Published online May 26, 2021. doi: 10.12998/wjcc.v9.i15.3758
Peer-review started: January 10, 2021
First decision: February 12, 2021
Revised: February 13, 2021
Accepted: March 8, 2021
Article in press: March 8, 2021
Published online: May 26, 2021
Processing time: 122 Days and 7.4 Hours
Lymphangiomatosis is a multisystem disorder that is rarely localized to the gastrointestinal tract. Lymphangiomatosis usually has no specific clinical presentation and is easily misdiagnosed. A case report and review of the literature on lymphangiomatosis associated with protein-losing enteropathy will help to improve the overall understanding of this disease.
We report a case of lymphangiomatosis of the bowel and other solid organs. A 78-year-old man presented with recurrent bowel bleeding and protein-losing enteropathy, as well as cystic lesions in the spleen, liver, and kidney. Imaging examinations revealed many cystic lesions on the spleen, liver, kidney, and thickened wall of the ascending colon, as well as pleural effusion and ascites. Colonoscopy revealed a strawberry mucosa, variable spontaneous bleeding, and surface erosion located in the terminal ileum. Several cystic masses with a translucent and smooth surface as well as diffuse white spots were located in the colon. A laterally spreading tumor (LST) was located in the ascending colon. Pathology indicated highly differentiated adenocarcinoma (LST) and lymphangiomatoid dilation, and D2-40 was positive. The final diagnosis was lymphangiomatosis. The patient underwent surgery for LST and then was administered thalidomide 75-150 mg/d. His condition, however, did not improve. He eventually died 6 mo after the initial diagnosis.
Lymphangiomatosis usually occurs diffusely and can involve many organs, such as the spleen, kidney, liver, lung, mesentery, and bowel. Recurrent bowel bleeding or protein-losing enteropathy is an important indicator that should alert clinicians about the possibility of this disease when it afflicts the bowel. Doctors should improve the medical understanding of lymphangiomatosis.
Core Tip: Lymphangiomatosis is a multisystem disorder that is rarely localized to the gastrointestinal tract. We report a case of lymphangiomatosis of the bowel and other solid organs (spleen, liver, and kidney). The condition was misdiagnosed because it did not present specific clinical indicators, and the treating doctors were not aware of this condition. After a series of imaging and pathologic examinations, the patient was correctly diagnosed and treated with thalidomide. Follow-up indicated that he died 6 mo after the initial diagnosis. This case emphasizes the importance of improving the understanding of lymphangiomatosis.
- Citation: Ding XL, Yin XY, Yu YN, Chen YQ, Fu WW, Liu H. Lymphangiomatosis associated with protein losing enteropathy: A case report. World J Clin Cases 2021; 9(15): 3758-3764
- URL: https://www.wjgnet.com/2307-8960/full/v9/i15/3758.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i15.3758
Lymphangiomatosis is an uncommon malformation of the lymphatic system. Lymphatic diseases can vary from small lymphangioma to generalized lymphangiomatosis, which is a rare condition and can have several clinical manifestations[1,2]. Lymphangiomatosis mainly occurs on the neck and head in children but can occur anywhere in the body, and abdominal lymphangiomatosis has also been reported[1,2]. It rarely affects the bowel[3]. Gastrointestinal bleeding, anemia, and abdominal pain are the most frequent symptoms[4], and protein-losing enteropathy (PLE) is a rare and severe complication[2,5].
In the present study, we report a rare case of lymphangiomatosis that manifested as PLE and recurrent bowel bleeding, as well as multiple organ injury to the spleen, liver, and kidney.
A 78-year-old man presented to our clinic with melena and weakness for 15 d.
The patient’s symptoms started 15 d before presentation but had worsened over the previous week. He presented to the gastrointestinal department with melena, weakness, and vomiting. He then presented with anemia (hemoglobin level: 38 g/L) and a positive fecal occult blood test.
The patient had undergone surgery and chemotherapy for a history of lymphatic sarcoma on the left side of the neck 20 years prior. He also had a more than 10-year history of gallstones and a 2-year history of diabetes. He had no other history of disease or allergic reactions to medication.
The patient’s personal and family history was typical.
Following physical examination, he presented an anemic appearance and pretibial edema. No other obvious abnormalities were observed during physical examination.
The laboratory values following admission indicated microcytic hypochromic anemia, with a hemoglobin value of 63 g/L, lymphocyte count of 0.33 × 109/L, lymphocyte percentage of 9.9%, albumin value of 20.68 g/L, and globulin value of 13.96 g/L. The laboratory examination for fecal occult blood was positive. The urinary protein was negative. No significant abnormalities were recorded in other tests.
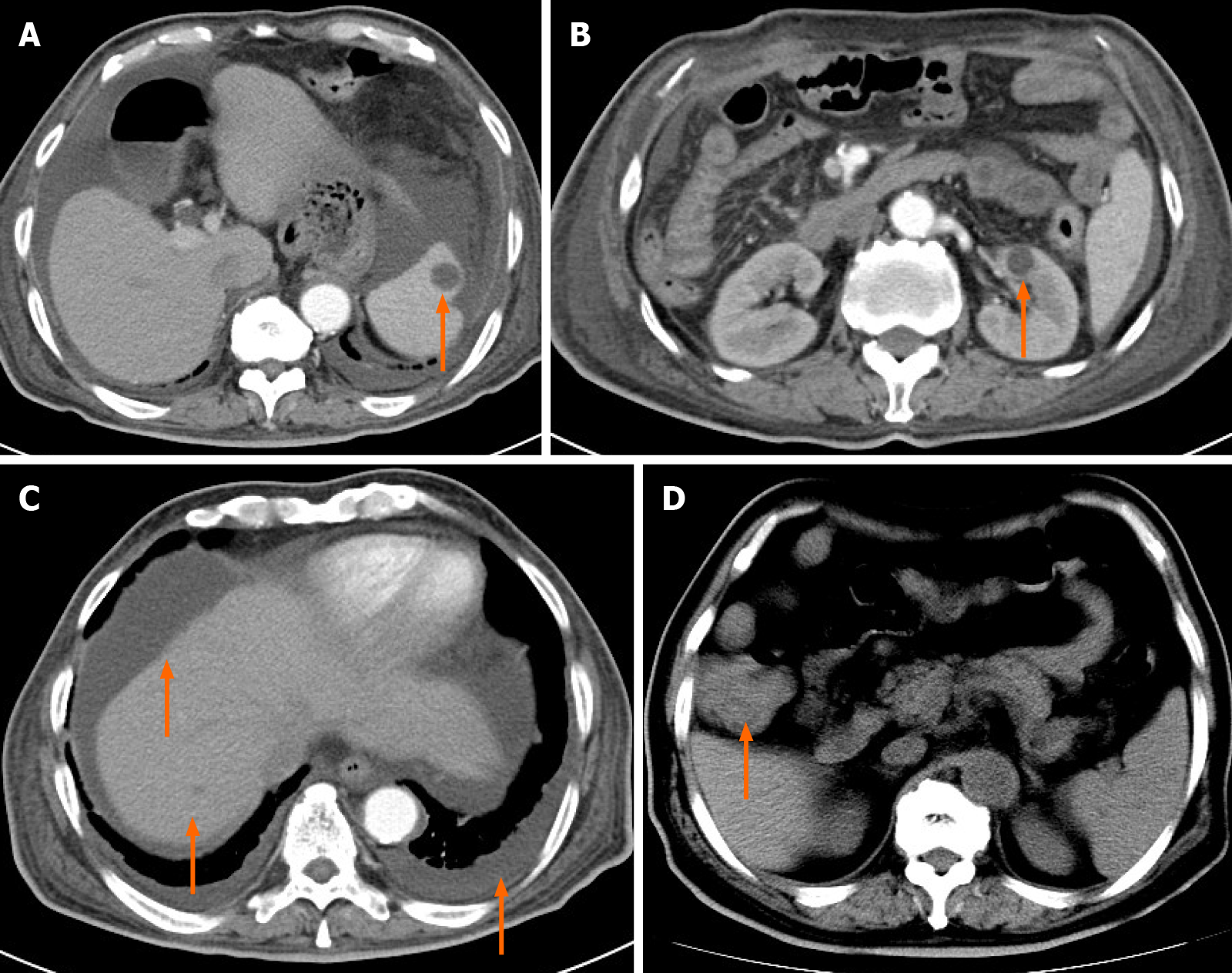
Abdominal computed tomography (CT) displayed multiple small cystic lesions without enhancement distributed in the spleen, kidney, liver, ascites, and pleural effusion (Figure 1A-C). CT also revealed thickening of the ascending colon wall (Figure 1D).
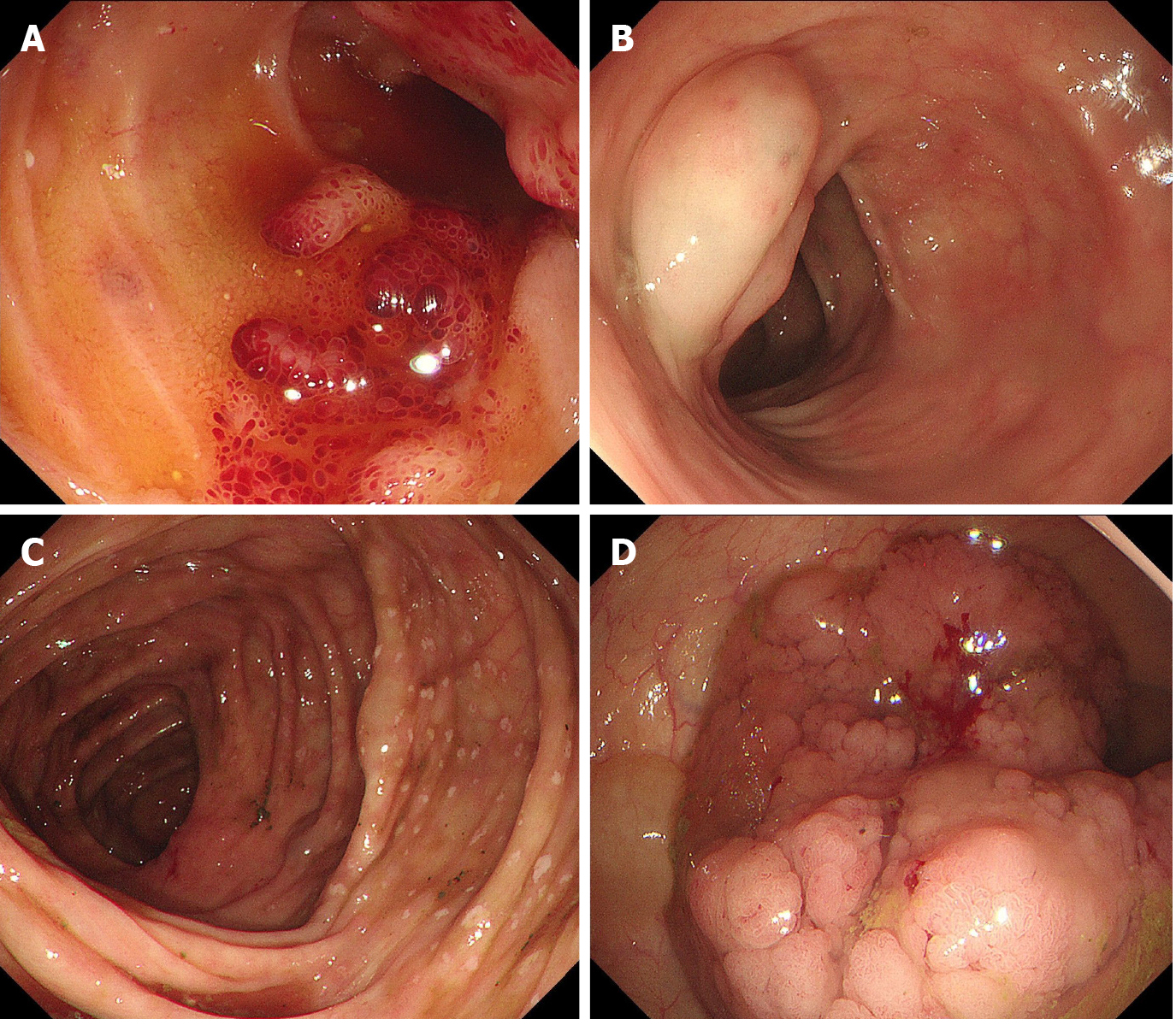
Colonoscopy revealed a strawberry mucosa, variable spontaneous bleeding, and surface erosion located in the terminal ileum and ileocecal valve (Figure 2A). Multiple cystic masses with a translucent and smooth surface, diffuse white spots located in the colon, and a laterally spreading tumor (LST) located in the ascending colon (Figure 2B-D) were also observed.
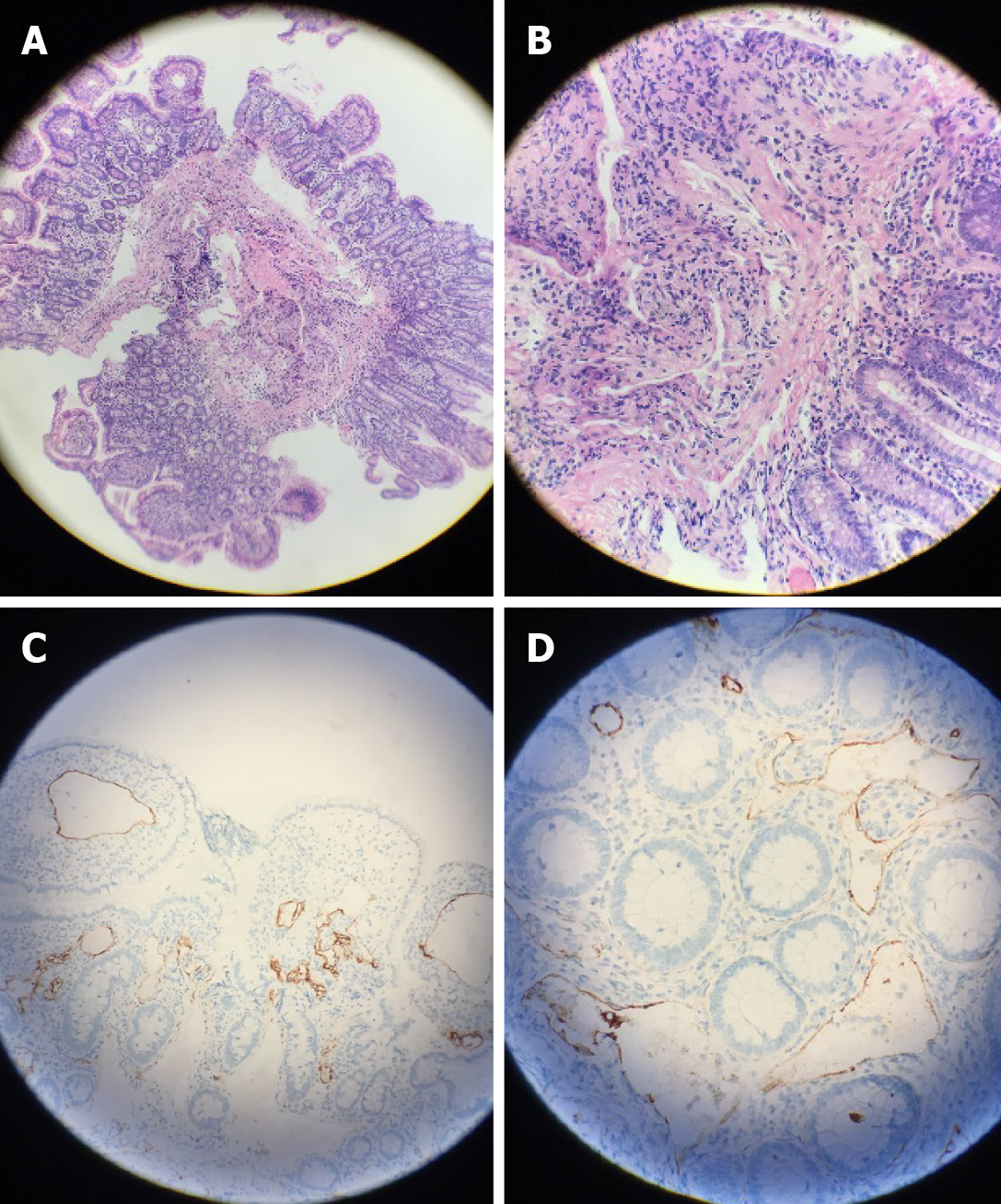
The biopsy of the LST indicated villous tubular adenoma with low-grade intraepithelial neoplasia as well as highly differentiated adenocarcinoma after surgery. Histological findings also showed a large amount of vascular hyperplasia and dilatation located in the muscularis mucosae and submucosa (Figure 3A and B), which were immunohistochemically positive for D2-40, a specific lymphatic endothelial marker (Figure 3C and D).
According to the history and histopathologic characteristics, the patient was diagnosed with lymphangiomatosis (intestine, colon, spleen, kidney, and liver) and colon cancer (LST).
The patient underwent radical right hemicolectomy after the first colonoscopy because of the large LST. However, he continued to present melena, hematochezia, and weakness after surgery. According to the history and imaging examinations, he was diagnosed with lymphangiomatosis. The patient was treated with a medium-chain triglyceride diet, thalidomide 75 mg/d, recurrent transfusion, albumin, and diuretics. His condition, however, did not improve. The dose of thalidomide was slowly increased to 150 mg/d.
Despite the patient taking thalidomide with good tolerance, he died because of recurrent bowel bleeding and consistent PLE. His hemoglobin values were 43-65 g/L despite recurrent transfusion. Similarly, his albumin values were 16.37-29.5 g/L despite recurrent albumin transfusion due to protein loss. The patient died 6 mo after the initial diagnosis.
Lymphangiomatosis is a general term for excessive growth of aberrant lymphatic vessels. The acknowledged main cause is a congenital malformation of the lymphatic system, resulting in abnormal dilatation and proliferation of the lymphatic channel and leading to the formation of lymphangioma[6]. Trauma, partial lymphatic obstruction, and inflammation can also lead to secondary lymphangioma[7]. In our case, the patient was an elderly man with a surgical history of lymphatic sarcoma on the left side of the neck, and we speculated that the cause of his condition was secondary.
The clinical symptoms of lymphangiomatosis are complicated and atypical. They include gastrointestinal bleeding, anemia, abdominal pain, perforation, and PLE, and whether it afflicts the bowels depends on the position and size of the spread[2,4,7]. Our patient presented gastrointestinal bleeding and PLE, which is rare.
Blood examination results showed a hemoglobin decrease and positive occult blood test. Additionally, PLE caused by lymphangiomas show a decrease in the albumin, and globulin values, lymphocyte count, and lymphocyte percentage also decrease. Our patient presented these changes. CT manifestations of lymphangiomatosis include multiple hypodense cysts occupying the spleen, liver, bones, and other organs[8]. CT scans also occasionally reveal ascites and pleural effusion. Our patient had cystic lesions in the spleen, liver, and kidney, as well as ascites and pleural effusion. Endoscopy is the most important examination for gastrointestinal lymphangio
The histological features of lymphangiomatosis are nonspecific, and a definitive diagnosis requires the hyperproliferation of normal lymphatic vessels with normal endothelium, predominantly in the context of submucosa, with disruption of the muscular layer and sometimes of the serosa. Regarding immunohistochemistry, D2-40 is a specific marker of lymphangioma. CD31-, CD34-, and VIII-related antigens and VEGFR3 are also helpful in the diagnosis[9].
Treatment is dictated by the nature of the symptoms, anatomic location, and the associated potential complications of treatments, including systemic therapy, endoscopic therapy, surgery, and radiation therapy[10]. Rapamycin is very effective in some cases of lymphangiomatosis[11]. In others, thalidomide may also be effective[12]. However, we only tried thalidomide because rapamycin was not available.
Our case required additional laboratory and imaging examinations, such as an ascites test, α-antitrypsin clearance rate which is difficult to popularize in clinic because of its complicated detection method, capsule endoscopy test, and lymphangiography, to diagnose the disease. Capsule endoscopy can show lesions in or changes to the intestine. Lymphangiography is an imaging technique that shows the lymphatic system. Performing lymphangiography could perhaps have indicated the disease of our patient and revealed that the disease was primary or secondary. Unfortunately, the patient rejected undergoing complete capsule endoscopy. Additionally, our hospital did not have the capability to perform lymphangiography, and he was too weak to travel to Beijing to undergo the procedure.
Only a few lymphangiomatosis cases have been reported to be associated with PLE to date[2,5]. PLE is a severe complication of lymphangiomatosis when it affects the gastrointestinal tract and is characterized by an excessive loss of proteins in the gastrointestinal tract due to impaired integrity of the mucosa. It is characterized by edema and hypoproteinemia. In severe cases, pleural effusion and peritoneal effusion may occur. Gastrointestinal lymphangiectasia and lymphangioma are the causes of protein-losing enteropathy. Endoscopy clearly revealed pathological changes in the intestine and colon, likely explaining why our patient had PLE[2,5]. PLE is an important indicator that should alert clinicians about the possibility of this disease when it affects the bowel.
Lymphangiomatosis usually occurs diffusely and can involve many organs, such as the spleen, kidney, liver, lung, mesentery, and bowel. Lymphangiomatosis is a rare disease that usually has no specific clinical presentation and can be easily misdiagnosed. A history of recurrent bowel bleeding or protein-losing enteropathy is an important indicator that should alert clinicians to the possibility of this disease. Doctors should improve their medical knowledge of lymphangiomatosis.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: Integrated Oncology Branch of China Anti-Cancer Association; Neuroendocrine Tumor Committee of Shandong Medical Association; and Qingdao Youth Digestion Committee.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ozen H S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Yuan YY
| 1. | Valakada J, Madhusudhan KS, Ranjan G, Garg PK, Sharma R, Gupta AK. Abdominal Lymphangiomatosis With Intestinal Lymphangiectasia Diagnosed by Magnetic Resonance Lymphangiography: A Case Report. Curr Probl Diagn Radiol. 2018;47:200-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Lin RY, Zou H, Chen TZ, Wu W, Wang JH, Chen XL, Han QX. Abdominal lymphangiomatosis in a 38-year-old female: case report and literature review. World J Gastroenterol. 2014;20:8320-8324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Ilhan M, Oner G, Alibeyoglu A, Yeğen G, Gök AF, Akyüz F, Bicen F. Primary intestinal lymphangiomatosis of the ileum in an adult-the role of surgical approach. J Surg Case Rep. 2016;2016:rjw133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Giuliani A, Romano L, Coletti G, Walid A Fatayer M, Calvisi G, Maffione F, Muolo C, Vicentini V, Schietroma M, Carlei F. Lymphangiomatosis of the ileum with perforation: A case report and review of the literature. Ann Med Surg (Lond). 2019;41:6-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Glöckler M, Severin T, Arnold R, Greiner P, Schwab KO, Uhl M, Schlensak C, Rössler J, Dittrich S. First description of three patients with multifocal lymphangiomatosis and protein-losing enteropathy following palliation of complex congenital heart disease with total cavo-pulmonary connection. Pediatr Cardiol. 2008;29:771-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Bellini C, Hennekam RC. Clinical disorders of primary malfunctioning of the lymphatic system. Adv Anat Embryol Cell Biol. 2014;214:187-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Ding XL, Yu YN, Jing X, Tian ZB, Liu H, Yin XY. Diagnostic values of digestive endoscopy in small intestinal lymphangioma. Zhonghua Weichang Neijing Dianzi Zazhi. 2017;4:119-122. [DOI] [Full Text] |
| 8. | Kwag E, Shim SS, Kim Y, Chang JH, Kim KC. CT features of generalized lymphangiomatosis in adult patients. Clin Imaging. 2013;37:723-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Mehmedovic Z, Mehmedovic M, Custovic MK, Sadikovic A, Mekic N. A rare case of giant mesenteric cystic lymphangioma of the small bowel in an adult: A case presentation and literature review. Acta Gastroenterol Belg. 2016;79:491-493. [PubMed] |
| 10. | Xiao NJ, Ning SB, Li T, Li BR, Sun T. Small intestinal hemolymphangioma treated with enteroscopic injection sclerotherapy: A case report and review of literature. World J Gastroenterol. 2020;26:1540-1545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Laforgia N, Schettini F, De Mattia D, Martinelli D, Ladisa G, Favia V. Lymphatic Malformation in Newborns as the First Sign of Diffuse Lymphangiomatosis: Successful Treatment with Sirolimus. Neonatology. 2016;109:52-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Pauzner R, Mayan H, Waizman A, Rozenman J, Farfel Z. Successful thalidomide treatment of persistent chylous pleural effusion in disseminated lymphangiomatosis [corrected]. Ann Intern Med. 2007;146:75-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |