Published online Jun 16, 2021. doi: 10.12998/wjcc.v9.i17.4178
Peer-review started: January 9, 2021
First decision: January 29, 2021
Revised: February 12, 2021
Accepted: April 22, 2021
Article in press: April 22, 2021
Published online: June 16, 2021
Processing time: 136 Days and 20.1 Hours
Functional bowel disorder (FBD) may be caused by a decrease in disaccharidase activity. Thus, the timely diagnosis of disaccharidase deficiency could lead to a better prognosis in patients with this condition.
To determine the potential value of intestinal disaccharidases glucoamylase, maltase, sucrase, and lactase in understanding the etiology and pathogenesis of FBD.
A total of 82 FBD patients were examined. According to the Rome IV criteria (2016), 23 patients had diarrhea-predominant irritable bowel syndrome (IBS), 33 had functional diarrhea, 10 had constipation-predominant IBS, 4 had functional constipation, and 12 had mixed IBS. The Dahlqvist method was used to measure disaccharidase activity in the brush-border membrane of mature enterocytes of the small intestine, in duodenal biopsies obtained during esophagogastroduodenoscopy.
Lactase deficiency was detected in 86.5% of patients, maltase deficiency in 48.7%, sucrase deficiency in 50%, and glucoamylase deficiency in 84.1%. The activities of all enzymes were reduced in 31.7% of patients, and carbohydrase deficiency was detected in 63.5% of patients. The low activity of enzymes involved in membrane digestion in the small intestine was found in 95.2% of patients.
In 78 of the 82 patients with FBD, gastrointestinal symptoms were associated with disaccharidase deficiency.
Core Tip: Our study has shown that patients with functional bowel disorders (FBDs) often had a deficiency of intestinal enzymes. We supposed that there can be a different mechanism for developing FBD: A damage of the small intestine mucous membrane may result in a decreased activity of the enzymes on the brush border of enterocytes, which leads to the disorder of carbohydrates' hydrolysis and the accumulation of osmoactive substances in the lumen of the small intestine, in its turn it causes a bacterial overgrowth in the small intestine, stool disorder, abdominal pain and bloating. The timely diagnosis of disaccharidase deficiency could lead to a better prognosis in patients with FBD.
- Citation: Dbar S, Akhmadullina O, Sabelnikova E, Belostotskiy N, Parfenov A, Bykova S, Bakharev S, Baulo E, Babanova A, Indeykina L, Kuzmina T, Kosacheva T, Spasenov A, Makarova A. Patients with functional bowel disorder have disaccharidase deficiency: A single-center study from Russia. World J Clin Cases 2021; 9(17): 4178-4187
- URL: https://www.wjgnet.com/2307-8960/full/v9/i17/4178.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i17.4178
Functional bowel disorder (FBD) clinically manifests as changes in stool frequency and consistency, sometimes accompanied by abdominal pain associated with defecation[1]. According to the Rome IV criteria (2016), FBD includes irritable bowel syndrome (IBS), functional diarrhea (FD), and functional constipation (FC). Their etiology is unknown and the pathogenesis is caused by visceral hypersensitivity and intestinal motility disorders, which are induced by dysfunction of the central nervous system and immune system, and alterations in intestinal microbiota[2]. Conventional light microscopy has shown that the intestine of patients with FBD lacks morphological changes. However, changes remain a possibility because functional pathology cannot exist in principle alone, as the function of the body is a reflection of structural changes[3]. The changes may exist at the molecular level, which would require different technology for detection. In addition, clinical symptoms of FBD have been observed in cases of intestinal digestion disorders caused by a deficiency of membrane enzymes in the small intestine mucosa[4].
According to the digestion theory stated by Ugolev in the 1960s, the final hydrolysis of oligosaccharides is carried out by disaccharidases/carbohydrases. Disaccharidases or carbohydrases are glycoside hydrolase enzymes located on the brush border of enterocytes, which break down disaccharides into monosaccharides that can be absorbed in the small intestine[4]. Carbohydrase deficiency of small intestine mucosa can lead to the development of IBS[5]. Various methods are used to diagnose disaccharidase deficiency with varying success, including breath tests, genetic tests, and even an oral trial of the various formulations of the enzyme[6,7]. To assess disaccharidase activity, the Dahlqvist method uses intestinal homogenate extracted from duodenal biopsies taken during esophagogastroduodenoscopy (EGD), which then is incubated with various disaccharide substrates[8]. This method with some modifications is currently considered the gold standard for the diagnosis of IBS[9].
At present, few studies have been conducted on intestinal disaccharidase activity. Most of these studies have been on lactase activity in both adults and children[10-12] and sucrase activity in children[13]. Maltase and glucoamylase activities have not been studied in adults. Therefore, the aim of this study was to determine the value of intestinal disaccharidases glucoamylase, maltase, sucrase, and lactase in understan
This was a prospective, real time, investigator initiated, observational, single-center study conducted for 1 year in Russia. The study protocol was approved by the local scientific and ethical committee. A written informed consent was obtained from all the patients/relatives before initiating the therapy. The patients/caretakers received information about the usage, advantages and disadvantages of treatment.
In total, 82 patients with FBD participated in this study, including 39 men and 43 women with an average age of 37.4 ± 8.9 years. The inclusion criteria were the provisions of the Roman IV criteria (2016). The exclusion criteria were: Patients with “alarming symptoms” (rectal bleeding, sudden weight loss, anemia, fever, night symptoms, disease onset over 50 years of age). In the anamnesis, 6 patients were taking non-steroidal anti-inflammatory drugs (NSAIDs) 2-3 times a week for headaches during a year, 5 patients were taking antibiotics (2-3 courses of 7 d for the last year), 18 patients had acute enteric infection, 10 patients had gastrointestinal (GI) symptoms triggered by diet, 28 had a stressful situation.
All patients underwent examinations that included EGD with duodenal biopsy specimens obtained for further histological tests and research on the enzyme activity of the small intestine mucosa, as well as ultrasound examination of the abdominal cavity, examination of the colon, and if indicated, the small intestine. These examinations ruled out the presence of organic diseases of the small and large intestine as well as severe concomitant diseases. Of the 82 patients, 23 had diarrhea-predominant IBS (IBS-D), 33 had FD, 10 had constipation-predominant IBS (IBS-C), 4 had FC, and 12 had mixed IBS (IBS-M). The first group of patients included 56 patients with diarrhea (IBS-D + FD), the second group included 14 patients with constipation (IBS-C + FC), and the third group comprised 12 patients with IBS-M (Table 1). In all, 15 (18.3%) patients suffered from the disease for 6 mo to 1 year, 36 (43.9%) for 1 year to 5 years, 14 (17.0%) for 5 years to 10 years, and 17 (20.8%) for 10 years to 23 years. A total of 18 (21.9%) patients had associated symptoms of acute enteric infection, 10 (12.2%) patients had GI symptoms triggered by diet, 6 (7.3%) with NSAIDs, and 5 (6.1%) with antibiotic therapy. Twenty-eight (34.1%) patients noticed a correlation between symptoms and stressful situations, and fifteen (18.4%) did not experience symptoms.
| Symptoms | Frequency of symptoms | ||
| 1st group, n = 56 | 2nd group, n = 14 | 3rd group, n = 12 | |
| Constipation | 0 | 14/100 | 3/25 |
| Mushy stools | 36/64.2 | 0/0 | 8/66.7 |
| Watery stool | 13/23.2 | 0/0 | 0/0 |
| Constipations alternating with diarrhea | 39/69.7 | 0/0 | 9/75 |
| Stool frequency 1-5 times/d | 51/91.0 | 0/0 | 10/100 |
| Stool frequency 5-10 times/d | 5/8.9 | 0/0 | 0/0 |
| Stool frequency more than 10 times/d | 1/1.7 | 0/0 | 0/0 |
| Abdominal pain | 36/64.2 | 8/57.1 | 9/75.0 |
| Weakness | 14/25.0 | 3/21.4 | 2/16.7 |
| Rumbling in the abdomen | 40/71.4 | 9/64.2 | 12/100 |
| Abdominal bloating | 30/53.5 | 8/57.1 | 11/91.6 |
When collecting data for anamnesis, specific attention was paid to food tolerance/ intolerance and the effect of food on GI symptoms. Laboratory and instrumental research methods were conducted within the scope of standards and algorithms used for the diagnosis of FBD. Instrumental studies, in addition to X-ray enterography, colonoscopy, and diagnostic ultrasound of the abdominal organs, also included EGD with duodenum biopsy (3-5 specimens). Biochemical evaluation of intestinal (membrane) disaccharidase activity and histological evaluation of the duodenal mucosa were performed as well. In the histological preparations, the height of the intestinal villi, depth of the crypts, and their ratio were evaluated. In preparations stained with the periodic-acid Schiff reaction, the state of the brush border of the enterocytes was evaluated, with attention paid to their presence in the epithelium of goblet cells and intraepithelial lymphocytes, as well as in Paneth cells located at the base of the crypts.
The activities of the carbohydrate-splitting enzymes lactase, glucoamylase, sucrase and maltase were determined by the Dahlqvist method[14]. The study was conducted in two stages. In the first stage, the substrate was subjected to hydrolysis and the concentration of the released glucose was determined by the Trinder method, using a spectrophotometer[15]. The biopsy specimens were placed in physiological saline and homogenized, followed by three consecutive enzymatic reactions. The first reaction was hydrolysis of disaccharides. The second reaction was oxidation of the glucose by glucose oxidase to gluconic acid and hydrogen peroxide. In the third reaction, atomic oxygen released from hydrogen peroxide by peroxidase reacted with phenol and 4-aminoantipyrine to form quinoneimine (Trinder reaction). Disaccharidase activity was evaluated by the color intensity, as measured with a spectrophotometer at a wavelength of 495 nm, and was indicated in nanograms of released glucose per milligram of tissue per min (ng of glucose/mg tissue × min).
The Dahlqvist method was used to determine disaccharidase activity in small intestine mucosa. The control group comprised 20 people not suffering from intestinal diseases, who were age- and sex-matched to the patients with IBS, including 10 men and 10 women with an average age of 33.9 ± 8.7 years. The inclusion criteria of the control group were: Absence of organic and functional diseases of the small and large intestine, and written informed consent. Patients in the control group did not report and/or have complaints of any stool disorder or signs of maldigestion and malabsorption syndrome. All patients in the control group had normal histological structure of the duodenal mucosa.
To establish reference intervals and determine the reliability and effectiveness of this technique, statistical analysis was carried out to calculate the sensitivity and specificity of each enzyme (Table 2). Additionally, the receiver operating characteristic curve and area under the curve were calculated for glucoamylase, maltase, sucrase, and lactase (Table 2). SPSS Statistics software was used to calculate the metrics. In the analysis, we used the results of disaccharidases activity of the patients with IBS and of the control group. Statistical analysis confirmed that the method used to measure the disaccharidase activity had high sensitivity and specificity, as well as high diagnostic efficiency.
| Disaccharidase | Cut-off | Sensitivity (%) | Specificity (%) | AUC |
| Glucoamylase | 159.5 | 96 | 84 | 0.9 |
| Maltase | 846 | 80 | 70 | 0.8 |
| Sucrase | 87.5 | 80 | 82 | 0.9 |
| Lactase | 32.5 | 90 | 87 | 0.9 |
The obtained data were processed with Statistica 12 statistical software. Nonparametric methods were implemented using the Mann-Whitney U, Kruskal-Wallis, and χ2 tests for analyses of data not normally distributed. The Kendall tau rank correlation coefficient (r), a nonparametric method, was used to study the interrelationships among the quantitative data not normally distributed. Differences were considered statistically significant at P < 0.05.
The GI symptoms in most patients were associated with consumption of products containing carbohydrates. X-ray enterography and endoscopic studies confirmed the absence of pathological changes in the intestine and other digestive organs, which met the Rome IV criteria for FBD. Histological examination of the small intestine biopsy specimens was performed for all patients. In most patients, the ratio of the height of the villi and depth of the crypt was greater than 3:1, and the number of intraepithelial lymphocytes did not exceed 20 per 100 enterocytes. The contours of the brush border were clear, and many Paneth cells were found at the base of the intestinal crypts. There was weak to moderate infiltration of lymphocytes and plasmocytes into the lamina propria of the small intestine mucosa. The above-mentioned data enabled us to exclude pathohistological signs of small bowel diseases (e.g., Celiac disease, Whipple disease), although the changes in a number of cases could be interpreted as weakly inactive duodenitis.
In the control group, lactase activity varied from 17 ng to 148 ng of glucose/mg tissue × min, glucoamylase activity varied from 100 ng to 1571 ng of glucose/mg tissue × min, maltase activity ranged from 558 ng to 1323 ng of glucose/mg tissue × min, and sucrase activity ranged from 91 ng to 348 ng of glucose/mg tissue × min. These values were within the normal range.
Table 3 shows the median, and the first and third quartiles in the control and IBS groups. There was significant variation in the enzyme activity in patients, but in all groups, the average activity was significantly lower than normal. The most pronounced decrease in activities of glucoamylase, sucrase, and lactase were detected in patients with diarrhea. In the group of patients with constipation, 5 had normal disaccharidase activity and 9 had decreased activity. In 5 patients, the diagnosis of IBS-M was confirmed by the occurrence of meteorism (flatulence) after consumption of products containing carbohydrates, and in 2 patients with glucoamylase and lactase deficiency, it was confirmed by the occurrence of abdominal pain and liquid stool. One patient with deficiencies of all studied enzymes had lost 15 kg within 5 years. At the same time, all patients, regardless of the enzyme activity index, complained of abdominal pain or discomfort of varying intensity. The activities of lactase (86.5%) and glucoamylase (84.1%) decreased most frequently in patients with FBD. The activities of maltase and sucrase decreased less frequently, and with approximately the same frequency in all three groups. Overall, a decrease in all studied disaccharidases was observed in approximately one-third (39.2%) of patients with FBD, while normal activity of all disaccharidases was found in only 4 of 82 (4.9%) patients (Table 4). Patients, who took NSAIDs (7.3%) and antibiotics (6.1%) had a decrease in all studied disaccharidases.
| Group | Carbohydrase activity, ng of glucose/mg tissue × min | |||
| Glucoamylase | Maltase | Sucrase | Lactase | |
| Control, n = 20 | 490 (231.5-699.25) | 887 (854.5-1146) | 124 (94-210) | 56 (43-77.25) |
| 1st, n = 56 | 103 (53-141)a | 625 (283-964) | 56 (31-84)a | 10 (6-12)a |
| 2nd, n = 14 | 116 (44-192.5)a | 571 (415.25-739.5)a | 61.5 (28-158.5)a | 19.5 (3.25-45.5)a |
| 3rd, n = 12 | 184 (125-236)a | 618 (373-673.75)a | 54.5 (38.75-87)a | 6 (4-20.25)a |
| Group | Decrease in enzyme activity | ||||
| Glucoamylase | Maltase | Sucrase | Lactase | All disaccharidases | |
| 1st, n = 56 | 47/83.9 | 30/53.5 | 29/51.7 | 55/98.2 | 21/37.5 |
| 2nd, n = 14 | 9/64.2 | 5/35.7 | 6/42.8 | 8/57.1 | 3/21.4 |
| 3rd, n = 12 | 12/100.0 | 5/41.6 | 6/50.0 | 8/66.7 | 2/16.7 |
| All, n = 82 | 69/84.1 | 40/48.7 | 41/50.0 | 71/86.5 | 26/31.7 |
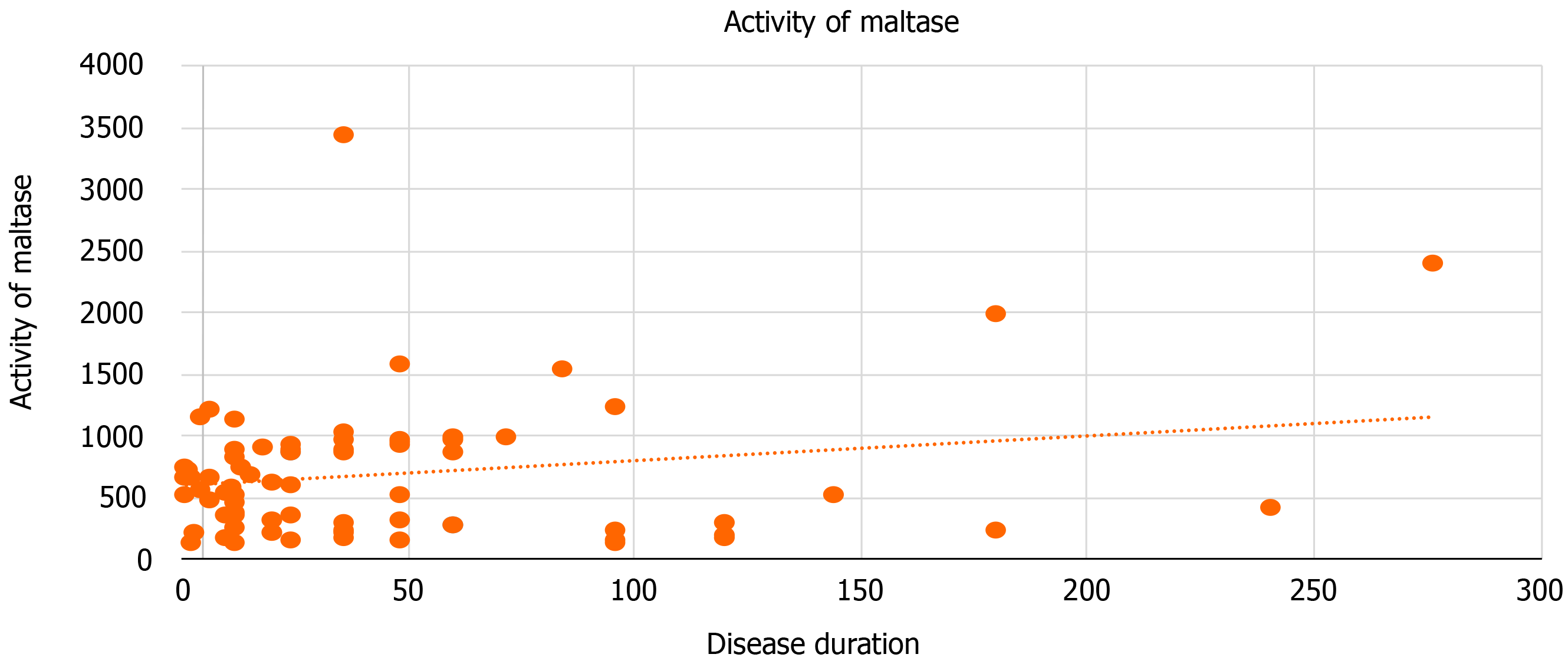
We performed a correlation analysis of the dependency of enzyme activity on the duration of clinical manifestations of the disease in the largest group of FBD patients with diarrhea. A moderate positive correlation was found between maltase activity and disease duration (Figure 1). Interrelationships with other enzymes were not detected (glucoamylase: r = -0.056, P = 0.67; sucrase: r = 0.009, P = 0.95; lactase: r =
A decrease in the activity of enzymes involved in membrane digestion leads to the accumulation of oligomers and monomers in the lumen of the small intestine. These oligosaccharides and monosaccharides, as a nutritious (culture) medium for microorganisms, contribute to small intestinal overgrowth in the lumen of the small intestine. Suppression of enzymatic activity is caused by acute enteric infection, NSAIDs, antibiotics, and other agents that damage the epithelial layer of the small intestine where enzymes are synthesized for membrane digestion.
According to conventional light microscopy data, damage to the structure of small intestine mucosa does not occur in patients with FBD, and alterations do not occur beyond minor inactive duodenitis. However, the decrease in disaccharidase activity indicates that some damage is occurring to epithelial cells at an ultrastructural level, which cannot be detected by light microscopy. Studies of intestinal enzymes in patients with FBD have mainly focused on lactase deficiency. Farup et al[16] reported a high number of cases of lactase deficiency in IBS patients, and noted the positive effect of implementing elimination diets. Cloarec et al[17] showed that the quantity of lactose malabsorbed in France was about 60%; however, most of the patients did not complain of milk intolerance, and their daily consumption of dairy products did not change after their diagnosis of lactose intolerance. The severity of symptoms in patients with lactase deficiency depends on the dose of lactose and expression of lactase, as well as the composition of intestinal microflora[18]. The histology of the small intestine mucosa in cases of lactose intolerance does not show specific alterations, even in cases of pronounced symptoms after the consumption of small amounts of a product[19].
IBS patients usually complain of lactose intolerance, or more often, milk intolerance. The study by Corlew-Roath and Di Palma showed that carbohydrate digestion disorder was equally found in patients with and without IBS, but patients without IBS responded better to changes in the diet[20]. A study by Saberi-Firoozi et al[21] in Southern Iran surveyed 1978 people over the age of 35 to determine the prevalence of IBS and lactose intolerance; in total, 562 people had symptoms of lactose intolerance, most of whom were females with IBS. Yang et al[22] compared cases of lactose intolerance in healthy volunteers and patients with IBS-D, and found that the frequency of lactose intolerance symptoms depended upon the consumed dose of lactose and was significantly higher in patients with IBS-D. Varjú et al[23] conducted a meta-analysis of studies published up to April 24, 2018, and found that lactose intolerance was more common among IBS patients compared to healthy individuals.
Sucrase deficiency has mainly been studied by pediatricians, who have noted a genetic predisposition and a correlation with damage to the mucous membrane due to various diseases, psychological stress, acute enteric infections, antibiotic therapy, and NSAIDs[24]. Sucrase breaks down sucrose to glucose and fructose and plays an important role in digesting starch oligomer to glucose. The Western diet is high in sucrose, contained by many products including fresh fruits such as peaches and bananas, as well as desserts such as biscuits and chocolate, in addition to many popular drinks[25]. Isomaltase cleaves the branched α-1, α-6 bonds of polysaccharides and oligosaccharides that cannot be cleaved by amylase or maltase[26]. Glucoamylase works at the border of the brush surface of enterocytes, and together with amylase, hydrolyzes oligosaccharides to glucose[27]. Isomaltase together with sucrase comprise the sucrase-isomaltase complex[28]. Maltase breaks down the maltose disaccharide to glucose[29]. Deficiency of any of these enzymes can lead to intolerance to everyday food products, and cause bloating and abdominal pain mimicking the symptoms of IBS[24]. Moreover, due to such symptoms occurring with high frequency, disaccha
Glucoamylase and maltase play important roles in the digestion of starch-containing products, which are consumed in large amounts in the modern diet. A decrease in the activity of these enzymes can lead to disorders of the GI tract.
In 78 of the 82 patients with FBD, GI symptoms were associated with disaccharidase deficiency, which developed after acute enteric infections, antibiotic therapy, NSAIDs, or other damaging agents. Decreased activity of membrane digestion enzymes was observed in most patients. Therefore, additional studies are necessary to determine the contribution of these enzymes to the symptoms associated with FBD. Our study indicates that the mechanism underlying FBD may be as follows. Damage to the mucous membrane of the small intestine leads to a decrease in the activity of enzymes on the brush border of enterocytes, resulting in disorders of carbohydrate hydrolysis and accumulation of osmoactive substances in the lumen of the small intestine. In turn, small intestinal bacterial overgrowth, stool disorder, abdominal pain, and bloating occurs. Additional diagnostic methods should be explored for patients with FBD.
Functional bowel disorder (FBD) clinically manifests as changes in stool frequency and consistency, sometimes accompanied by abdominal pain associated with defecation. FBDs are a very common in modern society. Many patients report an association their symptoms with the consumption foods that containing carbohydrates.
Few studies have been conducted on intestinal disaccharidase activity. Most of these studies have been on lactase activity in both adults and children and sucrase activity in children. Maltase and glucoamylase activities have not been studied in adults.
The aim of this study was to determine the value of intestinal disaccharidases glucoamylase, maltase, sucrase, and lactase in understanding the etiology and pathogenesis of FBDs.
When collecting data for anamnesis, specific attention was paid to food tolerance/ intolerance and the effect of food on gastrointestinal (GI) symptoms. Laboratory and instrumental research methods were conducted within the scope of standards and algorithms used for the diagnosis of FBD. Instrumental studies, in addition to X-ray enterography, colonoscopy, and diagnostic ultrasound of the abdominal organs, also included esophagogastroscopy with duodenum biopsy (3-5 specimens). Biochemical evaluation of intestinal (membrane) disaccharidase activity and histological evaluation of the duodenal mucosa were performed as well. In the histological preparations, the height of the intestinal villi, depth of the crypts, and their ratio were evaluated. In preparations stained with the periodic-acid Schiff reaction, the state of the brush border of the enterocytes was evaluated, with attention paid to their presence in the epithelium of goblet cells and intraepithelial lymphocytes, as well as in Paneth cells located at the base of the crypts. The activities of the carbohydrate-splitting enzymes lactase, glucoamylase, sucrase and maltase were determined by the Dahlqvist method.
In 78 of the 82 patients with FBD, GI symptoms were associated with disaccharidase deficiency, which developed after acute enteric infections, antibiotic therapy, non-steroidal anti-inflammatory drugs, or other damaging agents. Decreased activity of membrane digestion enzymes was observed in most patients. Therefore, additional studies are necessary to determine the contribution of these enzymes to the symptoms associated with FBD.
Our study indicates that the mechanism underlying FBD may be as follows. Damage to the mucous membrane of the small intestine leads to a decrease in the activity of enzymes on the brush border of enterocytes, resulting in disorders of carbohydrate hydrolysis and accumulation of osmoactive substances in the lumen of the small intestine. In turn, small intestinal bacterial overgrowth, stool disorder, abdominal pain, and bloating occurs. Additional diagnostic methods should be explored for patients with FBD.
A detailed study of this phenomenon would make it possible to create drugs that compensate for the deficiency of enzymes or stimulate their synthesis, which would significantly improve the treatment of patients with FBD.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Russia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gupta RA S-Editor: Gao CC L-Editor: A P-Editor: Li JH
| 1. | Drossman DA, Hasler WL. Rome IV-Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology. 2016;150:1257-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 1036] [Article Influence: 115.1] [Reference Citation Analysis (0)] |
| 2. | Mearin F, Lacy BE, Chang L, Chey WD, Lembo AJ, Simren M, Spiller R. Bowel Disorders. Gastroenterology. 2016;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1781] [Cited by in RCA: 1898] [Article Influence: 210.9] [Reference Citation Analysis (3)] |
| 3. | Sinagra E, Pompei G, Tomasello G, Cappello F, Morreale GC, Amvrosiadis G, Rossi F, Lo Monte AI, Rizzo AG, Raimondo D. Inflammation in irritable bowel syndrome: Myth or new treatment target? World J Gastroenterol. 2016;22:2242-2255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 83] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 4. | Parfenov AI. Enterology: a Guide for physicians. 2nd ed. Moscow: Medical Information Agency, 2009. |
| 5. | Henström M, Diekmann L, Bonfiglio F, Hadizadeh F, Kuech EM, von Köckritz-Blickwede M, Thingholm LB, Zheng T, Assadi G, Dierks C, Heine M, Philipp U, Distl O, Money ME, Belheouane M, Heinsen FA, Rafter J, Nardone G, Cuomo R, Usai-Satta P, Galeazzi F, Neri M, Walter S, Simrén M, Karling P, Ohlsson B, Schmidt PT, Lindberg G, Dlugosz A, Agreus L, Andreasson A, Mayer E, Baines JF, Engstrand L, Portincasa P, Bellini M, Stanghellini V, Barbara G, Chang L, Camilleri M, Franke A, Naim HY, D'Amato M. Functional variants in the sucrase-isomaltase gene associate with increased risk of irritable bowel syndrome. Gut. 2018;67:263-270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 6. | Opekun AR, Balesh AM, Shelby HT. Use of the Biphasic (13)C-Sucrose/Glucose Breath Test to Assess Sucrose Maldigestion in Adults with Functional Bowel Disorders. Biomed Res Int. 2016;2016:7952891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Puntis JW, Zamvar V. Congenital sucrase-isomaltase deficiency: diagnostic challenges and response to enzyme replacement therapy. Arch Dis Child. 2015;100:869-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Dahlqvist A. Assay of intestinal disaccharidases. Scand J Clin Lab Invest. 1984;44:169-172. [PubMed] [DOI] [Full Text] |
| 9. | Dahlqvist A. Method for assay of intestinal disaccharidases. Anal Biochem. 1964;7:18-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1443] [Cited by in RCA: 1380] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 10. | Bayless TM, Brown E, Paige DM. Lactase Non-persistence and Lactose Intolerance. Curr Gastroenterol Rep. 2017;19:23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 11. | Deng Y, Misselwitz B, Dai N, Fox M. Lactose Intolerance in Adults: Biological Mechanism and Dietary Management. Nutrients. 2015;7:8020-8035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 183] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 12. | Wanes D, Husein DM, Naim HY. Congenital Lactase Deficiency: Mutations, Functional and Biochemical Implications, and Future Perspectives. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Deb C, Campion S, Derrick V, Ruiz V, Abomoelak B, Avdella A, Zou B, Horvath K, Mehta DI. Sucrase-isomaltase Gene Variants in Patients With Abnormal Sucrase Activity and Functional Gastrointestinal Disorders. J Pediatr Gastroenterol Nutr. 2021;72:29-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Dahlqvist A. Specificity of the human intestinal disaccharidases and implications for hereditary disaccharide intolerance. J Clin Invest. 1962;41:463-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 160] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 15. | Trinder P. Determination of blood glucose using 4-amino phenazone as oxygen acceptor. J Clin Pathol. 1969;22:246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 412] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 16. | Farup PG, Monsbakken KW, Vandvik PO. Lactose malabsorption in a population with irritable bowel syndrome: prevalence and symptoms. A case-control study. Scand J Gastroenterol. 2004;39:645-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Cloarec D, Gouilloud S, Bornet F, Bruley des Varannes S, Bizais Y, Galmiche JP. [Lactase deficiency and lactose intolerance-related symptoms in adult healthy subjects from western France]. Gastroenterol Clin Biol. 1991;15:588-593. [PubMed] |
| 18. | Misselwitz B, Pohl D, Frühauf H, Fried M, Vavricka SR, Fox M. Lactose malabsorption and intolerance: pathogenesis, diagnosis and treatment. United European Gastroenterol J. 2013;1:151-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 19. | Ferguson A, MacDonald DM, Brydon WG. Prevalence of lactase deficiency in British adults. Gut. 1984;25:163-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Rana SV, Malik A. Breath tests and irritable bowel syndrome. World J Gastroenterol. 2014;20:7587-7601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (2)] |
| 21. | Saberi-Firoozi M, Khademolhosseini F, Mehrabani D, Yousefi M, Salehi M, Heidary ST. Subjective lactose intolerance in apparently healthy adults in southern Iran: Is it related to irritable bowel syndrome? Indian J Med Sci. 2007;61:591-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Yang J, Deng Y, Chu H, Cong Y, Zhao J, Pohl D, Misselwitz B, Fried M, Dai N, Fox M. Prevalence and presentation of lactose intolerance and effects on dairy product intake in healthy subjects and patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 2013; 11: 262-268. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 23. | Varjú P, Gede N, Szakács Z, Hegyi P, Cazacu IM, Pécsi D, Fábián A, Szepes Z, Vincze Á, Tenk J, Balaskó M, Rumbus Z, Garami A, Csupor D, Czimmer J. Lactose intolerance but not lactose maldigestion is more frequent in patients with irritable bowel syndrome than in healthy controls: A meta-analysis. Neurogastroenterol Motil. 2019;31:e13527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 24. | Gericke B, Amiri M, Naim HY. The multiple roles of sucrase-isomaltase in the intestinal physiology. Mol Cell Pediatr. 2016;3:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 25. | Ventura EE, Davis JN, Goran MI. Sugar content of popular sweetened beverages based on objective laboratory analysis: focus on fructose content. Obesity (Silver Spring). 2011;19:868-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 176] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 26. | Treem WR. Congenital sucrase-isomaltase deficiency. J Pediatr Gastroenterol Nutr. 1995;21:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 60] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Gray GM. Starch digestion and absorption in nonruminants. J Nutr. 1992;122:172-177. [PubMed] [DOI] [Full Text] |
| 28. | Diaz-Sotomayor M, Quezada-Calvillo R, Avery SE, Chacko SK, Yan LK, Lin AH, Ao ZH, Hamaker BR, Nichols BL. Maltase-glucoamylase modulates gluconeogenesis and sucrase-isomaltase dominates starch digestion glucogenesis. J Pediatr Gastroenterol Nutr. 2013;57:704-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Nichols BL, Baker SS, Quezada-Calvillo R. Metabolic Impacts of Maltase Deficiencies. J Pediatr Gastroenterol Nutr. 2018;66 Suppl 3:S24-S29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | El-Chammas K, Williams SE, Miranda A. Disaccharidase Deficiencies in Children With Chronic Abdominal Pain. JPEN J Parenter Enteral Nutr. 2017;41:463-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Viswanathan L, Rao SSC, Kennedy K, Sharma A, Yan Y, Jimenez E. Prevalence of Disaccharidase Deficiency in Adults With Unexplained Gastrointestinal Symptoms. J Neurogastroenterol Motil. 2020;26:384-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |