Published online May 26, 2021. doi: 10.12998/wjcc.v9.i15.3668
Peer-review started: December 4, 2020
First decision: January 25, 2021
Revised: February 6, 2021
Accepted: March 18, 2021
Article in press: March 18, 2021
Published online: May 26, 2021
Processing time: 158 Days and 1.1 Hours
Metachronous pulmonary and pancreatic metastases from colorectal cancer are rare. The diagnosis of pancreatic metastases is difficult and predominantly relies on computed tomography, pathology and immunohistochemistry. Here, we describe the use of next-generation sequencing (NGS) for determination of the origin of metastasis and prognostic prediction of colorectal cancer.
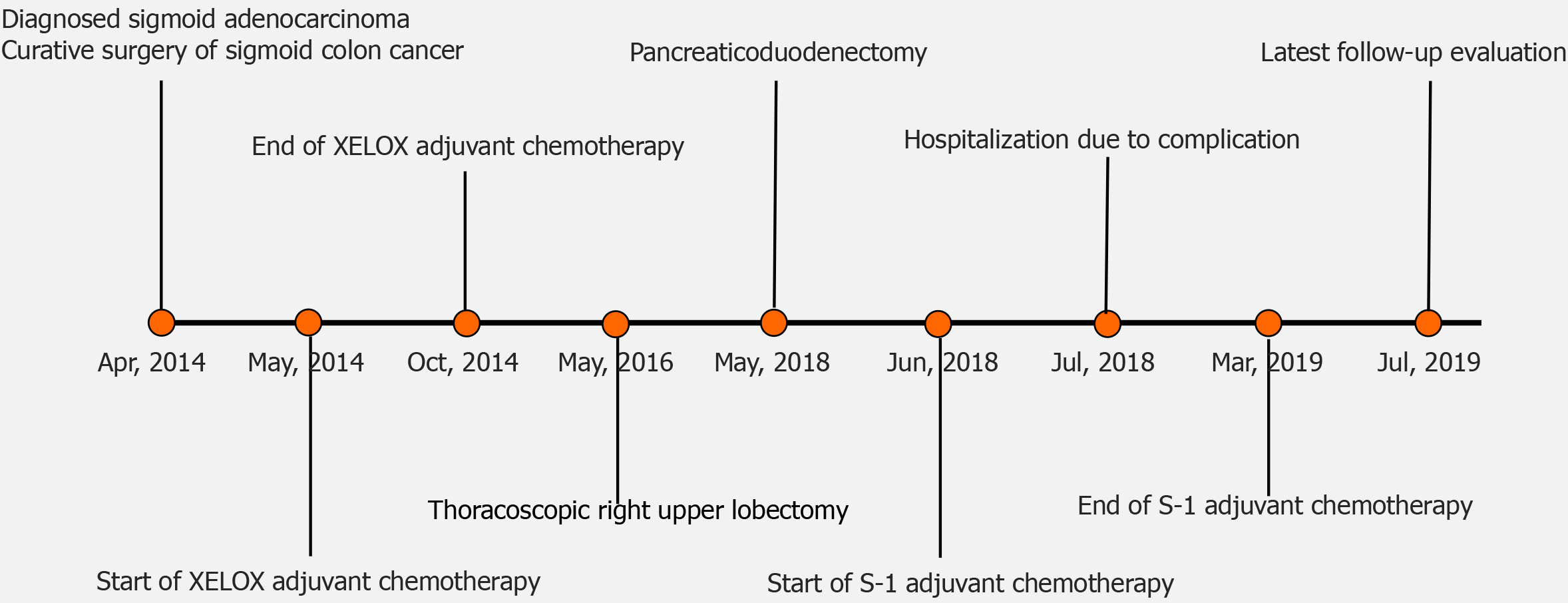
A 59-year-old man was diagnosed with sigmoid adenocarcinoma stage IIA (T3N0M0) and underwent surgery in April 2014, followed by XELOX adjuvant chemotherapy. The patient developed pulmonary metastasis in the right upper lung and underwent surgery in May 2016 without further adjuvant chemotherapy. In May 2018, pancreatic metastasis was found and he underwent pancreaticoduodenectomy. After surgery, he was treated with adjuvant S-1 chemotherapy from June 2018 to March 2019. Histopathological review of the specimens from all three lesions indicated consistent patterns characteristic of colon cancer. Concordant gene mutation profiles were observed across the three lesions that included oncogenic driver mutations most frequently seen in colon cancer (e.g., APC, TP53, KRAS and FBXW7). Blood circulating tumor (ct)DNA before adjuvant chemotherapy was undetectable with NGS, suggesting a favorable response to chemotherapy. The patient was alive and well at the latest follow-up visit, achieving a disease-free survival of 17 mo.
The genetic profiles of primary tumor, metastases and ctDNA may have clinical value in auxiliary diagnosis, prognosis and therapeutic decision-making.
Core Tip: We describe a rare case of metachronous pulmonary and pancreatic metastases from colorectal cancer. Immunohistochemistry and next-generation sequencing (NGS) results suggested that pancreatic and pulmonary metastases both originated from the primary colon cancer. To our knowledge, this is the first case to utilize NGS to detect the origin of pancreatic metastasis from colon cancer. NGS also provided useful information for predicting clinical outcome and therapeutic decision-making.
- Citation: Yang J, Tang YC, Yin N, Liu W, Cao ZF, Li X, Zou X, Zhang ZX, Zhou J. Metachronous pulmonary and pancreatic metastases arising from sigmoid colon cancer: A case report. World J Clin Cases 2021; 9(15): 3668-3674
- URL: https://www.wjgnet.com/2307-8960/full/v9/i15/3668.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i15.3668
Pancreatic metastases are rare tumor entities that account for 2%-4% of all pancreatic malignancies[1]. More often, metastatic lesions in the pancreas occur as part of a systemic spread, as noted in 6%-10% of cases at autopsy[2]. Less common are isolated metastases amenable to resection. The handful of cohort analyses of pancreatic metastasis reported a range of 18 cases over a span of 18 years[3] to 29 cases over 12 years[4]. Kidney (approximately 80% of cases) is by far the most common primary site, followed by colon (8%), melanoma (5%), and others[1]. As a result, there are even fewer data on pancreatic metastasis arising from colon cancer. This was illustrated by a comprehensive review by Sperti et al[2] who found 24 cases among published works indexed in PubMed and other search engines up to 2014. The scant data make it unlikely that prospective or case-controlled studies have been conducted to address key clinical issues such as prognostic factors and optimal treatment regimens. Instead, retrospective studies and cases have become the major source of reference for clinical decision-making. In this article, we add another case to this small reservoir of evidence. Our patient developed serial pulmonary and pancreatic metastases several years after removal of primary sigmoid colon adenocarcinoma. He was treated with pancreaticoduodenectomy and adjuvant S-1 chemotherapy, and was alive and well at the latest follow-up 17 mo after surgery despite initial postoperative complications.
A 59-year-old old man was referred to our hospital in May 2018 due to detection of a space-occupying mass in the head of the pancreas at another institution.
He was diagnosed with sigmoid adenocarcinoma stage IIA (T3N0M0) and underwent curative surgery in April 2014. After surgery, the patient was treated with XELOX adjuvant chemotherapy from May to October 2014. The patient developed pulmonary metastasis in the right upper lung, with lesions of 2.5 cm × 2.5 cm, and underwent thoracoscopic right upper lobectomy in May 2016. After surgery, he was not treated with further adjuvant chemotherapy (Figure 1).
The patient had no previous medical history.
The patient did not have relevant personal or family history.
The patient’s temperature was 36.9°C, heart rate 86 bpm, blood pressure 133/84 mmHg, and neurological examination suggested no abnormalities. An abdominal examination showed no palpable mass or tenderness.
Blood carcinoembryonic antigen (CEA) level was elevated to 23.17 ng/mL (normal range 0-5 ng/mL), and carbohydrate antigen 19-9 was elevated to 52.71 U/mL (normal range 0-40 U/mL).
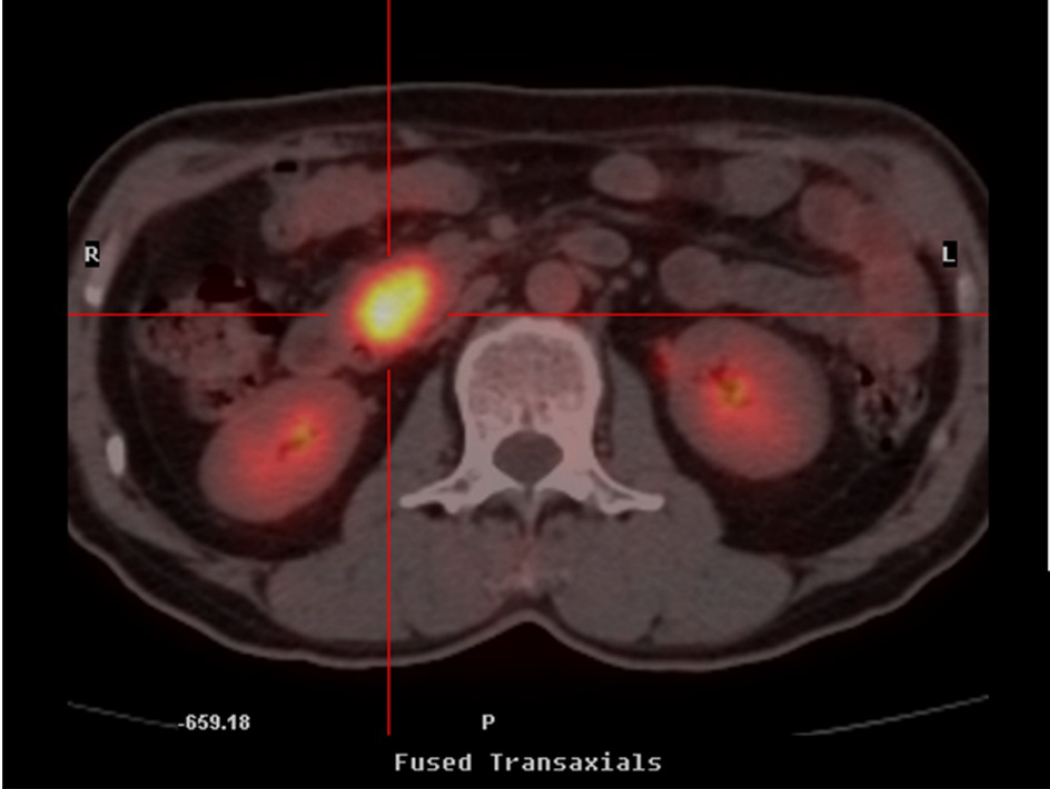
Positron emission tomography/computed tomography (CT) and ultrasound scans confirmed the presence of a space-occupying lesion with glucose hypermetabolism (2.3 cm in greatest dimension) in the head of the pancreas (Figure 2). Except for gallstones, no abnormalities were noted in the stomach, duodenum, common bile duct, or main pancreatic duct.
The final diagnosis of the presented case was pancreatic recurrence of colon cancer.
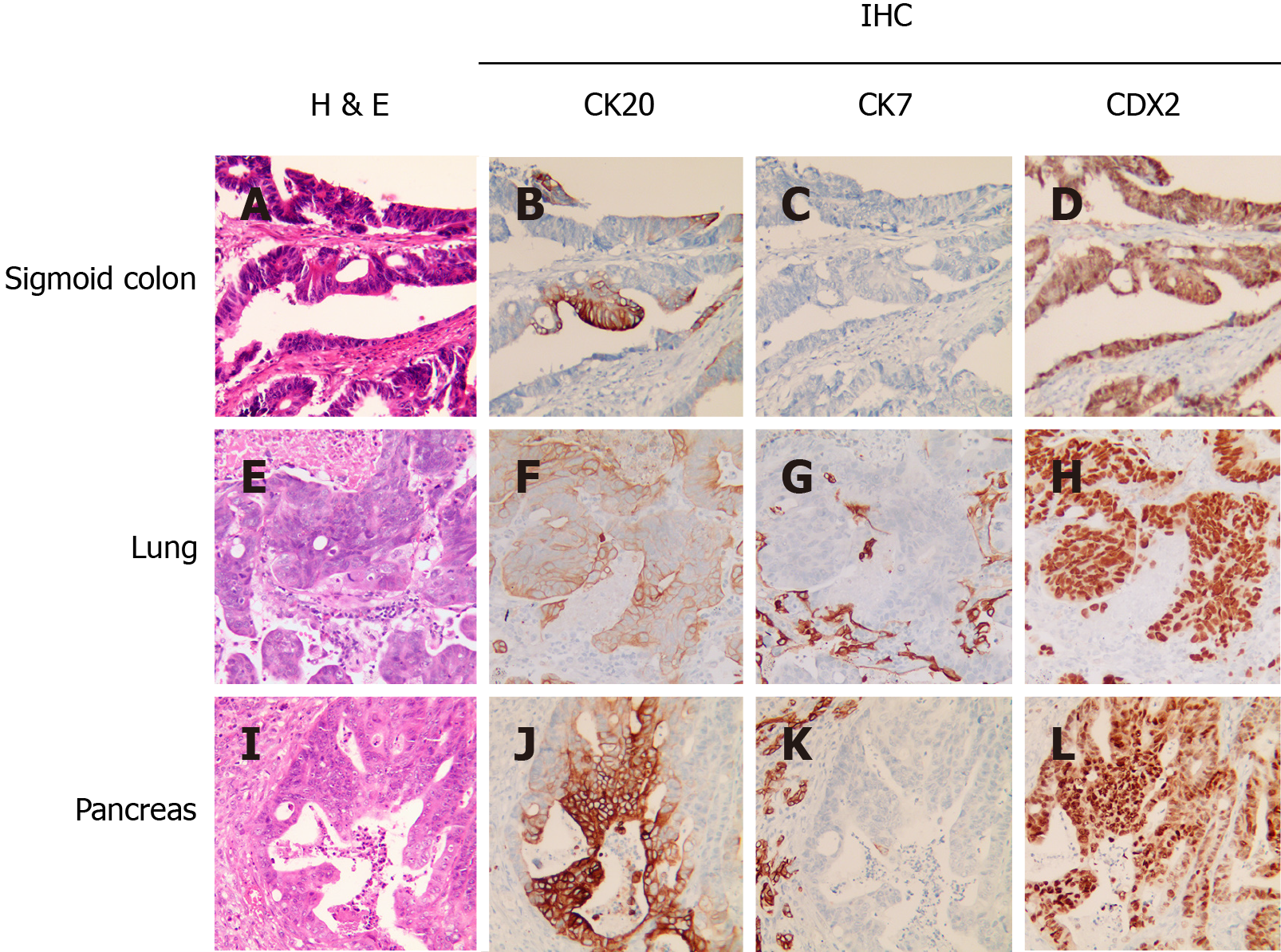
The patient was assessed as fit for surgery and received pancreaticoduodenectomy. A review of pathological specimens indicated moderately to poorly differentiated adenocarcinoma invading the duodenal submucosa, muscular layer, and serosa. All six resected lymph nodes were negative. There was no tumor involvement of the stomach, gallbladder or the surgical incisional edges of the duodenum and pancreas. The pancreatic specimen showed histopathological features consistent with those of the sigmoid and lung lesions, suggesting that the pancreatic lesion arose from primary colon cancer (Figure 3). Specimens from all three sites were also subjected to next-generation sequencing (NGS) targeting 520 cancer-related genes (OncoScreen Plus, Burning Rock, China) for mutational profiling. As shown in Table 1, of the 23 detected genomic alterations, 10 (43.5%) were present in all three specimens, including three double mutations in TP53, APC and FBXW7. Circulating tumor DNA (ctDNA) from blood collected before adjuvant chemotherapy tested negative for mutations in the same panel of 520 genes.
| Mutated gene and impact on protein sequence | Primary sigmoid cancer | Lung metastasis | Pancreatic resection |
| KRAS p.G12A | + | + | + |
| TP53 p.R248W | + | + | + |
| TP53 p.C275Y | + | + | + |
| APC p.E893* | + | + | + |
| APC p.L1488fs | + | + | + |
| PTEN p.T319fs | + | + | + |
| FBXW7 p.E664* | + | + | + |
| FBXW7 p.G597R | + | + | + |
| PIK3C2G p.V508A | + | + | + |
| LRP1B p.A3643D | + | + | + |
| PIK3CA p.G118D | + | ||
| ERCC5 p.T105M | + | ||
| FAT3 p.S1086G | + | + | |
| TSHR p.D487N | + | + | |
| ASXL1 p.G668S | + | ||
| INHBA p.K198N | + | ||
| FGF3 p.A87S | + | ||
| INPP4B p.K891M | + | ||
| MITF p.I220L | + | ||
| EZH2 p.D730fs | + | ||
| LRP1B p.P1191T | + | ||
| GNAS amplification | + | ||
| DAXX amplification | + |
The patient received adjuvant S-1 chemotherapy from June 2018 to March 2019. He experienced two rounds of complications over the following months due to abdominal pain and fever. CT scans showed bloody fluid oozing from the drain entrance and wounds mid-healing in the right abdomen. Supportive care was given to alleviate symptoms with sulperazon and omeprazole sodium, and both times the patient was discharged from hospital within 3 d upon recovery. There was no sign of new lesions upon the latest follow-up visit in July 2019, thereby achieving a disease-free interval of 17 mo after pancreatic metastasectomy (Figure 1).
Approximately 30% of colon cancer patients develop metastasis, but the pancreas appears to be an unusual site[5]. To date, there are only 17 case reports of primary colon cancer with metachronous metastases to the pancreas indexed in PubMed[1]. Few data have left many critical questions regarding disease management unanswered, including benefit and risk assessment of surgical resection, efficacy of surgery compared with chemotherapy, and prognostic factors. Evidence provided in reports of sporadic cases is therefore all the more relevant in this uncommon scenario.
The value of surgery in managing pancreatic metastasis has been evaluated in several recent studies[2,6,7]. Morbidity rates reportedly range from 30% to 48%, while mortality rates were cited at 0%-1.4%[6,8,9]. Considering also the well-documented benefit from resection of liver metastases of colorectal cancer[10], there is a rationale for surgery as a treatment option. However, caution is warranted as to whether surgery is superior to other treatment modalities. Therefore, more evidence is clearly needed to establish the optimal treatment for pancreatic metastasis.
Additionally, regular long-term follow-up appears necessary for metastatic colon cancer patients, as survival rate after pancreatic metastasis reportedly dropped from 88.2% at 1 year after surgery to 24.8% at 5 years[1]. It is unclear whether clinical outcome is associated with specific factors. A study of pancreatic metastases arising from renal cell carcinoma suggested that timing of metastasis, primary tumor lymph node status, and size and number of metastases are not associated with survival outcome[1]. Abnormally high postoperative CEA level is indicative of residual or recurrent disease from primary colon cancer, suggesting CEA levels as a potential prognostic factor[11].
Liquid biopsy is a minimally invasive and reliable method utilizing body fluids of cancer patients to provide important genetic information for complementary cancer diagnosis[12,13]. ctDNA in peripheral blood has been suggested as a surrogate marker for prognosis and treatment response in several cancer types[14,15]. An earlier study showed an inverse correlation between baseline blood ctDNA level and progression-free survival in response to second-line regorafenib chemotherapy in metastatic colorectal cancer[14]. In our case, ctDNA from blood specimens collected before S-1 chemotherapy tested negative for mutations in 520 cancer-related genes, which appears to be consistent with the favorable clinical outcome observed so far. Large-scale trials are ongoing to evaluate the utility of ctDNA status determined by comprehensive NGS panels in predicting the efficacy of surgery and/or chemotherapy in metastatic colon cancer, which will shed light on integrating ctDNA-based tumor monitoring into a more personalized version of the treatment framework[16].
We describe a rare case of pancreatic metastasis arising from colon cancer after resection of previous pulmonary metastasis. Histopathological features of the specimens from the primary and two metastatic lesions indicated a characteristic colorectal cancer pattern. NGS revealed concordant mutation profiles of the three lesions, highlighting its role in determining the tissue of origin for metastases. Our patient was treated with pancreatic metastasectomy and adjuvant S-1 chemotherapy and is alive 17 mo after surgery. An undetectable level of blood ctDNA was observed before the start of adjuvant chemotherapy, which could harbinger long-term survival. This case indicates that NGS could provide auxiliary diagnostic information for identifying metastatic tumors, and ctDNA detection in peripheral blood could be helpful in predicting clinical outcome. Solutions to significant questions, such as the superiority of surgery over other treatment options and the utility of ctDNA detection and predicting clinical outcome, will benefit from continued accumulation of evidence of this rare disease and insight from studies of similar medical conditions.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chakrabarti S S-Editor: Gao CC L-Editor: Webster JR P-Editor: Wang LL
| 1. | Hung JH, Wang SE, Shyr YM, Su CH, Chen TH, Wu CW. Resection for secondary malignancy of the pancreas. Pancreas. 2012;41:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Sperti C, Moletta L, Patanè G. Metastatic tumors to the pancreas: The role of surgery. World J Gastrointest Oncol. 2014;6:381-392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 100] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 3. | Wiltberger G, Bucher JN, Krenzien F, Benzing C, Atanasov G, Schmelzle M, Hau HM, Bartels M. Extended resection in pancreatic metastases: feasibility, frequency, and long-term outcome: a retrospective analysis. BMC Surg. 2015;15:126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Chikhladze S, Lederer AK, Kühlbrey CM, Hipp J, Sick O, Fichtner-Feigl S, Wittel UA. Curative-intent pancreas resection for pancreatic metastases: surgical and oncological results. Clin Exp Metastasis. 2020;37:313-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Riihimäki M, Hemminki A, Sundquist J, Hemminki K. Patterns of metastasis in colon and rectal cancer. Sci Rep. 2016;6:29765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 420] [Cited by in RCA: 680] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 6. | Reddy S, Wolfgang CL. The role of surgery in the management of isolated metastases to the pancreas. Lancet Oncol. 2009;10:287-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 167] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 7. | Adler H, Redmond CE, Heneghan HM, Swan N, Maguire D, Traynor O, Hoti E, Geoghegan JG, Conlon KC. Pancreatectomy for metastatic disease: a systematic review. Eur J Surg Oncol. 2014;40:379-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Sperti C, Pasquali C, Berselli M, Frison L, Vicario G, Pedrazzoli S. Metastasis to the pancreas from colorectal cancer: is there a place for pancreatic resection? Dis Colon Rectum. 2009;52:1154-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Huang Q, Zhou H, Liu C, Jin K, Fan K, Cheng H, Fan Z, Yang C, Liu L, Long J, Xu J, Ni Q, Hu Z, Yu X. Surgical Resection for Metastatic Tumors in the Pancreas: A Single-Center Experience and Systematic Review. Ann Surg Oncol. 2019;26:1649-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Akgül Ö, Çetinkaya E, Ersöz Ş, Tez M. Role of surgery in colorectal cancer liver metastases. World J Gastroenterol. 2014;20:6113-6122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 193] [Cited by in RCA: 190] [Article Influence: 17.3] [Reference Citation Analysis (1)] |
| 11. | Hall C, Clarke L, Pal A, Buchwald P, Eglinton T, Wakeman C, Frizelle F. A Review of the Role of Carcinoembryonic Antigen in Clinical Practice. Ann Coloproctol. 2019;35:294-305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 136] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 12. | Vacante M, Ciuni R, Basile F, Biondi A. The Liquid Biopsy in the Management of Colorectal Cancer: An Overview. Biomedicines. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 13. | Pantel K, Alix-Panabières C. Liquid biopsy and minimal residual disease - latest advances and implications for cure. Nat Rev Clin Oncol. 2019;16:409-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 720] [Article Influence: 120.0] [Reference Citation Analysis (0)] |
| 14. | Wong AL, Lim JS, Sinha A, Gopinathan A, Lim R, Tan CS, Soh T, Venkatesh S, Titin C, Sapari NS, Lee SC, Yong WP, Tan DS, Pang B, Wang TT, Zee YK, Soong R, Trnkova Z, Lathia C, Thiery JP, Wilhelm S, Jeffers M, Goh BC. Tumour pharmacodynamics and circulating cell free DNA in patients with refractory colorectal carcinoma treated with regorafenib. J Transl Med. 2015;13:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Song Y, Hu C, Xie Z, Wu L, Zhu Z, Rao C, Liu L, Chen Y, Liang N, Chen J, Yang N, Hu J, Zhao W, Tong G, Dong X, Zheng D, Jin M, Huang M, He Y, Rosell R, Lippi G, Mino-Kenudson M, Han-Zhang H, Mao X, Zhang L, Liu H, Field JK, Chuai S, Ye J, Han Y, Lu S; Written on behalf of AME Lung Cancer Collaborative Group. Circulating tumor DNA clearance predicts prognosis across treatment regimen in a large real-world longitudinally monitored advanced non-small cell lung cancer cohort. Transl Lung Cancer Res. 2020;9:269-279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 16. | Dasari A, Morris VK, Allegra CJ, Atreya C, Benson AB 3rd, Boland P, Chung K, Copur MS, Corcoran RB, Deming DA, Dwyer A, Diehn M, Eng C, George TJ, Gollub MJ, Goodwin RA, Hamilton SR, Hechtman JF, Hochster H, Hong TS, Innocenti F, Iqbal A, Jacobs SA, Kennecke HF, Lee JJ, Lieu CH, Lenz HJ, Lindwasser OW, Montagut C, Odisio B, Ou FS, Porter L, Raghav K, Schrag D, Scott AJ, Shi Q, Strickler JH, Venook A, Yaeger R, Yothers G, You YN, Zell JA, Kopetz S. ctDNA applications and integration in colorectal cancer: an NCI Colon and Rectal-Anal Task Forces whitepaper. Nat Rev Clin Oncol. 2020;17:757-770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 251] [Article Influence: 50.2] [Reference Citation Analysis (0)] |