Published online May 26, 2021. doi: 10.12998/wjcc.v9.i15.3680
Peer-review started: December 21, 2020
First decision: January 17, 2021
Revised: January 25, 2021
Accepted: March 15, 2021
Article in press: March 15, 2021
Published online: May 26, 2021
Processing time: 140 Days and 19.6 Hours
Erythropoiesis-stimulating agents (ESAs) have revolutionized the therapeutic strategy for anemia in chronic kidney disease. However, some cases are resistant or hyporesponsive to ESAs. Roxadustat is an oral hypoxia-inducible factor-prolyl hydroxylase inhibitor that stimulates erythropoiesis and regulates iron metabolism. Here, we describe a hemodialysis patient with refractory anemia who did not respond to traditional treatments and depended on blood transfusion for more than 1 year. After applying Roxadustat, the patient’s anemia improved significantly.
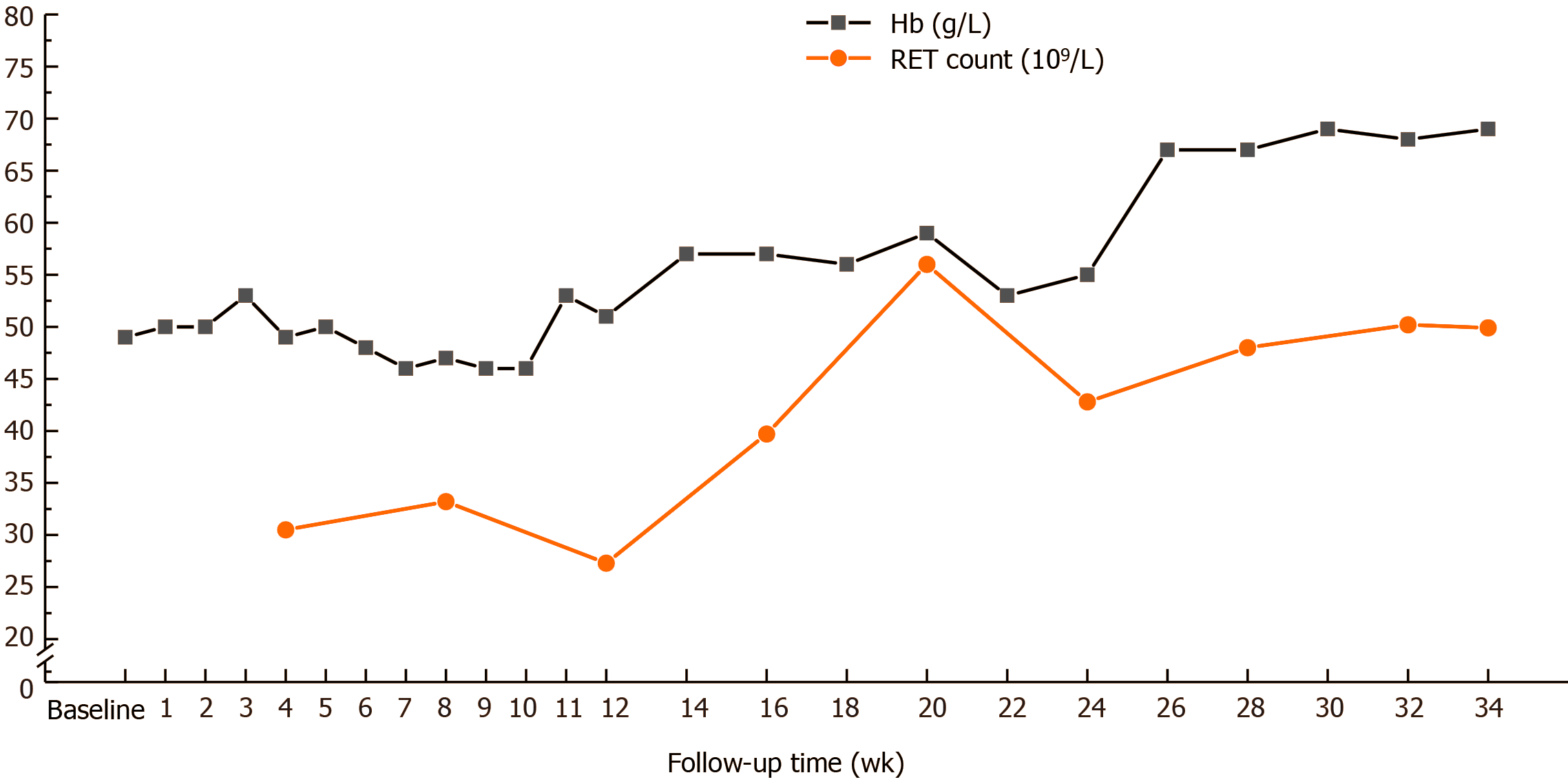
A 44-year-old man was diagnosed with uremia accompanied by severe anemia with a hemoglobin (Hb) level ranging from 30-40 g/L. His anemia did not improve after sufficient dialysis or high doses of active ESAs; other causes of anemia were excluded. The patient required approximately 600-1000 mL of red blood cell suspension every 15-30 d for more than 1 year. After accepting Roxadustat therapy, the patient’s anemia symptoms improved significantly; his Hb level gradually increased to 50 g/L, and no further blood transfusions were administered. His Hb level reached 69 g/L by the 34th week. Although a Hb level of 60-70 g/L cannot be considered satisfactory, he no longer required blood transfusions and his quality of life was substantially improved. Roxadustat showed good efficacy and safety in this case.
Roxadustat represents an innovative and effective agent for the clinical treatment of renal anemia caused by multiple complex factors.
Core Tip: Anemia is a common complication of chronic kidney disease, often in combination with increased morbidity and mortality. Treatment of anemia in advanced chronic kidney disease includes iron replacement and administration of erythropoiesis-stimulating agents (ESAs). However, anemia remains undertreated because of concerns of the safety of ESAs. Roxadustat is an oral hypoxia-inducible factor-prolyl hydroxylase inhibitor (HIF-PHI) that stimulates erythropoiesis and regulates iron metabolism. We present a case in which Roxadustat was used for the treatment of a blood transfusion-dependent maintenance hemodialysis patient. This case highlights that increasing HIF activity with small-molecule HIF-PHIs such as Roxadustat is an innovative therapeutic approach for anemia.
- Citation: Fei M, Wen XQ, Yu ZL, Kang T, Wu WH, Ou ST. Roxadustat as treatment for a blood transfusion-dependent maintenance hemodialysis patient: A case report and review of literature. World J Clin Cases 2021; 9(15): 3680-3688
- URL: https://www.wjgnet.com/2307-8960/full/v9/i15/3680.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i15.3680
Anemia is a common complication of chronic kidney disease (CKD) that is associated with increased rates of hospitalization, transfusion[1], death and other complications in CKD[2,3]. Currently, the cornerstone treatment of renal anemia is the use of erythropoiesis-stimulating agents (ESAs) and iron supplementation. Indeed, the administration of ESAs and iron supplementation are well-established and effective therapeutic approaches for treating renal anemia. However, the use of high-dose ESAs increases the risks of cardiovascular events and death, and some cases are resistant or hyporesponsive to ESAs, partly because of inflammation or iron depletion[4,5]. Roxadustat is a new oral anti-renal anemia medication that stabilizes hypoxia-inducible factor (HIF) subunits, promotes HIF activity, and induces the transcription of early-response target genes. It enhances the expression of endogenous erythropoietin (EPO), EPO receptor (EPOR), and proteins that promote iron absorption and transport[6,7], ultimately increasing hemoglobin levels, and improving iron homeostasis[8-12]. HIF prolyl hydroxylase inhibitors (HIF-PHIs) effectively improve anemia in CKD populations and constitute a new therapeutic approach for anemia in CKD. Here, we describe a case of a maintenance hemodialysis (MHD) patient with refractory renal anemia who required blood transfusions for more than 1 year and then received Roxadustat therapy. Ultimately, the patient’s anemia improved significantly and he no longer needed to receive blood transfusions.
Tiredness and shortness of breath.
The patient was 44 years of age when admitted to our hospital in May 2018 complaining of feeling tired and of being short of breath. More than 1 year earlier, laboratory evaluation revealed elevated serum creatinine (SCr) (unspecified details), but there was no formal diagnosis or treatment. Four months before admission, the patient started to feel tired and short of breath after activity, accompanied by foamy urine and fatigue. There was no further diagnosis or treatment. The patient was later admitted to the hospital for worsened symptoms.
Past medical history included hypertension that was controlled by irbesartan and abdominal surgery because of trauma more than 10 years previously.
His personal and family history were unremarkable.
Body temperature, blood pressure, heart rate, respiratory rate, oxygen saturation, chest examination, and abdominal examination were normal.
His renal function results included an SCr of 1006.1 μmol/L (reference range: 57-97 μmol/L) and a glomerular filtration rate of 5 mL/min (reference range: 90-110 mL/min). Routine blood tests showed a white blood cell count of 3.30 × 109/L [reference range: (3.5-9.5) × 109/L] and a hemoglobin (Hb) level of 33 g/L (reference range: 130-175 g/L). Iron metabolism indices revealed a serum ferritin (SF) level of 396.14 ng/mL (reference range: 25.00-280.00 ng/mL), a serum iron level of 12.4 μmol/L (reference range: 10.6-36.7 μmol/L), a total iron-binding capacity of 52.1 µmol/L (reference range: 50-77 µmol/L), transferrin saturation (TSAT) of 23.8%, and an intact parathyroid hormone level of 385 pg/mL (reference range: 8.70-79.60 pg/mL). The autoantibody profile, antineutrophil cytoplasmic antibody, viral hepatitis, bilirubin, Coombs’ test, and fecal occult bleeding test results were all negative.
A color Doppler ultrasound scan detected shrinkage of both kidneys. Other imaging, including computed tomography scans of the chest and cardiac ultrasound, were normal.
Based on the patient’s medical history and laboratory examinations, the patient was diagnosed with stage 5 chronic kidney disease (CKD-5D) complicated by severe anemia and hypertension.
After making a definitive diagnosis, approximately 2200 mL of a red blood cell suspension was administered to correct his anemia during his first admission to the hospital. At the same time, therapy consisting of subcutaneous injections of ESAs was started at an initial dose of 6000 U/time (twice a week, BIW). Regular hemodialysis was also begun (three times a week, TIW). The ESW dose was adjusted based on body weight and Hb level. The maximum dose of more than 300 U/kg per week was administered for more than 3 mo, but the patient’s anemia did not improve, with an Hb level always fluctuating between 30 and 40 g/L. No iron deficiency, obvious microinflammation, serious secondary hyperparathyroidism, or blood loss were observed. The patient received multiple blood transfusions and required approximately 600-1000 mL of red blood cell suspension every 15-30 d. At the same time, the patient received intravenous deferoxamine to reduce his iron load; the SF level fluctuated between 600 and 900 ng/mL and TSAT between 30% and 50% (Table 1). After MHD and massive blood transfusion for more than 1 year, on September 6, 2019, Roxadustat was given orally at an initial dose of 100 mg/dose (TIW); ESA therapy was simultaneously discontinued. After taking Roxadustat, the patient’s anemia improved significantly, and no further blood transfusion was performed. We adjusted the dose of Roxadustat during the course of treatment according to his Hb level, and we are currently administering the recommended maximum dose (150 mg TIW); his Hb level has increased to 69 g/L.
| Characteristics | |
| Age in yr | 44 |
| Sex | Male |
| Body weight in kg | 60 |
| Hb in g/L | 49 |
| SI in μmol/L | 24.0 |
| TIBC in μmol/L | 51.6 |
| TSAT, % | 46.51 |
| SF in ng/mL | 656.03 |
| iPTH in pg/mL | 385 |
| Dialysis method: hemodialysis/peritoneal dialysis | Hemodialysis |
| Duration of dialysis in yr | 1.33 |
| Dose of rhEPO injection in IU | 6000 |
| Frequency of rhEPO injection as times/wk | 3 |
| Blood pressure | |
| Systolic in mmHg | 157 |
| Diastolic in mmHg | 96 |
| CRP in mg/L | 4.61 |
| Blood lipids | |
| TC in mmol/L | 3.76 |
| TGs in mmol/L | 5.19 |
| LDL-C in mmol/L | 0.74 |
| HDL-C in mmol/L | 2.43 |
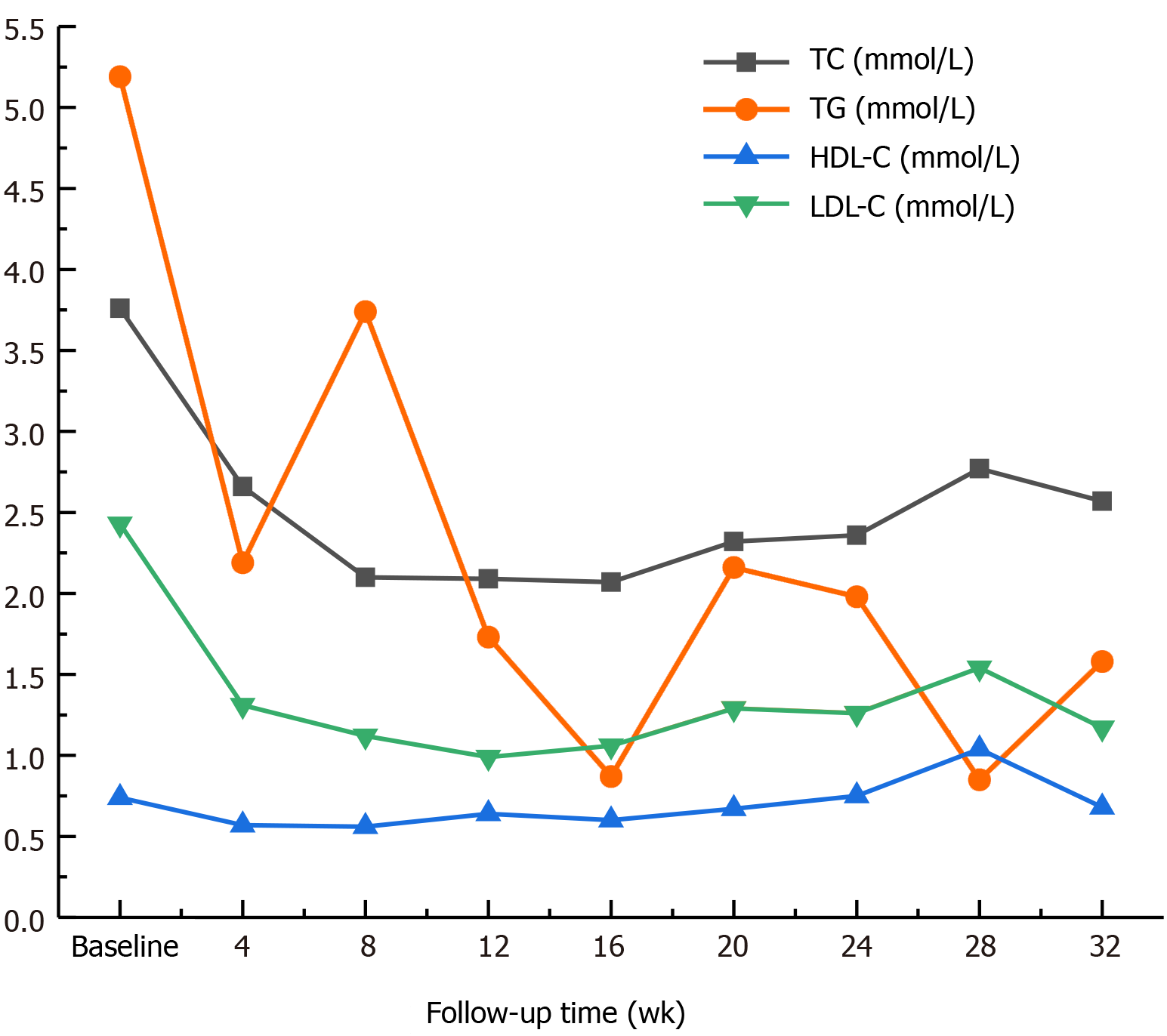
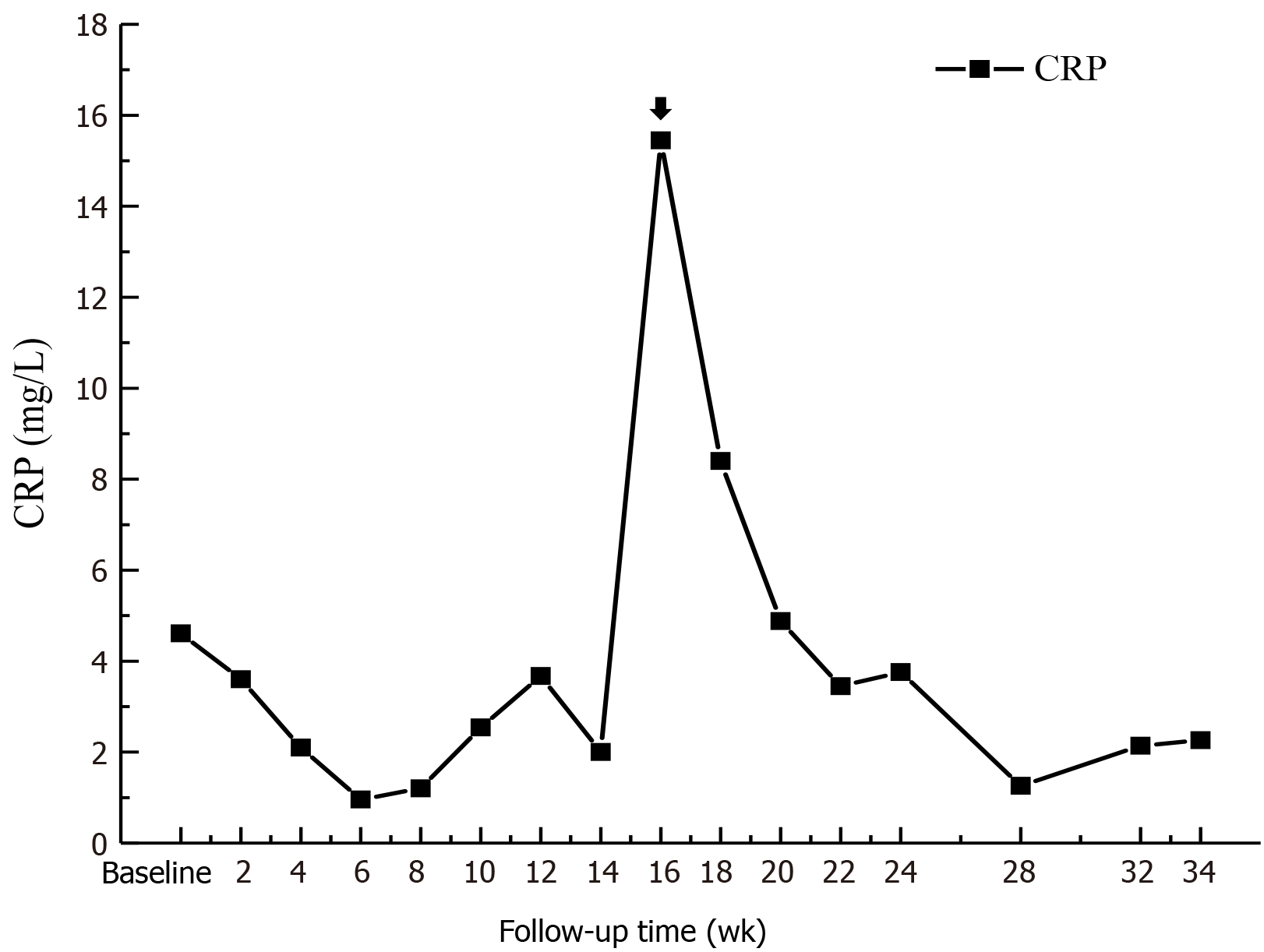
Before Roxadustat administration, his Hb level was 49 g/L (Table 1), which dropped to 46 g/L in the 2nd week after starting Roxadustat. A total of 400 mL of red blood cell suspension was infused, the Hb level increased to 62 g/L, and the patient was discharged. From the 3rd week after starting Roxadustat, the patient felt that his symptoms of weakness, fatigue, and shortness of breath were significantly relieved. His Hb level gradually increased to 50 g/L, and no more blood transfusions were performed. At the 5th week of Roxadustat treatment, the dosage was adjusted to 120 mg/time (TIW), and the patient’s anemia symptoms continued to improve. At week 12, the Hb level increased further and fluctuated between 50 and 60 g/L. His Hb level had reached 69 g/L by the 30th week, and his reticulocyte (RET) count also increased (Figure 1). The dosage was then adjusted to the maximum dose (150 mg/time, TIW). In addition, iron metabolism (Table 2) and blood lipid levels improved significantly (Figure 2), total triglycerides (TGs) gradually decreased to normal, total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) were lower than before Roxadustat treatment, and C-reactive protein (CRP), a marker of inflammation, was also reduced. At week 16, his CRP level was significantly elevated because of concurrent respiratory infections (Figure 3). However, this coinfection did not affect the response to Roxadustat. No adverse events, such as elevated blood pressure, nausea, vomiting, peripheral edema, myalgia, rash, elevated alanine aminotransferase, or metabolic acidosis, were observed during follow-up, but hyperkalemia was occasionally observed. The patient no longer required blood transfusions, and his quality of life (QoL) was substantially improved. Currently, his Roxadustat treatment is ongoing, and although his Hb is rising slowly, the level is expected to eventually reach the target value.
| Date | |||||||||
| Index | Baseline | Oct 2019 | Nov 2019 | Dec 2019 | Jan 2020 | Feb 2020 | Mar 2020 | Apr 2020 | May 2020 |
| SI in μmol/L | 24.00 | 47.00 | 40.90 | 24.70 | 51.40 | 18.60 | 43.5 | 24.4 | 36.5 |
| TIBC in μmol/L | 51.6 | 50.7 | 55.2 | 54.3 | 54.9 | 63.9 | 63.5 | 69.9 | 55.2 |
| TAST, % | 46.51 | 92.70 | 74.09 | 45.49 | 93.62 | 29.11 | 68.5 | 34.91 | 66.12 |
| SF in ng/mL | 656.03 | 724.84 | 798.02 | 574.53 | 432.47 | 420.11 | 337.21 | 570.57 | 320.16 |
Anemia is one of the hallmarks of CKD and it progresses with CKD stage, greatly influencing patient prognosis and QoL. The mechanism of anemia in patients with CKD is complicated. EPO deficiency and iron metabolism disorder play vital roles in renal anemia[13-15]. In addition, chronic inflammation, infection, uremic toxins, occult blood loss, oxidative stress, hyperparathyroidism, malnutrition, and other factors may further aggravate anemia[16-19]. Traditional treatment strategies for anemia in CKD include the use of ESAs, intravenous administration of iron, and blood transfusion[20,21]. ESAs effectively correct renal anemia and significantly reduce the need for blood transfusion[22,23]. However, approximately 5%-10% of patients have a low response or show resistance to ESAs. Iron deficiency, inadequate dialysis, inflammation, malnutrition, chronic blood loss, secondary hyperparathyroidism, aluminum toxicity, and hypersplenism may be important reasons for resistance to ESAs[24]. Despite the efficacy of ESAs for treating anemia, high doses may increase the risk of vascular thrombosis, myocardial infarction, heart failure, tumor progression or recurrence, and mortality[25]. Therefore, the current treatments of renal anemia have some limitations.
The patient in this case was diagnosed with CKD-5D accompanied by severe anemia. However, his anemia did not improve with sufficient dialysis and high doses of active ESAs. After excluding autoimmune diseases such as systemic lupus erythematosus, vasculitis, cancer, and hemolytic anemia, ESA resistance was considered to be responsible for his refractory anemia; bone marrow diseases could not be completely excluded. The patient needed massive blood transfusions, yet there is evidence that long-term, massive blood transfusion therapy may lead to secondary iron overload, liver cirrhosis, cardiomyopathy, eventual damage to multiple organs, and even death[26].
Roxadustat (FG-4592) is an HIF-PHI that stabilizes HIF- subunits, resulting in increased HIF transcriptional activity. This leads to functional activation of early-response target genes encoding proteins such as EPO, EPOR, enzymes of heme biosynthesis, and proteins that promote iron absorption and transport, resulting in coordinated erythropoiesis[12,27-30]. Clinical trials have demonstrated that successful anemia management can be achieved with Roxadustat in both dialysis-dependent and nondialysis-dependent-CKD patients[29,30]. In addition, a phase 3, randomized, double-blind study in Japan reported that Roxadustat maintained target hemoglobin in patients undergoing hemodialysis and that it was noninferior to darbepoetin alfa[31]. Compared with traditional ESAs, Roxadustat is well tolerated, with fewer adverse events[8-11]. In this case, the patient’s Hb level and RET count simultaneously increased after Roxadustat therapy, and although his Hb level did not reach the target value, his anemia symptoms were significantly improved. Moreover, he no longer required blood transfusions, thus avoiding iron overload as well as the economic burden of the procedure; his iron metabolism improved as well. A poor response to ESAs has been associated with inflammation[32,33], and inflammation is known to increase levels of hepcidin, resulting in functional iron deficiency. HIF promotes expression of adenosine receptor genes, with anti-inflammatory effects[34-36]. In this case, the patient’s inflammation improved significantly, and coinfection did not affect his Hb response to Roxadustat, which is consistent with previous clinical research[29,30]. Moreover, Roxadustat reduced nonfasting TC levels compared with rhEPO[12,29,30], which might be partly mediated by HIF-dependent effects on acetyl coenzyme A, with a vital role in the first step of cholesterol synthesis[37]. Downregulation of 3-hydroxy-3-methyl glutaryl coenzyme A reductase, which is required for cholesterol synthesis, may also be involved[38,39]. Chen et al[30] found that TC levels decreased by 23% with Roxadustat therapy, with levels of TGs, LDL-C and high-density lipoprotein cholesterol (HDL-C) decreasing. In our case, the patient’s TG level decreased to normal, and his TC, HDL-C, and LDL-C levels were lower than before treatment, which is consistent with previous studies. In other studies, hypertension, acute pancreatitis, hyperkalemia, metabolic acidosis and diarrhea were found to be possibly related to Roxadustat treatment. Nevertheless, a phase III trial in Chinese dialysis patients previously treated with ESAs did not raise significant safety concerns[29,30]. In our case, no obvious adverse events were observed. Del Vecchio and Locatelli[40] reported dose-dependent effects of Roxadustat on erythropoiesis and iron metabolism in American dialysis patients. We adjusted the dose of Roxadustat according to the patient’s Hb level, and we are currently administering the recommended maximum dose of 2.5 mg/kg (150 mg TIW); his Hb level has increased to 69 g/L. The patient is being followed to assess further effects, but eliminating the need for blood transfusion represents the first step to successful treatment.
Roxadustat is a new drug that can effectively improve renal anemia through a new mechanism involving multiple pathways, and more than 80% of patients treated with Roxadustat achieve normal levels of blood indices[29,30]. Therefore, Roxadustat constitutes a new, effective method for the clinical treatment of renal anemia in CKD.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ohashi N S-Editor: Gao CC L-Editor: Filipodia P-Editor: Wang LL
| 1. | Seliger S, Fox KM, Gandra SR, Bradbury B, Hsu VD, Walker L, Chiou CF, Fink JC. Timing of erythropoiesis-stimulating agent initiation and adverse outcomes in nondialysis CKD: a propensity-matched observational study. Clin J Am Soc Nephrol. 2010;5:882-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Levin A. The treatment of anemia in chronic kidney disease: understandings in 2006. Curr Opin Nephrol Hypertens. 2007;16:267-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Hörl WH. Anaemia management and mortality risk in chronic kidney disease. Nat Rev Nephrol. 2013;9:291-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 4. | Vlagopoulos PT, Tighiouart H, Weiner DE, Griffith J, Pettitt D, Salem DN, Levey AS, Sarnak MJ. Anemia as a risk factor for cardiovascular disease and all-cause mortality in diabetes: the impact of chronic kidney disease. J Am Soc Nephrol. 2005;16:3403-3410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 210] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 5. | KDOQI. ; National Kidney Foundation. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Anemia in Chronic Kidney Disease. Am J Kidney Dis. 2006;47:S11-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 372] [Article Influence: 19.6] [Reference Citation Analysis (1)] |
| 6. | Fan C, Iacobas DA, Zhou D, Chen Q, Lai JK, Gavrialov O, Haddad GG. Gene expression and phenotypic characterization of mouse heart after chronic constant or intermittent hypoxia. Physiol Genomics. 2005;22:292-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Peyssonnaux C, Nizet V, Johnson RS. Role of the hypoxia inducible factors HIF in iron metabolism. Cell Cycle. 2008;7:28-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 165] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 8. | Besarab A, Provenzano R, Hertel J, Zabaneh R, Klaus SJ, Lee T, Leong R, Hemmerich S, Yu KH, Neff TB. Randomized placebo-controlled dose-ranging and pharmacodynamics study of roxadustat (FG-4592) to treat anemia in nondialysis-dependent chronic kidney disease (NDD-CKD) patients. Nephrol Dial Transplant. 2015;30:1665-1673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 223] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 9. | Besarab A, Chernyavskaya E, Motylev I, Shutov E, Kumbar LM, Gurevich K, Chan DT, Leong R, Poole L, Zhong M, Saikali KG, Franco M, Hemmerich S, Yu KH, Neff TB. Roxadustat (FG-4592): Correction of Anemia in Incident Dialysis Patients. J Am Soc Nephrol. 2016;27:1225-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 202] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 10. | Provenzano R, Besarab A, Sun CH, Diamond SA, Durham JH, Cangiano JL, Aiello JR, Novak JE, Lee T, Leong R, Roberts BK, Saikali KG, Hemmerich S, Szczech LA, Yu KH, Neff TB. Oral Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitor Roxadustat (FG-4592) for the Treatment of Anemia in Patients with CKD. Clin J Am Soc Nephrol. 2016;11:982-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 228] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 11. | Provenzano R, Besarab A, Wright S, Dua S, Zeig S, Nguyen P, Poole L, Saikali KG, Saha G, Hemmerich S, Szczech L, Yu KH, Neff TB. Roxadustat (FG-4592) Versus Epoetin Alfa for Anemia in Patients Receiving Maintenance Hemodialysis: A Phase 2, Randomized, 6- to 19-Week, Open-Label, Active-Comparator, Dose-Ranging, Safety and Exploratory Efficacy Study. Am J Kidney Dis. 2016;67:912-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 201] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 12. | Chen N, Qian J, Chen J, Yu X, Mei C, Hao C, Jiang G, Lin H, Zhang X, Zuo L, He Q, Fu P, Li X, Ni D, Hemmerich S, Liu C, Szczech L, Besarab A, Neff TB, Peony Yu KH, Valone FH. Phase 2 studies of oral hypoxia-inducible factor prolyl hydroxylase inhibitor FG-4592 for treatment of anemia in China. Nephrol Dial Transplant. 2017;32:1373-1386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 154] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 13. | van Nooten FE, Green J, Brown R, Finkelstein FO, Wish J. Burden of illness for patients with non-dialysis chronic kidney disease and anemia in the United States: review of the literature. J Med Econ. 2010;13:241-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Babitt JL, Lin HY. Mechanisms of anemia in CKD. J Am Soc Nephrol. 2012;23:1631-1634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 669] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 15. | Kali A, Charles MV, Seetharam RS. Hepcidin - A novel biomarker with changing trends. Pharmacogn Rev. 2015;9:35-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Verdalles U, Abad S, Vega A, Ruiz Caro C, Ampuero J, Jofre R, Lopez-Gomez JM. Factors related to the absence of anemia in hemodialysis patients. Blood Purif. 2011;32:69-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Macdougall IC. Role of uremic toxins in exacerbating anemia in renal failure. Kidney Int Suppl. 2001;78:S67-S72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Wu CJ, Chen CY, Lai TS, Wu PC, Chuang CK, Sun FJ, Liu HL, Chen HH, Yeh HI, Lin CS, Lin CJ. The role of indoxyl sulfate in renal anemia in patients with chronic kidney disease. Oncotarget. 2017;8:83030-83037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Tanaka M, Komaba H, Fukagawa M. Emerging Association Between Parathyroid Hormone and Anemia in Hemodialysis Patients. Ther Apher Dial. 2018;22:242-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 20. | Fuller DS, Robinson BM, Locatelli F, Pisoni RL. Patterns of Erythropoiesis-Stimulating Agent Use in European Hemodialysis Patients: The Dialysis Outcomes and Practice Patterns Study. Nephron. 2018;140:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Azmandian J, Abbasi MR, Pourfarziani V, Nasiri AA, Ossareh S, Ezzatzadegan Jahromi S, Sanadgol H, Amini S, Shahvaroughi Farahani A. Comparing Therapeutic Efficacy and Safety of Epoetin Beta and Epoetin Alfa in the Treatment of Anemia in End-Stage Renal Disease Hemodialysis Patients. Am J Nephrol. 2018;48:251-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Eschbach JW, Abdulhadi MH, Browne JK, Delano BG, Downing MR, Egrie JC, Evans RW, Friedman EA, Graber SE, Haley NR. Recombinant human erythropoietin in anemic patients with end-stage renal disease. Results of a phase III multicenter clinical trial. Ann Intern Med. 1989;111:992-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 493] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 23. | Möcks J. Cardiovascular mortality in haemodialysis patients treated with epoetin beta - a retrospective study. Nephron. 2000;86:455-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Kubasch AS, Platzbecker U. Setting Fire to ESA and EMA Resistance: New Targeted Treatment Options in Lower Risk Myelodysplastic Syndromes. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Bennett CL, Becker PS, Kraut EH, Samaras AT, West DP. Intersecting guidelines: administering erythropoiesis-stimulating agents to chronic kidney disease patients with cancer. Semin Dial. 2009;22:1-4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Adler M, Herrera-Gómez F, Martín-García D, Gavid M, Álvarez FJ, Ochoa-Sangrador C. The Impact of Iron Supplementation for Treating Anemia in Patients with Chronic Kidney Disease: Results from Pairwise and Network Meta-Analyses of Randomized Controlled Trials. Pharmaceuticals (Basel). 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Becker K, Saad M. A New Approach to the Management of Anemia in CKD Patients: A Review on Roxadustat. Adv Ther. 2017;34:848-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Gupta N, Wish JB. Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitors: A Potential New Treatment for Anemia in Patients With CKD. Am J Kidney Dis. 2017;69:815-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 318] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 29. | Chen N, Hao C, Liu BC, Lin H, Wang C, Xing C, Liang X, Jiang G, Liu Z, Li X, Zuo L, Luo L, Wang J, Zhao MH, Cai GY, Hao L, Leong R, Liu C, Neff T, Szczech L, Yu KP. Roxadustat Treatment for Anemia in Patients Undergoing Long-Term Dialysis. N Engl J Med. 2019;381:1011-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 417] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 30. | Chen N, Hao C, Peng X, Lin H, Yin A, Hao L, Tao Y, Liang X, Liu Z, Xing C, Chen J, Luo L, Zuo L, Liao Y, Liu BC, Leong R, Wang C, Liu C, Neff T, Szczech L, Yu KP. Roxadustat for Anemia in Patients with Kidney Disease Not Receiving Dialysis. N Engl J Med. 2019;381:1001-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 408] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 31. | Akizawa T, Iwasaki M, Yamaguchi Y, Majikawa Y, Reusch M. Phase 3, Randomized, Double-Blind, Active-Comparator (Darbepoetin Alfa) Study of Oral Roxadustat in CKD Patients with Anemia on Hemodialysis in Japan. J Am Soc Nephrol. 2020;31:1628-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 143] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 32. | Gomes AC, Gomes MS. Hematopoietic niches, erythropoiesis and anemia of chronic infection. Exp Hematol. 2016;44:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Bradbury BD, Critchlow CW, Weir MR, Stewart R, Krishnan M, Hakim RH. Impact of elevated C-reactive protein levels on erythropoiesis- stimulating agent (ESA) dose and responsiveness in hemodialysis patients. Nephrol Dial Transplant. 2009;24:919-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Bartels K, Grenz A, Eltzschig HK. Hypoxia and inflammation are two sides of the same coin. Proc Natl Acad Sci USA. 2013;110:18351-18352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 164] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 35. | Bowser JL, Lee JW, Yuan X, Eltzschig HK. The hypoxia-adenosine link during inflammation. J Appl Physiol (1985). 2017;123:1303-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 36. | Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364:656-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1400] [Cited by in RCA: 1563] [Article Influence: 111.6] [Reference Citation Analysis (0)] |
| 37. | Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2552] [Cited by in RCA: 2899] [Article Influence: 152.6] [Reference Citation Analysis (0)] |
| 38. | Nguyen AD, McDonald JG, Bruick RK, DeBose-Boyd RA. Hypoxia stimulates degradation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase through accumulation of lanosterol and hypoxia-inducible factor-mediated induction of insigs. J Biol Chem. 2007;282:27436-27446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 39. | Hwang S, Nguyen AD, Jo Y, Engelking LJ, Brugarolas J, DeBose-Boyd RA. Hypoxia-inducible factor 1α activates insulin-induced gene 2 (Insig-2) transcription for degradation of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase in the liver. J Biol Chem. 2017;292:9382-9393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 40. | Del Vecchio L, Locatelli F. Roxadustat in the treatment of anaemia in chronic kidney disease. Expert Opin Investig Drugs. 2018;27:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |