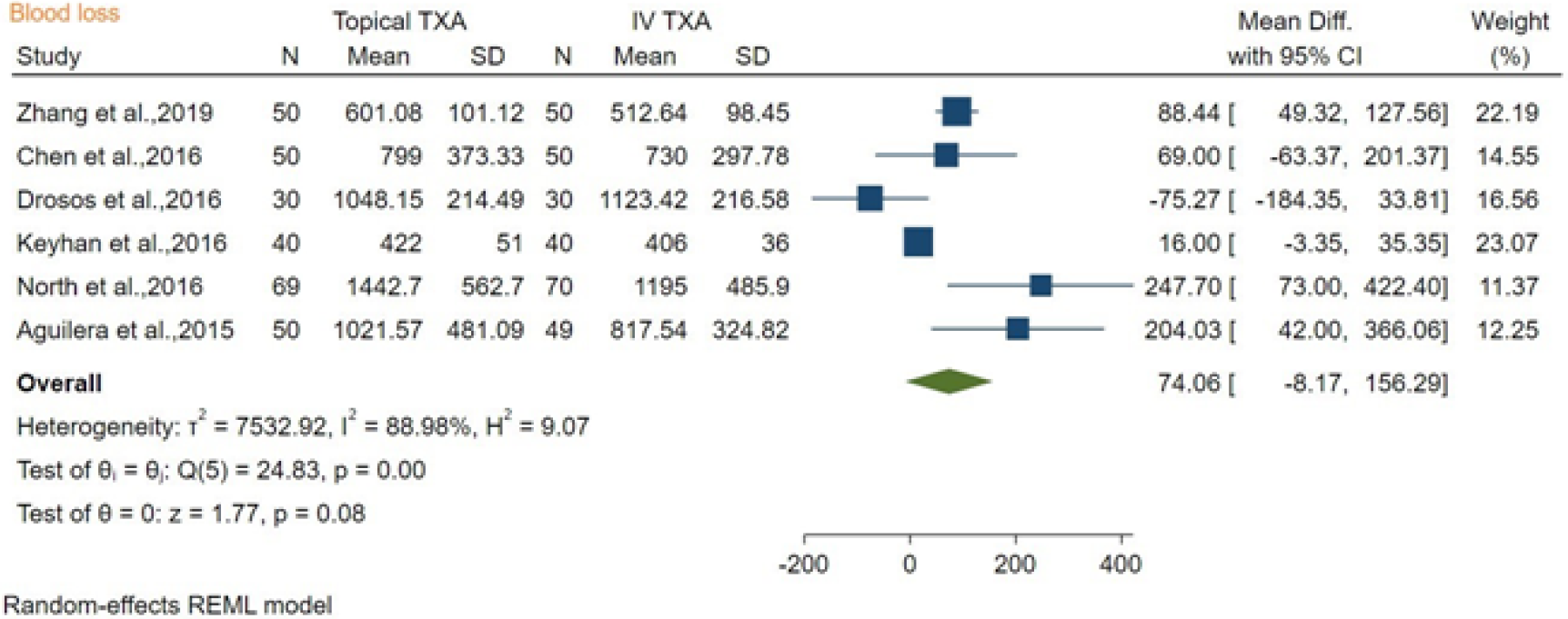
The blood loss after topical TXA vs IV TXA was compared among six studies about bone surgery, and the mean difference was 74.06 mL (mean difference [MD]: 74.06, 95%CI: -8.17 to 156.39; P = 0.08), with high heterogeneity found (I2 = 88.98%). Our results showed there was no significant difference between topical TXA and IV TXA in reducing blood loss in bone surgery (Figure 4).
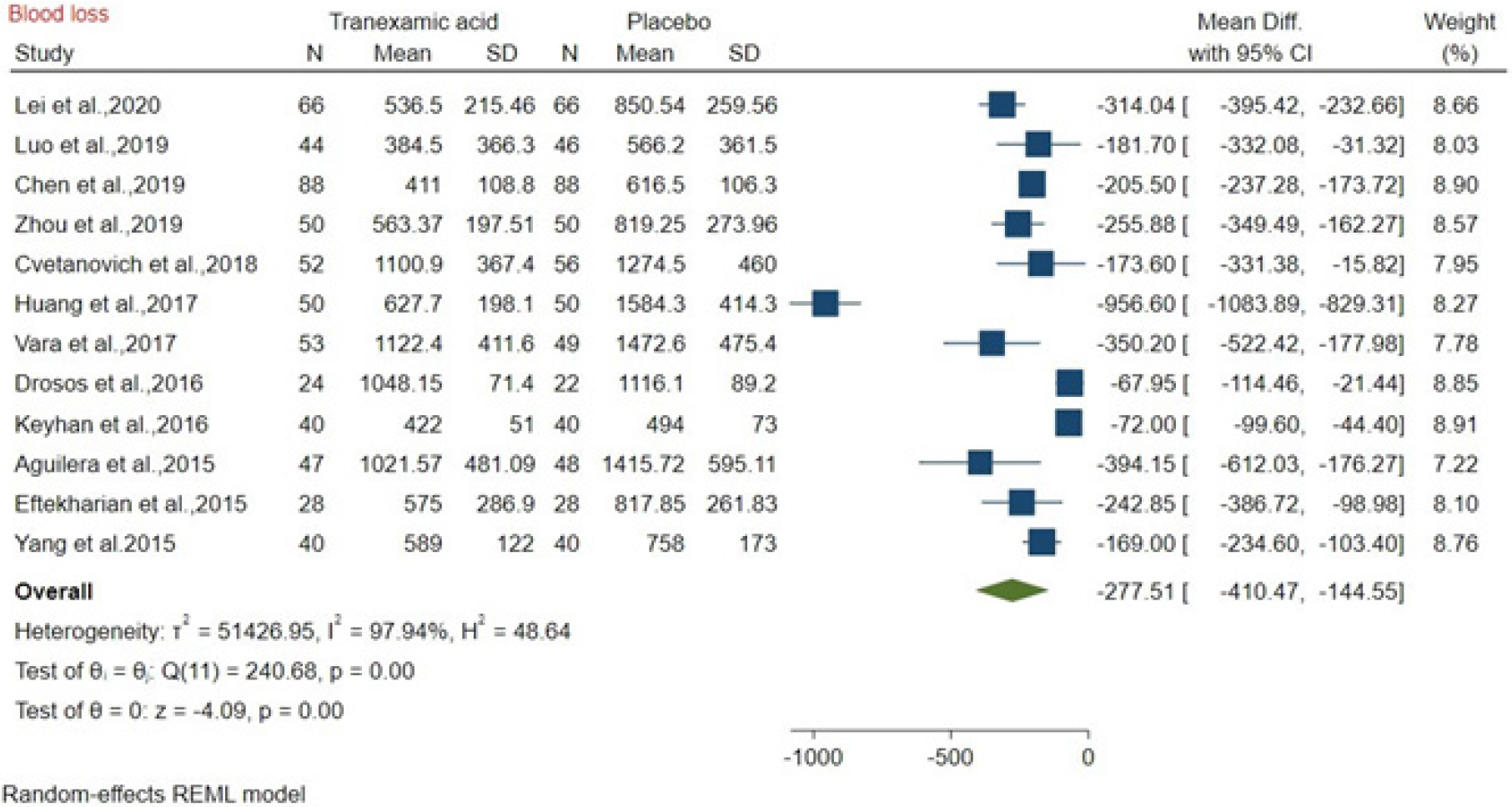
The blood loss after TXA vs placebo administration was compared among 12 studies about bone surgery, and the mean difference was -277.51 mL (MD: -277.51, 95%CI: -410.47 to -144.5; P = 0.00), with high heterogeneity (I2 = 97.94%). The results showed that TXA reduced 277.51 mL of blood loss compared to placebo (Figure 5).
The present meta-analysis showed that TXA reduced 277.51 mL of blood loss compared to placebo in bone surgery, and there was no significant difference between topical TXA and IV TXA in reducing blood loss. Moreover, TXA significantly reduced blood transfusion compared to placebo in bone surgery and there was no significant difference between topical TXA and IV TXA. In a systematic review and meta-analysis study with a sample size of 10488 patients[35], regardless of the type of TXA administration, it was shown that 30% of patients only needed an injection. These results were consistent with our study. If a theoretical comparison is made between the topical TXA and IV TXA, the topical TXA would result in a 90% reduction in plasma concentra
Much blood loss is common in bone surgery, which is a major source of mortality, and blood transfusions are often required during the perioperative period. However, blood transfusions may lead to increased length of hospital stay, a raised risk of infection, and an increased medical cost[42-44]. TXA prevents the conversion of plasminogen to plasmin, thus preventing fibrinolysis and blood loss[4]. Thus, it is clinically significant to use TXA to reduce blood loss and transfusion in bone surgery, which might be beneficial for wound healing.
However, our study also had some limitations. First, the optimal dose and timing of the topical TXA were not evaluated in our study due to lack of clinical guideline for TXA and inconsistency in dose and timing of TXA across studies, which remain to be evaluated in the further research. Second, significant heterogeneity was detected in blood loss and our findings remain to be further verified by more well-designed studies.
We found that the use of both topical and intravenous TXA are effective in reducing blood loss and might be beneficial for wound healing in bone surgery. Given the consideration of smaller dose and less medical cost, topical TXA is recommended for bone surgery. However, more studies are needed to further verify our findings in the future.
Tranexamic acid (TXA) as an anti-fibrinolytic drug has been available for over half a century and Topical use of TXA is more and more popular today.
Although TXA is regularly used in surgeons, a comprehensive guideline on safe topical doses and methods for TXA administration has remained controversial.
This study evaluated the efficacy of topical vs intravenous TXA in reducing blood loss and promoting wound healing in bone surgery.
From the electronic resources, PubMed, Cochrane Library, Embase, ISI, and Scopus were used to perform a literature search over the last 10 years between 2010 and 2020. EndNote™ X8 was used for managing the electronic resource. Searches were performed with mesh terms. The data were retracted blindly by two independent reviewers. Random effects were used to deal with potential heterogeneity and I2 showed heterogeneity. Chi-square (I2) tests were used to quantify the extent of heterogeneity (P < 0.01 was considered statistically significant). The efficacy of topical TXA in reducing blood loss and promoting wound healing in bone surgery was compared with intravenous TXA and placebo.
According to the research design, 1360 potentially important research abstracts and titles were discovered in our electronic searches, and eighteen papers remained in agreement with our inclusion criteria required. It was found that TXA reduced 277.51 mL of blood loss compared to placebo, and there was no significant difference between topical TXA and IV TXA in reducing blood loss in bone surgery. Our analysis also showed that TXA significantly reduced blood transfusion compared to placebo and there was no significant difference between topical TXA and IV TXA.
This meta-analysis showed that both topical and intravenous TXA are effective in reducing blood loss and might be beneficial for wound healing in bone surgery. Given the consideration of smaller dose and less medical cost, topical TXA is recommended for bone surgery.
Both topical and intravenous TXA are effective in reducing blood loss and might be beneficial for wound healing in bone surgery.
Manuscript source: Unsolicited manuscript
Specialty type: Chemistry, medicinal
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Leite CBG S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89:219-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3887] [Cited by in RCA: 3205] [Article Influence: 213.7] [Reference Citation Analysis (0)] |
| 2. | Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound Healing: A Cellular Perspective. Physiol Rev. 2019;99:665-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1040] [Cited by in RCA: 1652] [Article Influence: 275.3] [Reference Citation Analysis (0)] |
| 3. | Tengborn L, Blombäck M, Berntorp E. Tranexamic acid--an old drug still going strong and making a revival. Thromb Res. 2015;135:231-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 216] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 4. | Björlin G, Nilsson IM. The effect of antifibrinolytic agents on wound healing. Int J Oral Maxillofac Surg. 1988;17:275-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Huang F, Wu D, Ma G, Yin Z, Wang Q. The use of tranexamic acid to reduce blood loss and transfusion in major orthopedic surgery: a meta-analysis. J Surg Res. 2014;186:318-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 6. | Hu M, Liu ZB, Bi G. Efficacy and safety of tranexamic acid in orthopaedic trauma surgery: a meta-analysis. Eur Rev Med Pharmacol Sci. 2019;23:11025-11031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 7. | Amer KM, Rehman S, Amer K, Haydel C. Efficacy and Safety of Tranexamic Acid in Orthopaedic Fracture Surgery: A Meta-Analysis and Systematic Literature Review. J Orthop Trauma. 2017;31:520-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Nishiwaki T, Oya A, Fukuda S, Nakamura S, Nakamura M, Matsumoto M, Kanaji A. Curved periacetabular osteotomy via a novel intermuscular approach between the sartorius and iliac muscles. Hip Int. 2018;28:642-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | CRASH-2 collaborators, Roberts I, Shakur H, Afolabi A, Brohi K, Coats T, Dewan Y, Gando S, Guyatt G, Hunt BJ, Morales C, Perel P, Prieto-Merino D, Woolley T. The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH-2 randomised controlled trial. Lancet 2011; 377: 1096-1101, 1101.e1-1101. e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 678] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 10. | Roberts I, Belli A, Brenner A, Chaudhri R, Fawole B, Harris T, Jooma R, Mahmood A, Shokunbi T, Shakur H; CRASH-3 trial collaborators. Tranexamic acid for significant traumatic brain injury (The CRASH-3 trial): Statistical analysis plan for an international, randomised, double-blind, placebo-controlled trial. Wellcome Open Res. 2018;3:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105-2116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1038] [Cited by in RCA: 901] [Article Influence: 112.6] [Reference Citation Analysis (0)] |
| 12. | Eikebrokk TA, Vassmyr BS, Ausen K, Gravastrand C, Spigset O, Pukstad B. Cytotoxicity and effect on wound re-epithelialization after topical administration of tranexamic acid. BJS Open. 2019;3:840-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Lin C, Qi Y, Jie L, Li HB, Zhao XC, Qin L, Jiang XQ, Zhang ZH, Ma L. Is combined topical with intravenous tranexamic acid superior than topical, intravenous tranexamic acid alone and control groups for blood loss controlling after total knee arthroplasty: A meta-analysis. Medicine (Baltimore). 2016;95:e5344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Tuttle JR, Feltman PR, Ritterman SA, Ehrlich MG. Effects of Tranexamic Acid Cytotoxicity on In Vitro Chondrocytes. Am J Orthop (Belle Mead NJ). 2015;44:E497-E502. [PubMed] |
| 15. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18665] [Cited by in RCA: 17545] [Article Influence: 1096.6] [Reference Citation Analysis (1)] |
| 16. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. Cochrane bias methods group; cochrane statistical methods group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:5928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18487] [Cited by in RCA: 24867] [Article Influence: 1776.2] [Reference Citation Analysis (3)] |
| 17. | Lei YT, Xie JW, Huang Q, Huang W, Pei FX. The antifibrinolytic and anti-inflammatory effects of a high initial-dose tranexamic acid in total knee arthroplasty: a randomized controlled trial. Int Orthop. 2020;44:477-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | Luo X, He S, Lin Z, Li Z, Huang C, Li Q. Efficacy and Safety of Tranexamic Acid for Controlling Bleeding During Surgical Treatment of Intertrochanteric Fragility Fracture with Proximal Femoral Nail Anti-rotation: A Randomized Controlled Trial. Indian J Orthop. 2019;53:263-269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Chen F, Jiang Z, Li M, Zhu X. Efficacy and safety of perioperative tranexamic acid in elderly patients undergoing trochanteric fracture surgery: a randomised controlled trial. Hong Kong Med J. 2019;25:120-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Zhang YM, Yang B, Sun XD, Zhang Z. Combined intravenous and intra-articular tranexamic acid administration in total knee arthroplasty for preventing blood loss and hyperfibrinolysis: A randomized controlled trial. Medicine (Baltimore). 2019;98:e14458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Zhou XD, Zhang Y, Jiang LF, Zhang JJ, Zhou D, Wu LD, Huang Y, Xu NW. Efficacy and Safety of Tranexamic Acid in Intertrochanteric Fractures: A Single-Blind Randomized Controlled Trial. Orthop Surg. 2019;11:635-642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 22. | Cvetanovich GL, Fillingham YA, O’Brien M, Forsythe B, Cole BJ, Verma NN, Romeo AA, Nicholson GP. Tranexamic acid reduces blood loss after primary shoulder arthroplasty: a double-blind, placebo-controlled, prospective, randomized controlled trial. JSES Open Access. 2018;2:23-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 23. | Huang Z, Xie X, Li L, Huang Q, Ma J, Shen B, Kraus VB, Pei F. Intravenous and Topical Tranexamic Acid Alone Are Superior to Tourniquet Use for Primary Total Knee Arthroplasty: A Prospective, Randomized Controlled Trial. J Bone Joint Surg Am. 2017;99:2053-2061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 24. | Vara AD, Koueiter DM, Pinkas DE, Gowda A, Wiater BP, Wiater JM. Intravenous tranexamic acid reduces total blood loss in reverse total shoulder arthroplasty: a prospective, double-blinded, randomized, controlled trial. J Shoulder Elbow Surg. 2017;26:1383-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 25. | Goyal N, Chen DB, Harris IA, Rowden NJ, Kirsh G, MacDessi SJ. Intravenous vs Intra-Articular Tranexamic Acid in Total Knee Arthroplasty: A Randomized, Double-Blind Trial. J Arthroplasty. 2017;32:28-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Chen JY, Chin PL, Moo IH, Pang HN, Tay DK, Chia SL, Lo NN, Yeo SJ. Intravenous versus intra-articular tranexamic acid in total knee arthroplasty: A double-blinded randomised controlled noninferiority trial. Knee. 2016;23:152-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 27. | Drosos GI, Ververidis A, Valkanis C, Tripsianis G, Stavroulakis E, Vogiatzaki T, Kazakos K. A randomized comparative study of topical versus intravenous tranexamic acid administration in enhanced recovery after surgery (ERAS) total knee replacement. J Orthop. 2016;13:127-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Keyhani S, Esmailiejah AA, Abbasian MR, Safdari F. Which Route of Tranexamic Acid Administration is More Effective to Reduce Blood Loss Following Total Knee Arthroplasty? Arch Bone Jt Surg. 2016;4:65-69. [PubMed] |
| 29. | North WT, Mehran N, Davis JJ, Silverton CD, Weir RM, Laker MW. Topical vs Intravenous Tranexamic Acid in Primary Total Hip Arthroplasty: A Double-Blind, Randomized Controlled Trial. J Arthroplasty. 2016;31:928-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Aguilera X, Martínez-Zapata MJ, Hinarejos P, Jordán M, Leal J, González JC, Monllau JC, Celaya F, Rodríguez-Arias A, Fernández JA, Pelfort X, Puig-Verdie Ll. Topical and intravenous tranexamic acid reduce blood loss compared to routine hemostasis in total knee arthroplasty: a multicenter, randomized, controlled trial. Arch Orthop Trauma Surg. 2015;135:1017-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 31. | Eftekharian H, Vahedi R, Karagah T, Tabrizi R. Effect of tranexamic acid irrigation on perioperative blood loss during orthognathic surgery: a double-blind, randomized controlled clinical trial. J Oral Maxillofac Surg. 2015;73:129-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Gillespie R, Shishani Y, Joseph S, Streit JJ, Gobezie R. Neer Award 2015: A randomized, prospective evaluation on the effectiveness of tranexamic acid in reducing blood loss after total shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24:1679-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 33. | Lacko M, Cellar R, Schreierova D, Vasko G. Comparison of intravenous and intra-articular tranexamic acid in reducing blood loss in primary total knee replacement. Eklem Hastalik Cerrahisi. 2017;28:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Yang Y, Lv YM, Ding PJ, Li J, Ying-Ze Z. The reduction in blood loss with intra-articular injection of tranexamic acid in unilateral total knee arthroplasty without operative drains: a randomized controlled trial. Eur J Orthop Surg Traumatol. 2015;25:135-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 35. | Ker K, Edwards P, Perel P, Shakur H, Roberts I. Effect of tranexamic acid on surgical bleeding: systematic review and cumulative meta-analysis. BMJ. 2012;344:e3054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 711] [Cited by in RCA: 645] [Article Influence: 49.6] [Reference Citation Analysis (1)] |
| 36. | Abrishami A, Wong J, El-Beheiry H, Hasan S, Chung F. Intra-articular application of tranexamic acid for perioperative blood loss in total knee arthroplasty: a randomized controlled trial. Can J Anesth. 2009;56:138. |
| 37. | McCormack PL. Tranexamic acid: a review of its use in the treatment of hyperfibrinolysis. Drugs. 2012;72:585-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 455] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 38. | Pabinger I, Fries D, Schöchl H, Streif W, Toller W. Tranexamic acid for treatment and prophylaxis of bleeding and hyperfibrinolysis. Wien Klin Wochenschr. 2017;129:303-316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 39. | Montroy J, Hutton B, Moodley P, Fergusson NA, Cheng W, Tinmouth A, Lavallée LT, Fergusson DA, Breau RH. The efficacy and safety of topical tranexamic acid: A systematic review and meta-analysis. Transfus Med Rev. 2018;Online ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 40. | Drakos A, Raoulis V, Karatzios K, Doxariotis N, Kontogeorgakos V, Malizos K, Varitimidis SE. Efficacy of Local Administration of Tranexamic Acid for Blood Salvage in Patients Undergoing Intertrochanteric Fracture Surgery. J Orthop Trauma. 2016;30:409-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 41. | Baric D, Unic D, Rudez I, Bacic-Vrca V, Planinc M, Jonjic D. Systemic usage of tranexamic acid is superior to topical: Randomized placebo-controlled trial. Interact Cardiovasc Thorac Surg. 2011;12:S92. |
| 42. | Hill GE, Frawley WH, Griffith KE, Forestner JE, Minei JP. Allogeneic blood transfusion increases the risk of postoperative bacterial infection: a meta-analysis. J Trauma. 2003;54:908-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 382] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 43. | Saleh A, Small T, Chandran Pillai AL, Schiltz NK, Klika AK, Barsoum WK. Allogenic blood transfusion following total hip arthroplasty: results from the nationwide inpatient sample, 2000 to 2009. J Bone Joint Surg Am. 2014;96:e155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 44. | Shokoohi A, Stanworth S, Mistry D, Lamb S, Staves J, Murphy MF. The risks of red cell transfusion for hip fracture surgery in the elderly. Vox Sang. 2012;103:223-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |