Published online May 26, 2021. doi: 10.12998/wjcc.v9.i15.3716
Peer-review started: December 28, 2020
First decision: January 9, 2021
Revised: February 17, 2021
Accepted: March 24, 2021
Article in press: March 24, 2021
Published online: May 26, 2021
Processing time: 134 Days and 6 Hours
Sarcomatoid carcinoma of the pancreas (SCP) is a rare type of pancreatic neoplasm, and only a few cases have been described in the literature. Histologically, it is composed mostly of atypical spindle cells with apparent sarcomatous features.
This is a report of a 61-year-old Chilean woman who underwent medical investigation for acute abdominal pain. Computed tomography identified a solid tumor in the tail of the pancreas with features suspicious of malignancy. En-bloc distal pancreatectomy and splenectomy were performed to excise the tumor. Histo
The case presented herein is a patient with an SCP with a rare presentation and long-term survival after surgery despite not receiving adjuvant chemotherapy.
Core Tip: Sarcomatoid carcinoma of the pancreas (SCP) is an extremely rare and aggressive histologic subtype of undifferentiated pancreatic carcinoma. The prognosis of this neoplasm is similar to or even worse than that of typical pancreatic ductal adenocarcinoma (PDAC). However, the clinical course and surgical outcomes of SCP remain poorly characterized owing to its rarity. Because there is no standard regimen for treating SCP, patients with this disease are administered the same regimens as those with more common PDACs. In the present study, we report a case of SCP; although some patients have a rapid recurrence and early death, long-term survival may be possible.
- Citation: Toledo PF, Berger Z, Carreño L, Cardenas G, Castillo J, Orellana O. Sarcomatoid carcinoma of the pancreas — a rare tumor with an uncommon presentation and course: A case report and review of literature. World J Clin Cases 2021; 9(15): 3716-3725
- URL: https://www.wjgnet.com/2307-8960/full/v9/i15/3716.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i15.3716
Pancreatic cancer is considered a disease with uniformly poor outcomes[1]. The worldwide 5-year survival rate for pancreatic cancer patients is approximately 6%[2]. Pancreatic ductal adenocarcinoma (PDAC) is by far the most common solid pancreatic tumor, which represents 85 to 90% of all pancreatic neoplasms; thus, most attributes of pancreatic cancer are related to this tumor[3]. However, several morphological variants of PDAC are recognized in the latest (2019) World Health Organization (WHO) classification of pancreatic tumors based on distinctive histologic features[2,4]. Sarcomatoid carcinoma of the pancreas (SCP) is among these variants. SCP is an extremely uncommon tumor that accounts for 0.1% to 5.7% of all pancreatic malig
We report an exceptional case of SCP detected in a patient who underwent consultation in our emergency room with acute abdominal pain. The patient has survived for a long time to date without disease recurrence despite not receiving chemotherapy. We, therefore, discuss this case and review the relevant literature.
A 61-year-old female was admitted to our hospital suffering from 48 h of acute abdominal pain, characterized by epigastralgia without radiation and no response to spasmolytics or analgesics.
The patient had been suffering intermittent episodes of mild discomfort of the gastrointestinal tract such as bearable diffuse abdominal pain and feeling of flatulence that persisted for one year. The pain pattern was not related to defecation or eating, there was no nausea, vomiting, weight loss, melaena, change in bowel habit, urinary symptoms, or fever. She was managed conservatively as thought to be a functional gastrointestinal disorder.
She describes the pain as aggravating suddenly and sharp in nature. She presented to the emergency department after 48 h of the pain acutely worsened. The pain was in the epigastrium and across the anterior abdomen, was sharp and constant without radiation.
She had no antecedents of alcohol, tobacco, or drug abuse.
She had a medical history of arterial hypertension and trigeminal neuralgia and had no surgical history. In her family history, there were two cases of colorectal cancer (mother and sister) without other illnesses.
The patient experienced epigastric tenderness upon palpation, although she had no rebound tenderness, muscle tension, or a palpable mass. She had no other relevant findings.
Laboratory test results including complete blood count, liver function tests, serum amylase and lipase, biochemistry, were within normal ranges.
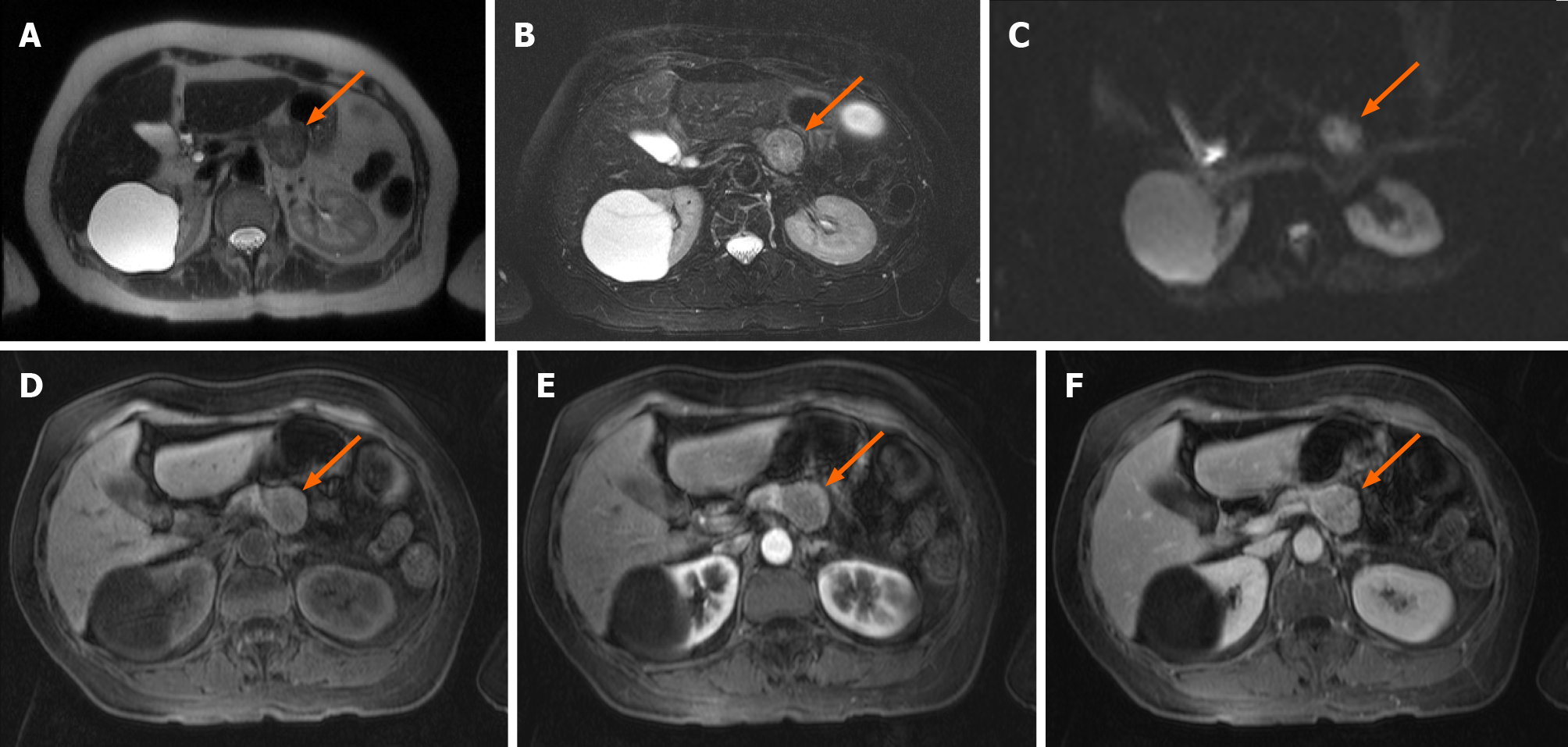
An abdominal computed tomography (CT) scan showed a solid mass of the tail of the pancreas that contacted the lesser curvature of the gastric body. Magnetic resonance imaging (MRI) showed a pancreatic head, uncinate process, neck, and body of normal morphology. A solid nodular mass 29 mm in diameter was confirmed in the pancreatic tail, hypointense in T1, heterogeneous with hyperintense areas in T2, with enhance
Complementary imaging studies for staging were performed. Thorax CT revealed 10 solid nodules between 3-6 mm distributed in both lungs, which, due to their distribution, were suspicious of secondary implants.
Video-thoracoscopy was performed, and these nodules had the characteristic appearance of benign anthracotic nodules, a type of pneumoconiosis caused by repeated exposure to air pollution or coal dust particles[7]. Biopsies were performed, and the benign nature was confirmed by histology.
Given these findings of no extra-abdominal disease, surgery was performed. Distal pancreatectomy with en bloc splenectomy was performed. Following surgery, the patient recovered successfully and was discharged from the hospital after 5 d.
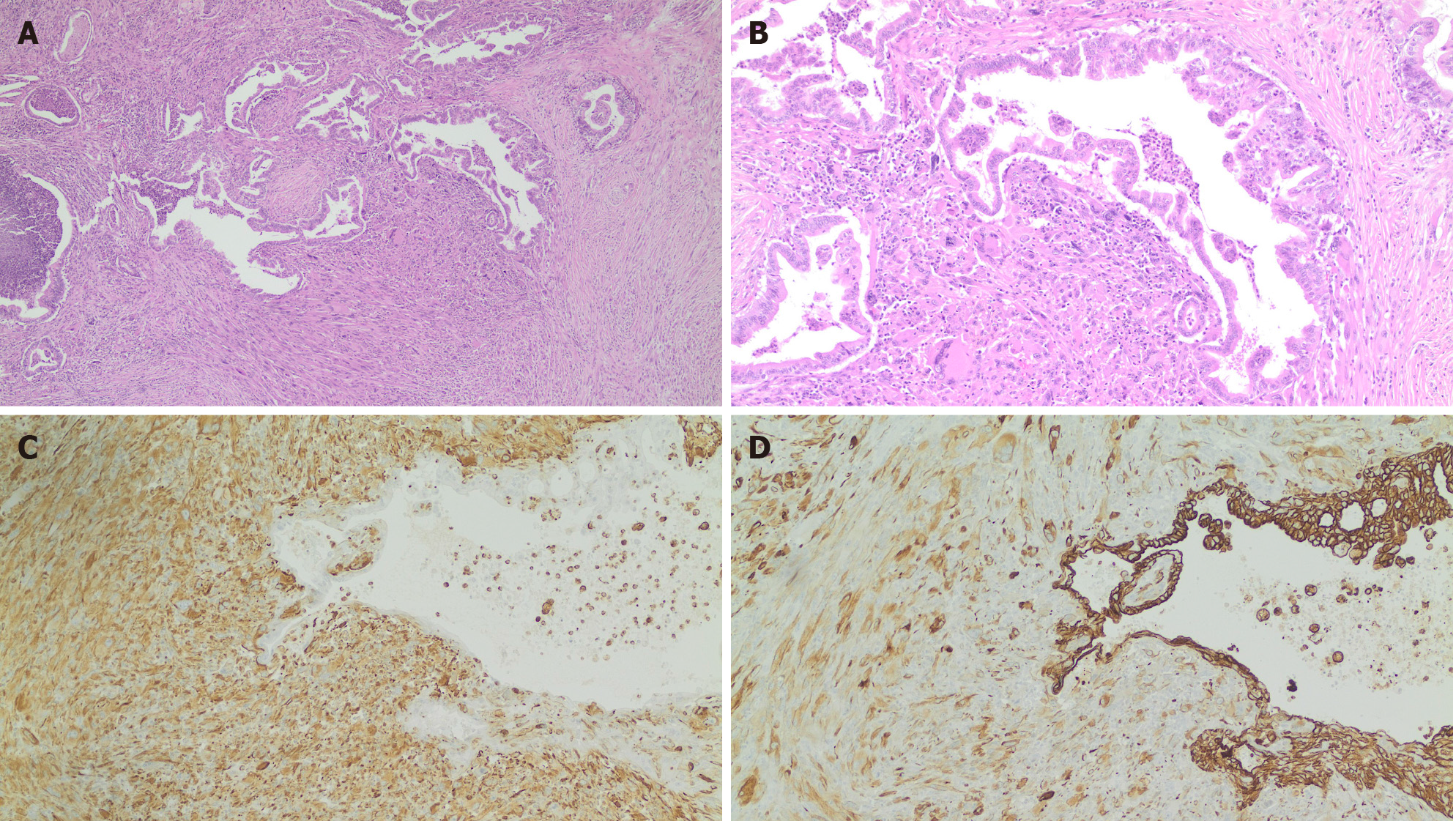
Gross examination of the resected specimen revealed the tumor was localized in the tail of the pancreas, measured 3.2 cm × 2.9 cm, and consisted of a solid mass. Margins of surgical resection were free of tumor. Microscopically, the tumor was consistent with ductal adenocarcinoma with sarcomatoid features (Figure 2). Immunohistochemistry showed that the tumor had both epithelial and mesenchymal markers that were positive for pan-cytokeratin (Figure 2D), vimentin (Figure 2C), and smooth muscle actin (SMA) and negative for CD68. Thus, a diagnosis of SCP was confirmed.
The tumor was confined to the tail of the pancreas with no invasion to the spleen. All surgical margins were free of tumor tissue. There was no evidence of perineural invasion but lymphovascular permeation of one of thirty peripancreatic lymph nodes were positive for metastatic cancer.
The oncologic committee disclosure was that the patient should receive postoperative adjuvant chemotherapy with gemcitabine and capecitabine. Unfortunately, this could not be carried out for extra medical reasons since the patient's medical insurance did not cover this treatment.
As our patient did not have access to adjuvant chemotherapy, we performed follow-up every 6 mo with general laboratory exams and imaging of the abdomen. The last image obtained was an abdominal CT after 35 mo of curative surgery, which did not reveal tumor recurrence.
Sarcomatoid carcinomas are uncommon aggressive histologic variants of carcinoma. Although they may rarely arise in almost any organ, the lung, breast, and kidney are the most common primary sites[8]. Several terms have been used to describe this malignancy, including carcinosarcoma, pseudosarcoma, pseudocarcinoma, and spindle cell carcinoma[9]. The multiple names demonstrate the varied understanding of this disease, which these terms have been often used interchangeably, and their definitions vary among the reports[10]. According to the WHO classification (5th edition, 2019) of pancreatic tumors assigns SCP under the category of undifferentiated carcinomas (UCP)[4]. UCP is a subtype of PDAC representing a set of rare tumors that accounts for as many as 5% of all pancreatic malignancies[11]. UPC is categorized into two different types: undifferentiated carcinoma [with three variants: anaplastic undifferentiated carcinoma, sarcomatoid carcinoma (SCP), and carcinosarcoma] and undifferentiated carcinoma with osteoclast-like giant cells[5,12]. Hence, we present a case of SCP that is an extremely rare type of tumor, with only a few cases reported in the literature[5,9,10,13-21,31-36]
SCP is defined histologically as a poorly differentiated tumor composed by the proliferation of spindle cells with evidence of epithelial differentiation. Sarcomatoid carcinomas can exhibit a monophasic or biphasic appearance. The monophasic pattern often referred to as spindle cell carcinoma, is akin to a soft tissue sarcoma without epithelioid areas. The biphasic pattern features a mixture of mesenchymal-like and epithelial-like cells with a transition zone. The sarcomatous tissue of these tumors shows evidence of epithelial differentiation, such as epithelial markers and epithelial ultrastructural features, rather than a specific line of mesenchymal differentiation[14].
The diagnosis often represents a clinicopathologic challenge, and immunohistochemistry plays a key role in the histopathological diagnosis where an epithelial immunohistochemical profile assembles PDAC[6,22]. In immunohistochemistry, undifferentiated cells often express both broad lineage carcinoma (pan-cytokeratin) and sarcoma (vimentin and desmin) markers and display a loss of E-cadherin[12]. Its pathogenesis remains unclear[23,24].
Owing to the rarity of the disease, the clinical course, surgical outcomes, and optimal treatment strategies for SCP are poorly characterized[5].
To date, the largest study to analyze the histological spectrum of pancreatic carcinoma with sarcoma-like transformation was reported in 1977 by AlguacilGarcia and Weiland[25] who identified four distinctive histological types of sarcoma-like carcinoma based on light microscopic analysis only. Of twelve cases they reported an average survival of 8.3 mo for patients with nonresectable lesions.
In addition to our patient, 16 cases of SCP with confirmed both epithelial and sarcomatoid elements have been reported (Table 1).
| Ref. | Age (yr)/ gender | Involved part of the pancreas | Tumor extension | Therapeutic schedule | Tumor size, cm | Sarcomatoid component | Follow-up time/results |
| Cresson et al[31], 1987 | 69/male | Head and tail | NA | Mitomycin, adriamycin, and 3000 rads of external radiation to the stomach | NA | Tubular structures, desmosomes, and hemijunctions under electron microscope | 5 mo/hemorrhage after surgery of metastasis in the jejunum |
| Higashi et al[19], 1999 | 74/male | Head | Head of the pancreas and the adjacent duodenum, with blood vessel and perineural sarcomatoid | Pylorus preserving pancreatoduodenectomy | 4.5 × 4 × 3 | CK AE1/AE3 (+), EMA (+), MUC1 (-), ARA (+), S100 (+), SMA (+), desmin (–), vimentin (–) | 3 mo/died after surgery of peritoneal carcinomatosis |
| Darvishian et al[32], 2002 | 74/male | Head | Peripancreatic adipose tissue and the duodenal wall. | Pancreatoduodenectomy | 4.0 × 3.0 | Vimentin (+), CK (+), CEA (+), SMA (+), desmin (+) and CD68 (-) | 4 mo/alive and well |
| De la Riva et al[33], 2006 | 72/female | Head | NA | NA | NA | CK and vimentin (+) | 9 mo/deceased with hepatic metastasis |
| Kim et al[21], 2006 | 73/female | Body and tail | Local invasion. With retroperitoneal lymph node with metastasis | Pancreatectomy with splenectomy and colonic segmental resection | 20 ×15 ×13 | CK (-), Vimentin (+), CD68 (+) | 4 mo/deceased secondary to hepatic and peritoneal metastases |
| Ren et al[13], 2013 | 48/male | Tail | Free surgical margins | Surgery N/A. Digital subtraction angiography interventional chemotherapy was then implemented. Gemcitabine, oxaliplatin, and floxuridine were intravenously injected via the superior mesenteric artery and celiac trunk artery. | 10 cm × 8 cm × 3.5 | Vimentin, α-1-antichymotrypsin, CK-19, CK-18, and pan-CK (+). CD68 and lysozyme (-) | 36 mo/alive and well |
| Yao et al[15], 2013 | 48/male | Tail | Free surgical margins | Laparoscopic spleen-preserving left pancreatectomy, adjuvant gemcitabine 1 cycle | 10 × 8 × 5 | CK 18 and vimentin (+) | 3 mo/tumor recurrence and death |
| Kane et al[9], 2014 | 85/male | Body | Local invasion with free surgical margins | A distal pancreatectomy, splenectomy, and partial gastrectomy | 3.3 × 3.0 × 2.6 | Pan-CK, CK5.2 (+), S100, SMA, EMA (-) | 26 mo/alive and well |
| Lai et al[34], 2015 | 55/male | Body and tail | NA | Distal pancreatectomy, splenectomy, and colonic segmental resection | 14 | CK, CK7, and vimentin (+) | NA |
| Nambiar et al[35], 2017 | 41/male | Head and uncinate | Liver metastasis | Gemcitabine | 2.2 × 2.1 | CK (+) and vimentin (+) | 1 mo/on chemotherapy when reported |
| Ruess et al[36], 2017 | 73/female | Head of pancreas | Free surgical margins | Extended pylorus-preserving pancreatoduodenectomy | 4.2 | Pan-CK1/3 (+), CK7 (+), CK19 (+). Vimentin (+). S100 (+) | 4 mo/death after surgery |
| Xie et al[16], 2018 | 63/male | Head of pancreas | Invasion of the distal common bile duct. Local invasion of the peripheral nerves. The lymph nodes, blood vessels, and resection margins were free from tumor tissue. | Pancreatoduodenectomy. 15 d of thymopeptides (1 mg per day). | 2.5 × 2 × 1.8 cm | Vimentin (+), CK7 (+), and CK19 (+) | 16 mo/hepatic metastasis |
| Bukhari and Joudeh[17], 2019 | 64/male | Head | Free surgical margins | Pancreatoduodenectomy with cholecystectomy and adjuvant gemcitabine | 2.4 × 2 × 1.9 | CAM 5.2 (-), vimentin (+) | 19 mo/alive and well |
| Zhou et al[14], 2019 | 59/male | Head | Pancreatic head with extension into the main pancreatic duct. Free surgical margins. Three out of 23 lymph nodes were positive for metastasis | Pancreatoduodenectomy | 2.5 × 2.5 × 2.0 | CK19 (+) and vimentin (+) | 6 mo/liver metastasis and peritoneal metastasis |
| Kimura et al[10], 2020 | 58/male | Body | Three lymph nodes out of 40 with direct invasion | Distal pancreatectomy with splenectomy. A six-month course of gemcitabine | 5 | CK (+) and vimentin (+). PSmad2/3, snail, and fibronectin | 120 mo (10 yr)/alive and well |
| Omrani et al[18], 2020 | 73/male | Tail | NA | En bloc resection of the tail of the pancreas, spleen, a part of the stomach, and postoperative adjuvant chemotherapy with gemcitabine | 10 | OCG were positive for CK19 and CK7 | 120 mo (10 yr)/colonic metastasis |
| Our case | 61/female | Tail | Free surgical margins, one lymph node compromised | Distal pancreatectomy and en-bloc splenectomy | 3.2 × 2.9 | Pan-CK (+) and vimentin (+) | 35 mo/alive and well |
Although SCP and “Carcinosarcoma” have different pathologic features, both share similar clinical features. Carcinosarcomas are considered to be “truly” biphasic neoplasms composed of intermingled carcinomatous and sarcomatous components, which have epithelial and mesenchymal differentiation. These two components are typically separated without a transition zone[14].
In previously published reports, the terms SPC and Carcinosarcoma have been often used interchangeably, and their definitions vary among the reports. On this basis, we excluded some articles in our summary of case reports (Table 1), when the termino
Recent publications have described the clinical and radiological features of UCP. Shiihara et al[6] aimed to identify the detailed clinicopathological features of UCP and revealed that these patients likely have abdominal pain or discomfort as an initial symptom, whereas jaundice was less common. It tends to present more commonly in men vs women with a ratio of 2.5:1 and occurs more frequently in the head of the pancreas[25]. Zhao et al[26] reported the radiologic features of SCP and found that the mean size of SCP was 5.1 cm, and most of the lesions appeared to be round or ellipsoidal in shape and were ill-defined. Vascular invasion by CT and MRI was reported in 5 of 10 lesions[26]. At the time of diagnosis, a bulky tumor is frequently detected, with the involvement of organs in the vicinity[27]. One of the imaging key signs for PDAC is the abrupt “cut-off” of the main pancreatic duct (MPD), with upstream MPD dilatation and substantial pancreatic atrophy[28]. Zhao et al[26] reported that eight of ten patients with SCP had upstream dilatation of the MPD. Among them, in three patients MPD was compressed by the lesions and no atrophy of the distal pancreatic parenchyma. In the other five patients, upstream MPD dilatation and distal pancreatic parenchyma atrophy were detected synchronously in only two patients while no atrophy was detected in the remaining three patients[26].
Because there is no standard regimen for treating SCP, patients with this disease are administered the same regimens as those with more common PDACs. Gemcitabine has been reported to be effective in the event of portal vein thrombosis or tumor recurrence, whereas a cisplatin/etoposide/ifosfamide (VIP) regimen was also found to produce notable results[6]. Imaoka et al[29] conducted a multicenter retrospective cohort study to investigate the efficacy of chemotherapy in patients with UCP (n = 50) showing a median overall survival (OS) of 4.08 mo. The most frequently used first-line treatment regimens were gemcitabine, S-1, and gemcitabine plus nab-paclitaxel. Although there was no significant difference in OS among these first-line regimens, gemcitabine plus nab-paclitaxel significantly improved median progression-free survival compared with gemcitabine alone[29].
Although treatment for PDAC remains challenging, complete R0 surgical extirpation is the only chance of cure[5]. Although SCP shares similar molecular carcinogenesis with PDAC, its prognosis is much worse[6]. Despite aggressive surgical management, the median postoperative survival has consistently been reported as less than 1 year, and almost all recurrences involve unresectable multiple metasta
Furthermore, the impact of adjuvant chemotherapy on the survival of SCP has not been well defined. Imaoka et al[29] report that a paclitaxel-containing regimen would offer relatively longer survival in patients with unresectable UCP.
Given its aggressive biological behavior and poor prognosis, it is of prime importance to make early diagnoses for patients with SCP[22]. Although some patients have a rapid recurrence and early death, long-term survival has been reported[5,10]. Blair et al[5] reported 8 cases of SCP, of which two experienced long-term survival (> 5 years), with the longest surviving nearly 16 years despite the presence of lymph node metastasis representing the longest survival time of SPC patients in the literature. Nevertheless, both long-term survivors had the tumor in the body/tail of the pancreas, underwent R0 resections, and received adjuvant therapy.
There are two reports of exceptional survival after ten years of follow-up. One of them received adjuvant chemotherapy with gemcitabine who remained free of tumor recurrence and metastasis for 10 years but after this period the patient presented a colonic obstruction due to metastatic disease[18]. In the other case of SCP with a stage T3N1M0, after surgery the patient completed a 6-month course of adjuvant chemotherapy with gemcitabine and was then followed up with abdomen CT. At 10 years after the operation, the authors report he is alive with no recurrence[10].
SCP reported in the present paper is a very rare case of primary pancreatic neoplasm. Based on the limited number of reported cases, the prognosis is poor. To our knowledge, the good evolution of our patient, tumor-free survival of 35 mo after surgery despite not receiving adjuvant chemotherapy treatment, is rather exceptional particularly after having lymphovascular invasion. Although our patient had a smaller tumor size compared to the other long-term survival cases, Paal et al[25] reported in 35 cases of UCP that overall tumor size is not a reliable prognostic indicator. In this case, the clinical presentation with acute abdominal pain aided in obtaining a relatively early diagnosis and better surgical results.
Sarcomatoid carcinoma is a rare aggressive tumor with a poor prognosis. With an early diagnosis with early surgical eradication of the tumor and adjuvant chemo
We are very grateful to the patient who provided informed consent for publication of the case.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: American Society for Gastrointestinal Endoscopy, No. 172297; and Sociedad Chilena de Gastroenterología.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Chile
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Nie M, Tian C S-Editor: Gong ZM L-Editor: A P-Editor: Yuan YY
| 1. | Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1397] [Cited by in RCA: 1734] [Article Influence: 192.7] [Reference Citation Analysis (1)] |
| 2. | McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24:4846-4861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1338] [Cited by in RCA: 1262] [Article Influence: 180.3] [Reference Citation Analysis (39)] |
| 3. | Mostafa ME, Erbarut-Seven I, Pehlivanoglu B, Adsay V. Pathologic classification of "pancreatic cancers": current concepts and challenges. Chin Clin Oncol. 2017;6:59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2447] [Article Influence: 489.4] [Reference Citation Analysis (3)] |
| 5. | Blair AB, Burkhart RA, Griffin JF, Miller JA, Weiss MJ, Cameron JL, Wolfgang CL, He J. Long-term survival after resection of sarcomatoid carcinoma of the pancreas: an updated experience. J Surg Res. 2017;219:238-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Shiihara M, Higuchi R, Izumo W, Furukawa T, Yamamoto M. A Comparison of the Pathological Types of Undifferentiated Carcinoma of the Pancreas. Pancreas. 2020;49:230-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Mirsadraee M. Anthracosis of the lungs: etiology, clinical manifestations and diagnosis: a review. Tanaffos. 2014;13:1-13. [PubMed] |
| 8. | Hornick JL. Biphasic Tumors and Tumors With Mixed Patterns. In: Practical Soft Tissue Pathology: a Diagnostic Approach. Elsevier. 2019;249-267. [DOI] [Full Text] |
| 9. | Kane JR, Laskin WB, Matkowskyj KA, Villa C, Yeldandi AV. Sarcomatoid (spindle cell) carcinoma of the pancreas: A case report and review of the literature. Oncol Lett. 2014;7:245-249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Kimura T, Fujimoto D, Togawa T, Ishida M, Iida A, Sato Y, Goi T. Sarcomatoid carcinoma of the pancreas with rare long-term survival: a case report. World J Surg Oncol. 2020;18:105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Clark CJ, Graham RP, Arun JS, Harmsen WS, Reid-Lombardo KM. Clinical outcomes for anaplastic pancreatic cancer: a population-based study. J Am Coll Surg. 2012;215:627-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Haeberle L, Esposito I. Pathology of pancreatic cancer. Transl Gastroenterol Hepatol. 2019;4:50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 130] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 13. | Ren CL, Jin P, Han CX, Xiao Q, Wang DR, Shi L, Wang DX, Chen H. Unusual early-stage pancreatic sarcomatoid carcinoma. World J Gastroenterol. 2013;19:7820-7824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Zhou DK, Gao BQ, Zhang W, Qian XH, Ying LX, Wang WL. Sarcomatoid carcinoma of the pancreas: A case report. World J Clin Cases. 2019;7:236-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Yao J, Qian JJ, Zhu CR, Bai DS, Miao Y. Laparoscopic left pancreatectomy for pancreatic sarcomatoid carcinoma: A case report and review of the literature. Oncol Lett. 2013;6:568-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Xie Y, Xiang Y, Zhang D, Yao X, Sheng J, Yang Y, Zhang X. Sarcomatoid carcinoma of the pancreas: A case report and review of the literature. Mol Med Rep. 2018;18:4716-4724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Bukhari N, Joudeh A. Early Stage Anaplastic Sarcomatoid Carcinoma of The Pancreas, A Case Report. Am J Case Rep. 2019;20:597-601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Omrani S, Hajri M, Ferjaoui W, Guizani R, Talbi G, Gharbi L, Bayar R, khalfallah MT. Pancreatic sarcomatoid carcinoma : An unusual evolution. Med Case Rep Rev. 2020;3:1-2. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Higashi M, Takao S, Sato E. Sarcomatoid carcinoma of the pancreas: a case report with immunohistochemical study. Pathol Int. 1999;49:453-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Hu QL, Li HQ, Xia TY. A case of sarcomatoid carcinoma of the pancreas. Shijie Huaren Xiaohua Zazhi. 2015;23:707-710. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Kim KH, Kang DY, Lee MK, Yang HW, Han HY. Sarcomatoid Carcinoma of the Pancreas - A Case Report. Korean J Pathol. 2006;40:306-310. |
| 22. | Yepuri N, Pruekprasert N, Naous R. High-grade malignant pancreatic neoplasm with sarcomatoid features. AME Case Rep. 2018;2:39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Huey RW, Makawita S, Xiao L, Matamoros A, Estrella JS, Overman MJ, Varadhachary GR, Raghav K. Sarcomatoid carcinoma presenting as cancers of unknown primary: a clinicopathological portrait. BMC Cancer. 2019;19:965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Alguacil-Garcia A, Weiland LH. The histologic spectrum, prognosis, and histogenesis of the sarcomatoid carcinoma of the pancreas. Cancer. 1977;39:1181-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 25. | Paal E, Thompson LD, Frommelt RA, Przygodzki RM, Heffess CS. A clinicopathologic and immunohistochemical study of 35 anaplastic carcinomas of the pancreas with a review of the literature. Ann Diagn Pathol. 2001;5:129-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 92] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Zhao S, Su W, Deng L, Chen Y, Zuo C, Shao C, Ren F. Pancreatic sarcomatoid carcinoma: CT, MRI, and 18F-FDG PET/CT features. Clin Radiol 2020; 75: 397.e7-397. e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Hoshimoto S, Matsui J, Miyata R, Takigawa Y, Miyauchi J. Anaplastic carcinoma of the pancreas: Case report and literature review of reported cases in Japan. World J Gastroenterol. 2016;22:8631-8637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Elbanna KY, Jang HJ, Kim TK. Imaging diagnosis and staging of pancreatic ductal adenocarcinoma: a comprehensive review. Insights Imaging. 2020;11:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 29. | Imaoka H, Ikeda M, Maehara K, Umemoto K, Ozaka M, Kobayashi S, Terashima T, Inoue H, Sakaguchi C, Tsuji K, Shioji K, Okamura K, Kawamoto Y, Suzuki R, Shirakawa H, Nagano H, Ueno M, Morizane C, Furuse J. Clinical outcomes of chemotherapy in patients with undifferentiated carcinoma of the pancreas: a retrospective multicenter cohort study. BMC Cancer. 2020;20:946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Gelos M, Behringer D, Philippou S, Mann B. Pancreatic carcinosarcoma. Case report of multimodal therapy and review of the literature. JOP. 2008;9:50-55. [PubMed] |
| 31. | Cresson DH, Reddick RL. Sarcomatoid carcinoma of the pancreas presenting as gastric carcinoma: clinicopathologic and ultrastructural findings. J Surg Oncol. 1987;36:268-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 32. | Darvishian F, Sullivan J, Teichberg S, Basham K. Carcinosarcoma of the Pancreas. Arch Pathol Lab Med. 2002;126:1114-1117. [RCA] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | De la Riva S, Muñoz-Navas MA, Betés M, Súbtil JC, Carretero C, Sola JJ. Sarcomatoid carcinoma of the pancreas and congenital choledochal cyst. Gastrointest Endosc. 2006;64:1005-1006; discussion 1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Lai CW, Chen CW, Lee YH, Chen JH. Sarcomatoid carcinoma of the pancreas. Tzu Chi Med J. 2015;27:46-47. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 35. | Nambiar RK, Roshni S, Lijeesh AL, Mony RP. Sarcomatoid carcinoma of pancreas with liver metastases – A case report with review of literature. J Med Ther. 2017;1:1-3. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | Ruess DA, Kayser C, Neubauer J, Fichtner-Feigl S, Hopt UT, Wittel UA. Carcinosarcoma of the Pancreas: Case Report With Comprehensive Literature Review. Pancreas. 2017;46:1225-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |