Published online May 26, 2021. doi: 10.12998/wjcc.v9.i15.3662
Peer-review started: November 11, 2020
First decision: January 24, 2021
Revised: February 6, 2021
Accepted: March 9, 2021
Article in press: March 9, 2021
Published online: May 26, 2021
Processing time: 173 Days and 23.4 Hours
Fine-needle biopsy is an accurate and cost-efficient tool for the assessment of thyroid nodules. It includes two primary methods: Fine-needle capillary biopsy (FNCB) and fine-needle aspiration biopsy. Needle tract seeding (NTS) is a rare complication of thyroid fine-needle biopsy mainly caused by fine-needle aspiration biopsy rather than FNCB. Here, we present an extremely rare case of a papillary thyroid carcinoma (PTC) patient with FNCB-derived NTS.
We report a 32-year-old woman with PTC who showed subcutaneous NTS 1 year after FNCB and thyroidectomy. NTS was diagnosed based on clinical manifestations, biochemistry indices, and imaging (computed tomography and ultrasound). Pathological identification of PTC metastases consistent with the puncture path is the gold standard for diagnosis. Surgical resection was the main method used to treat the disease. After surgery, thyroid function tests and ultrasound scans were performed every 3-6 mo. To date, no evidence of tumor recurrence has been observed.
FNCB is a safe procedure as NTS is rare, and can be easily removed surgically with no recurrence. Accordingly, NTS should not limit the usefulness of FNCB.
Core Tip: Needle tract seeding (NTS) of tumor cells is rare. Given that NTS is mainly caused by fine-needle aspiration biopsy rather than fine-needle capillary biopsy (FNCB), the FNCB-derived deposit of tumor cells has never been reported in papillary thyroid carcinoma. Here, we present a case of NTS caused by FNCB in a papillary thyroid carcinoma patient and performed whole-genome sequencing of the NTS-derived lesion. In this case, subtle changes in the patient’s thyroglobulin and thyroglobulin antibodies were observed. Importantly, the information presented here will help improve the understanding of this disease.
- Citation: Shi LH, Zhou L, Lei YJ, Xia L, Xie L. Needle tract seeding of papillary thyroid carcinoma after fine-needle capillary biopsy: A case report. World J Clin Cases 2021; 9(15): 3662-3667
- URL: https://www.wjgnet.com/2307-8960/full/v9/i15/3662.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i15.3662
Fine-needle biopsy (FNB) is a reliable, accurate, and cost-effective technique to diagnose thyroid nodules, but it can lead to potential complications[1]. Of note, needle tract seeding (NTS) of tumor cells is a rare consequence[2,3]. Given that NTS is primarily caused by fine-needle aspiration biopsy rather than fine-needle capillary biopsy (FNCB), the FNCB-derived deposit of tumor cells has never been reported in papillary thyroid carcinoma (PTC)[4]. Here, we present a case of NTS caused by FNCB in a PTC patient and the follow-up of clinical characteristics after the removal of seeding lesions. A genetic investigation was performed by whole-genome sequencing (WGS) of the NTS-derived lesion. In this study, we determined that FNCB should be carefully performed to minimize the potential of NTS in the clinical setting[2].
A 32-year-old woman was admitted to our hospital due to the presence of anterior neck nodules for 3 years.
The patient presented with a 3-year history of a 3-mm subcutaneous nodule in the right side of her neck that was initially diagnosed as a sebaceous cyst. As the nodule was stable in terms of size, morphology, and color, the patient was followed-up without treatment. As the patient was willing to have the nodule removed, she was admitted to our hospital.
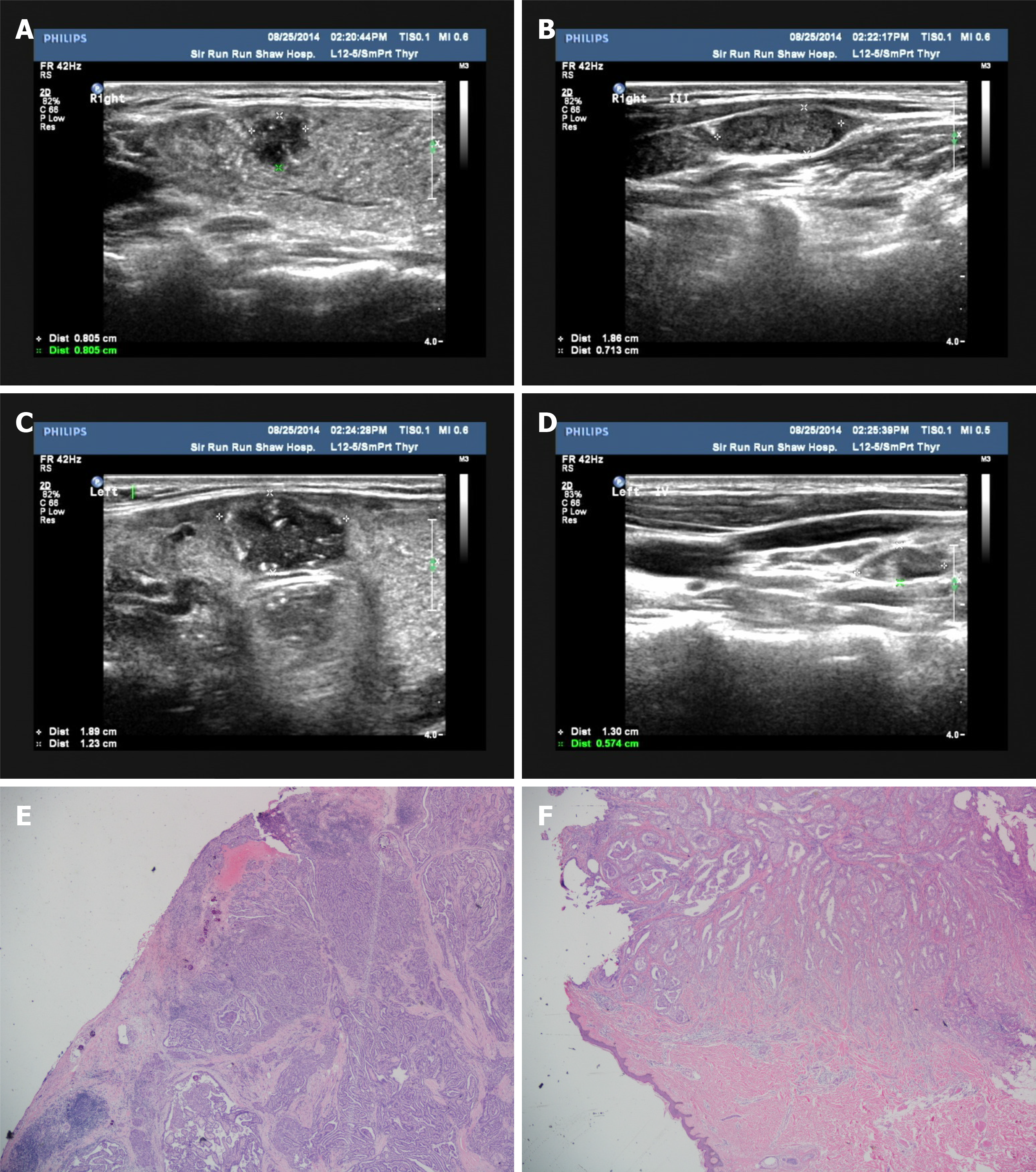
The patient underwent ultrasound-guided FNCB (22-gauge needles) of bilateral thyroid nodules and lateral cervical lymph nodes performed by Zhou L in 2014 (ultrasound performance is shown in Figure 1A-D). The pathologist reported that the nodules and lymph nodes were tumorous. Subsequently, total thyroidectomy followed by bilateral modified neck dissection, including bilateral levels II, III, IV, and VI, were performed. Histologically, the largest of the three PTC nodules was 2 cm × 1.6 cm and showed extra-thyroidal extension (Figure 1E), and 29 of 134 lymph nodes were malignant. Subsequently, 100-mCi radioactive iodine therapy was administered. Imaging examinations, including ultrasound and functional scans, showed no sign of a tumor.
No specific personal or family history of the disease was recorded.
An old, well-healed traverse surgical scar on the middle of the neck was observed. A hard 3-mm subcutaneous mass with poor mobility was palpable on the right side of the neck, and there were no other positive signs on physical examination.
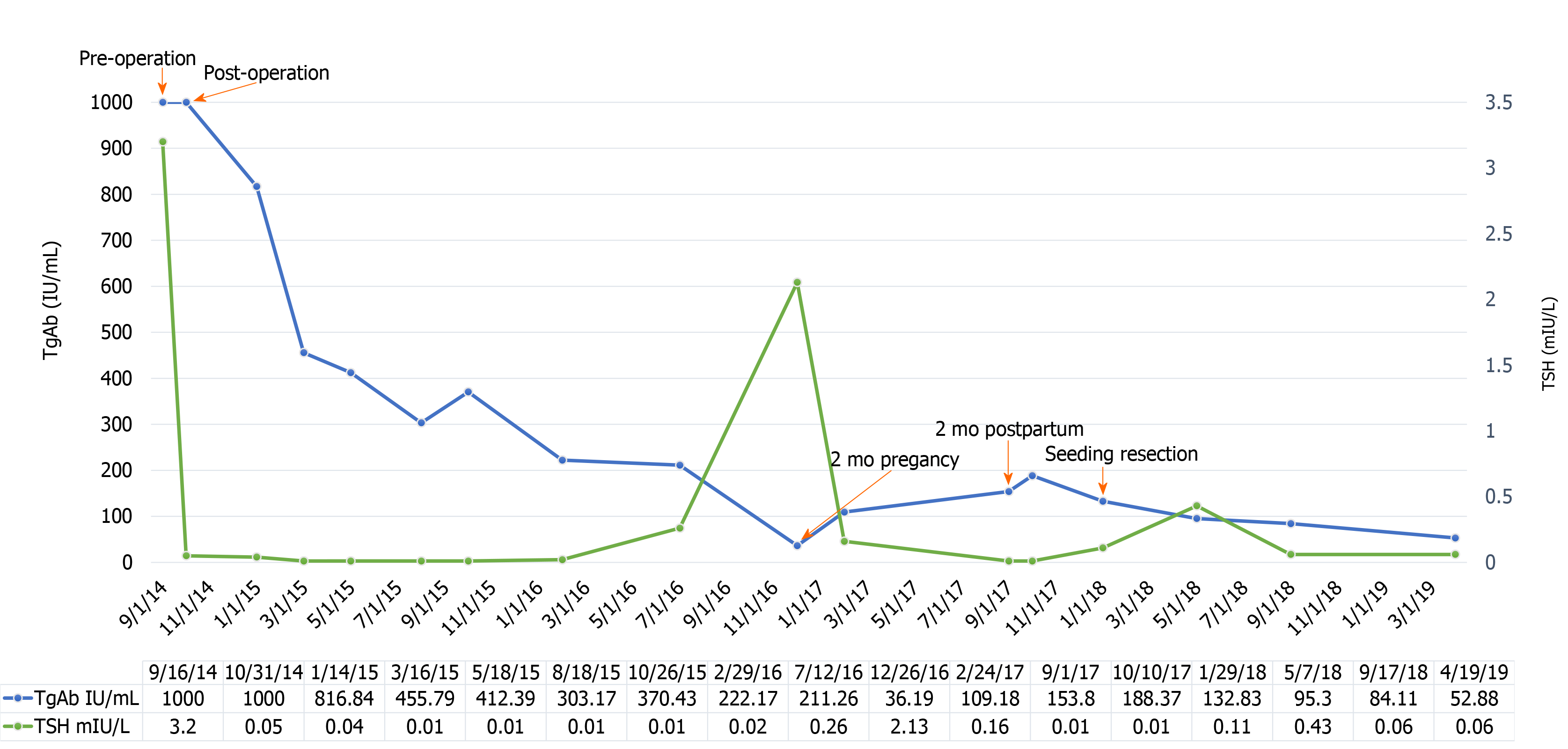
After thyroid surgery, thyroid-stimulating hormone (TSH) levels were maintained between 0.01 mIU/L and 0.04 mIU/L with TSH suppressive therapy, and thyroglobulin (Tg) was stable below 0.03 ng/mL. However, the Tg antibody (Tg-Ab) level tended to fluctuate during various stages of the disease. Initially, Tg-Ab decreased persistently but then began to increase when the patient was pregnant for 2 mo.
All routine imaging examinations were unremarkable.
The diagnosis before surgical resection was a sebaceous cyst.
The subcutaneous nodule was removed. During surgery, a deposit was observed in the skin and sternocleidomastoid muscle, consistent with the location of the puncture needle path of the right lateral cervical lymph nodes. The linear array and site of the nodule indicated that seeding most likely resulted from the needle biopsy.
Microscopic examination revealed a non-enveloped tumor with a large number of luminal structures in the dermis. The walls of the tubes were composed of a single layer of columnar cells with lightly stained nuclei and eosinophilic lumen, and infiltration of cells with strong nuclear staining was detected (Figure 1F). Based on the immunohistochemical results showing positive staining for thyroid transcription factor and Tg, the lesions were pathologically diagnosed as PTC metastases. In addition, WGS was performed to analyze the NTS-derived lesion, and coiled-coil domain containing 6 (CCDC6)-rearranged during transfection (RET) fusion was detected. When the subcutaneous nodule was removed, the Tg-Ab level declined rapidly and showed a downward trend in the following days (Figure 2). To date, no evidence of tumor recurrence has been observed.
FNB has been used to evaluate thyroid nodules for over 50 years[5]. Worldwide, thousands of FNBs for thyroid nodules are performed annually, with few reported cases of NTS[2,6] (approximately 20 cases)[7-12]. Previously, Ito et al[9] uncovered a higher incidence of NTS in PTC patients (0.14%) than expected, as several NTS cases were asymptomatic[12]. The most typical clinical sign of NTS was the presence of linearly arranged masses between the penetrated point and primary tumor[8,10]. However, some cases only exhibited small subcutaneous nodules at the penetrated point[7,12]. In the present case, the surgeon did not separate the skin and subcutaneous tissue layer during surgery, and most of the implants were located in the subcutaneous tissue layer. Therefore, we believe that the subcutaneous nodule caused by FNCB was possibly greater than that in the initial surgery. Generally, the clinical features (e.g., Tg, Tg-Ab, and others) of NTS in PTC patients are not fully understood. In this case, the Tg-Ab level increased with the development of the subcutaneous lesion but decreased immediately after surgical resection. This suggests that the Tg-Ab level may be an earlier biomarker in some NTS patients, but further investigations are warranted.
Several needle-associated risks of NTS, such as damage caused by the needle size, number of passes, withdrawing the needle without releasing suction, and injecting the tumor at the time of biopsy, have been well-documented[2,4,9]. Technically, FNB can be performed with aspiration using a syringe (fine-needle aspiration biopsy) or without aspiration (FNCB). Theoretically, NTS is rarely caused by FNCB as FNCB minimizes tissue and cell trauma[13]. However, our case indicates that FNCB is not as safe as we expected. Given that tumor cells are easily spread by aspiration, especially using a needle with a large diameter, non-aspiration techniques (i.e., FNCB) with a 23-G or a smaller fine needle are recommended[14,15]. In any case, an excessive number of passes should be avoided[16].
Mechanistic investigation of thyroid cancer cell implantation and growth provides a better understanding of the pathogenesis underlying NTS. Several genetic mutations have been identified in thyroid cancers, such as in RAS, BRAF, P53, and TSHR genes[17,18]. Khan et al[18] reported that RET/PTC3 rearrangements were significantly associated with gender, lymph node metastasis, and elevated TSH levels. Similarly, our female patient presented with Hashimoto thyroiditis, an elevated TSH level, and lymph node metastasis. In addition, RET mutations and rearrangements closely associated with tumor proliferation, invasion, and migration may be risk factors for NTS. RET fusions or rearrangements are somatic juxtapositions of 5' sequences from other genes with 3' RET sequences encoding a tyrosine kinase[19]. RET rearrangements (at least 13 different RET fusions) occur in approximately 2.5%-73.0% of sporadic PTC patients[20,21]. The most prevalent RET fusions are CCDC6-RET (also known as RET/PTC1) and nuclear receptor co-activator 4 (NCOA4)-RET (also known as RET/PTC3)[22-25]. In our case, the fusion type was ERC1-RET, which has not been previously reported.
For medical exploration and rigorous evaluation, WGS was performed to analyze the NTS-derived lesion in the patient free of charge. After the NTS-derived lesion was removed, no evidence of tumor recurrence was observed. Therefore, the patient is satisfied with the treatment and results and is currently being followed-up in our hospital.
FNB is a sensitive and specific technique for diagnosing thyroid nodules preoperatively, thereby facilitating the determination of optimal treatment plans for PTC[26,27]. Although NTS is a potential complication of FNB, surgical resection of NTS-derived lesions offers a favorable prognosis. Serum biomarkers and genetic characteristics could help in the treatment and follow-up of PTC patients with NTS.
We thank the patient for providing written informed consent for publication of any associated data on this case.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Kao NH, Kim DI, Mazeh H S-Editor: Gao CC L-Editor: Webster JR P-Editor: Li JH
| 1. | Polyzos SA, Anastasilakis AD. Rare potential complications of thyroid fine needle biopsy. Hippokratia. 2011;15:116-119. [PubMed] |
| 2. | Hales MS, Hsu FS. Needle tract implantation of papillary carcinoma of the thyroid following aspiration biopsy. Acta Cytol. 1990;34:801-804. [PubMed] |
| 3. | Ito Y, Asahi S, Matsuzuka F, Nakamura Y, Amino N, Miyauchi A. Needle tract implantation of follicular neoplasm after fine-needle aspiration biopsy: report of a case. Thyroid. 2006;16:1059-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Polyzos SA, Anastasilakis AD. A systematic review of cases reporting needle tract seeding following thyroid fine needle biopsy. World J Surg. 2010;34:844-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Soderstrom N. Puncture of goiters for aspiration biopsy. Acta Med Scand. 1952;144:237-244. [PubMed] |
| 6. | Block MA, Miller JM, Kini SR. The potential impact of needle biopsy on surgery for thyroid nodules. World J Surg. 1980;4:737-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Shinohara S, Yamamoto E, Tanabe M, Maetani T, Kim T. Implantation metastasis of head and neck cancer after fine needle aspiration biopsy. Auris Nasus Larynx. 2001;28:377-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Karwowski JK, Nowels KW, McDougall IR, Weigel RJ. Needle track seeding of papillary thyroid carcinoma from fine needle aspiration biopsy. A case report. Acta Cytol. 2002;46:591-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Ito Y, Tomoda C, Uruno T, Takamura Y, Miya A, Kobayashi K, Matsuzuka F, Kuma K, Miyauchi A. Needle tract implantation of papillary thyroid carcinoma after fine-needle aspiration biopsy. World J Surg. 2005;29:1544-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Tamiolakis D, Antoniou C, Venizelos J, Lambropoulou M, Alexiadis G, Ekonomou C, Tsiminikakis N, Alifieris E, Papadopoulos N, Konstandinidis T, Kouskoukis C. Papillary thyroid carcinoma metastasis most probably due to fine needle aspiration biopsy. A case report. Acta Dermatovenerol Alp Pannonica Adriat. 2006;15:169-172. [PubMed] |
| 11. | Basu A, Sistla SC, Siddaraju N, Verma SK, Iyengar KR, Jagdish S. Needle tract sinus following aspiration biopsy of papillary thyroid carcinoma: a case report. Acta Cytol. 2008;52:211-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Na CH, Kim DJ, Kim MS, Shin BS. Cutaneous Implantation Metastasis of Papillary Thyroid Carcinoma Following Fine Needle Aspiration Biopsy. Ann Dermatol. 2017;29:123-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Rizvi SA, Husain M, Khan S, Mohsin M. A comparative study of fine needle aspiration cytology versus non-aspiration technique in thyroid lesions. Surgeon. 2005;3:273-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Hayashi T, Hirokawa M, Higuchi M, Kudo T, Ito Y, Miyauchi A. Needle Tract Implantation Following Fine-Needle Aspiration of Thyroid Cancer. World J Surg. 2020;44:378-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Faquin WC, Bongiovanni M, Sadow PM. Update in thyroid fine needle aspiration. Endocr Pathol. 2011;22:178-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Kuzan TY, Canbey Goret C. Comparison of Number of Passes and Cytopathological Specimen Adequacy for Thyroid Fine-Needle Aspiration Biopsy in the Absence of an On-Site Pathologist. Eur Thyroid J. 2020;9:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013;13:184-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 852] [Cited by in RCA: 1052] [Article Influence: 87.7] [Reference Citation Analysis (0)] |
| 18. | Khan MS, Pandith AA, Ul Hussain M, Iqbal M, Khan NP, Wani KA, Masoodi SR, Mudassar S. Lack of mutational events of RAS genes in sporadic thyroid cancer but high risk associated with HRAS T81C single nucleotide polymorphism (case-control study). Tumour Biol. 2013;34:521-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Mulligan LM. RET revisited: expanding the oncogenic portfolio. Nat Rev Cancer. 2014;14:173-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 376] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 20. | Lu Z, Zhang Y, Feng D, Sheng J, Yang W, Liu B. Targeted next generation sequencing identifies somatic mutations and gene fusions in papillary thyroid carcinoma. Oncotarget. 2017;8:45784-45792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Cancer Genome Atlas Research Network. . Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159:676-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1650] [Cited by in RCA: 2187] [Article Influence: 218.7] [Reference Citation Analysis (0)] |
| 22. | Santoro M, Moccia M, Federico G, Carlomagno F. RET Gene Fusions in Malignancies of the Thyroid and Other Tissues. Genes (Basel). 2020;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 23. | Cheung CC, Carydis B, Ezzat S, Bedard YC, Asa SL. Analysis of ret/PTC gene rearrangements refines the fine needle aspiration diagnosis of thyroid cancer. J Clin Endocrinol Metab. 2001;86:2187-2190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 91] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Romei C, Ciampi R, Elisei R. A comprehensive overview of the role of the RET proto-oncogene in thyroid carcinoma. Nat Rev Endocrinol. 2016;12:192-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 256] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 25. | Abdullah MI, Junit SM, Ng KL, Jayapalan JJ, Karikalan B, Hashim OH. Papillary Thyroid Cancer: Genetic Alterations and Molecular Biomarker Investigations. Int J Med Sci. 2019;16:450-460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 218] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 26. | Le AR, Thompson GW, Hoyt BJ. Thyroid Fine-needle aspiration biopsy: an evaluation of its utility in a community setting. J Otolaryngol Head Neck Surg. 2015;44:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Guille JT, Opoku-Boateng A, Thibeault SL, Chen H. Evaluation and management of the pediatric thyroid nodule. Oncologist. 2015;20:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |