Published online May 26, 2021. doi: 10.12998/wjcc.v9.i15.3655
Peer-review started: November 4, 2020
First decision: February 12, 2021
Revised: February 25, 2021
Accepted: March 12, 2021
Article in press: March 12, 2021
Published online: May 26, 2021
Processing time: 188 Days and 9.1 Hours
Paradoxical psoriasis induced by tumor necrosis factor alpha antagonists is a rare side effect of those drugs and has similarities with and differences from classical psoriasis in clinical and pathological characteristics. Treating severe paradoxical psoriasis is challenging because the reported cases are rare, with treatment experience being only anecdotal.
We report 2 cases of paradoxical psoriasis caused by infliximab. Both cases manifested with a significant number of pustular lesions and had protracted and complicated clinical courses. In case 1, secukinumab alone could not control the eruptions, but colchicine supplementation markedly decreased disease activity. In case 2 miscellaneous medications were administered, including the systemic drug acitretin, the immunosuppressive drug cyclosporine, and the biologic agent ustekinumab. However, multiple applications of those medications failed to prevent new lesions from occurring. Both cases showed moderate-to-high anti-nuclear antibody titers.
Based on these cases, moderate-to-high anti-nuclear antibody titer seems to be a risk factor for paradoxical psoriasis. In addition, extensive pustular presentation may be a negative prognostic indicator and may portend a protracted clinical course refractory to therapy.
Core Tip: In this study, we report 2 cases of paradoxical psoriasis caused by infliximab. The data indicated that moderate-to-high anti-nuclear antibody titer was a risk factor for paradoxical psoriasis. In addition, extensive pustular presentation might be a negative prognostic indicator and portends a protracted clinical course refractory to therapy.
- Citation: Xia P, Li YH, Liu Z, Zhang X, Jiang Q, Zhou XY, Su W. Recalcitrant paradoxical pustular psoriasis induced by infliximab: Two case reports. World J Clin Cases 2021; 9(15): 3655-3661
- URL: https://www.wjgnet.com/2307-8960/full/v9/i15/3655.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i15.3655
Tumor necrosis factor alpha (TNF-α) is a cytokine produced by a variety of endothelial and immune cells. It plays an important role in the pathogenesis of various diseases. Since the 1990s, TNF-α antagonists have been used in the treatment of Crohn's disease, ulcerative colitis, rheumatoid arthritis, psoriasis, and other inflammatory diseases, achieving remarkable efficacy. However, the long-term safety of TNF-α antagonists remains uncertain. In 2004, researchers firstly reported a patient with Crohn's disease who developed psoriasis-like rashes during infliximab treatment[1]. As infliximab can also treat psoriasis, this phenomenon seemed paradoxical.
Statistics show that about 0.6%-5.3% of patients administered TNF-α antagonists develop paradoxical psoriasis, with infliximab being the most common cause[2]. Overall, 70% of paradoxical psoriasis cases occur among patients administered infliximab for rheumatoid arthritis or Crohn's disease[3,4]. It should be noted that when patients on infliximab for psoriasis develop rashes that differ morphologically from the original skin lesions, or if the skin lesions worsen with treatment, paradoxical psoriasis should be considered[5].
The clinical characteristics and pathology of paradoxical psoriasis seem to differ from those of classical psoriasis[2,4]. First, paradoxical psoriasis occurs most often on the palms, soles, and scalp, with a relatively short disease course. In addition, a significant number of skin lesions resemble early guttate psoriasis, with 40% of the lesions being pustular. Furthermore, although the pathology of paradoxical psoriasis resembles that of its classical counterpart, with psoriasiform hyperplasia and decreased thickness of the granular cell layer, it also demonstrates spongiosis and mild interface dermatitis in most cases.
The treatment and prognosis of paradoxical psoriasis vary depending on the size of the involved area and the type of lesions. As the reported cases are rare and treatment experience is only anecdotal, we summarized the information of 2 cases of paradoxical psoriasis caused by infliximab with protracted and complicated clinical courses.
Case 1: A 32-year-old unmarried, nulliparous female, presented with generalized pustular psoriasis of 2 wk duration.
Case 2: A 37-year-old male presented with generalized pustules and itchy erythema of more than 20 d duration.
Case 1: Prior to this pustular eruption, the patient had received infliximab injection treatment for 3 years because her psoriatic lesions gradually worsened and were accompanied by pain in both knees. At the beginning of this treatment, the rash and joint pain improved rapidly and significantly. At the eighth infliximab treatment session, the rash relapsed and 10-15 mg/wk methotrexate was supplemented, but the rash on the anterior part of the legs failed to respond. This time, after 18 sessions of infliximab treatment, the psoriatic lesions flared up significantly with generalized pustulosis and left knee joint pain.
Case 2: Twenty days prior to this presentation, the patient began to show corn-kernel size vesicles scattered across the chest, abdomen, bilateral forearms, and flexed sides of both thighs. He was diagnosed with varicella, and acyclovir was administered orally and topically without improvement. The lesions were surrounded by a red halo and studded with a tense vesicle that contained clear fluid and developed into a pustule. After the pustules dried up, the lesions became a scaly erythema that varied from the size of a grain of rice to the size of a nail. The rash gradually spread to the face, limbs, and the tips of the fingers and toes.
Case 1: The patient had psoriasis vulgaris for more than 10 years and received many treatments, including traditional herbal medicine (no details), methotrexate (unknown dosage), coal tar, glucocorticoid ointment, therapeutic baths, and ultraviolet phototherapy. The rash temporarily subsided after these treatments, but frequently recurred. The past medical history also included a 3-year course of iridocyclitis in which oral corticosteroids were administered, and gradually reduced to 5 mg/d at presentation.
Case 2: The past medical history included a 2-year course of Crohn's disease, for which 11 injections of infliximab were administered (5 mg/kg at baseline, 2, and 6 wk, and every 8 wk afterward).
Case 1: No positive family history was noted.
Case 2: The patient denied any past dermatologic history and a family history of psoriasis.
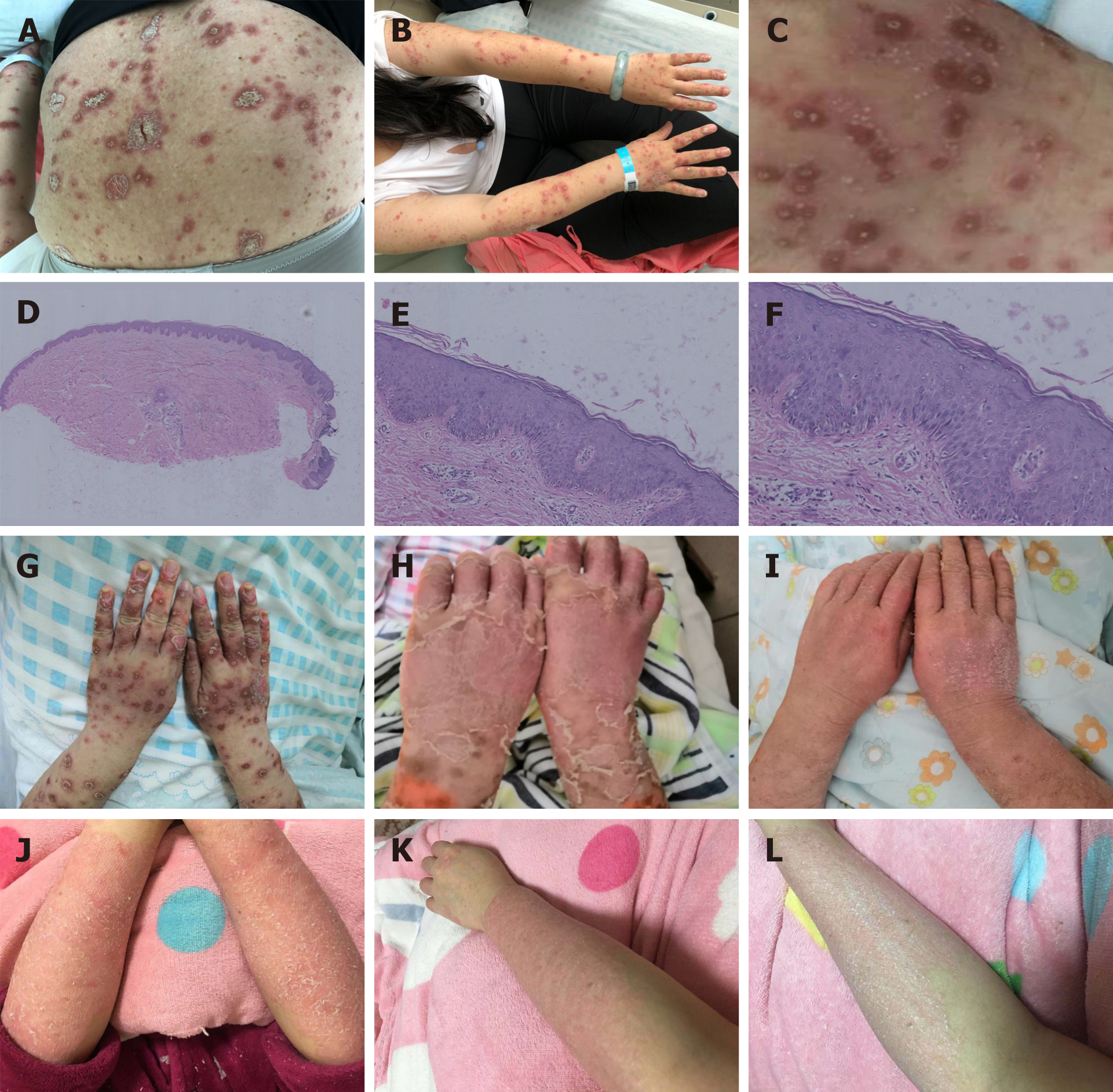
Case 1: The patient had no fever, the systemic examination was unremarkable. Dermatological examination showed erythema and plaques on the scalp hairline with abundant silvery white scales on the surface. Erythema, papules, and plaques on the trunk and limbs ranged from the size of a mung bean to the size of a nail, and were covered with dark yellow crusts and scales. Numerous pustules could be seen on the rashes on the dorsal aspect of the hands and forearms (Figure 1A-C).
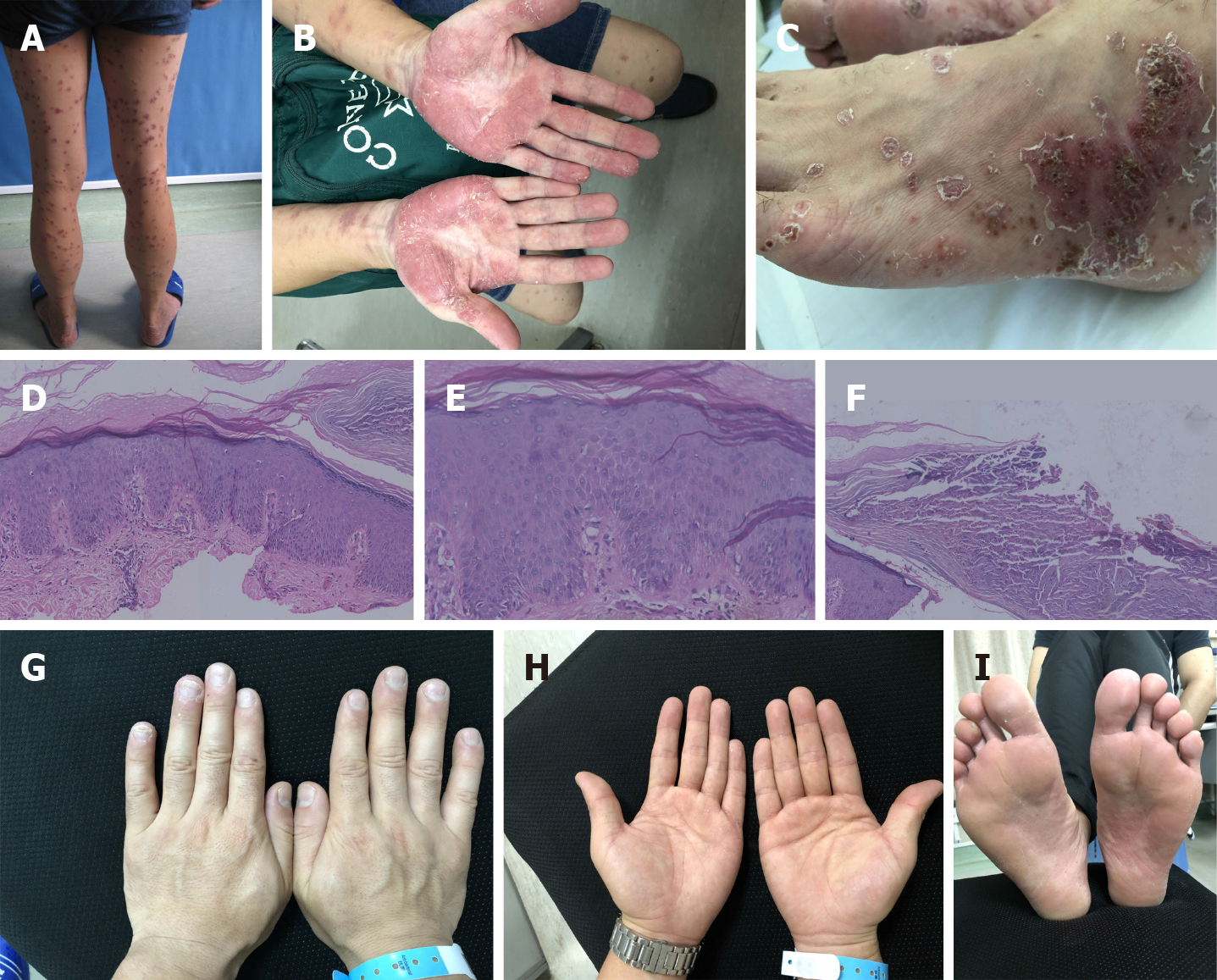
Case 2: Physical examination was normal. Dermatological examination showed pustular papules and plaques and confluent erythema involving the head, trunk, and limbs. Some of the lesions were fused and covered with collar-like white scales, while others were wet with exudation. Infiltrative erythema was distributed symmetrically on both the soles and palms, on which there were light yellow pustules and chaff-like scales. Purulent lakes were also noted as a result of fusion of the pustules. Dried-up pustules were present with brown yellow crusts on the surface. A large number of adherent light yellow scales were seen on the scalp (Figure 2A-C).
Case 1: The liver and kidney profiling with electrolytes were within the normal ranges, but the patient developed a moderately increased anti-nuclear antibody titer at the fifth treatment, and the titers increased gradually and persisted at 1:320 until the outbreak of pustulosis (Table 1). In addition, the neutrophil to lymphocyte (N/L) ratio, C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR) were increased after the pustulosis rash. Blood TNF-α and interleukin 8 (IL-8) levels were also elevated (Table 2). Viral and bacterial cultures of pustular lesions were negative.
| Time of infliximab treatment | Baseline | 8 wk | Outbreak of pustulosis | During 3 mo of follow-up |
| Case 1 | Negative | 1: 100 | 1: 320 | Negative |
| Case 2 | Negative | – | 1: 1000 | 1:320 |
| Rash | N/L | CRP, 0-5 mg/L | ESR, 20 mm/H | Cytokines | ||||
| Before | After | Before | After | Before | After | Before | After | |
| Case 1 | 1.20 | 4.28 | < 3.23 | 30.1 | 27 | 105 | TNF-α: 165.00 pg/mL, IL-8: normal | TNF-α: 202.00 pg/mL, IL-8: 81.80 pg/mL |
| Case 2 | 4.07 | 4.58 | < 3.23 | 10.4 | 12 | 23 | – | TNF-α: 84.80 pg/mL |
Case 2: The patient had normal liver and kidney profiles, but had a high anti-nuclear antibody titer that decreased gradually after stopping infliximab therapy (Table 1). The N/L ratio, CRP, ESR, and TNF-α were also increased after the pustulosis rash (Table 2).
Case 1: Skin biopsy of the non-pustule area of the left thigh showed psoriasiform epidermal hyperplasia and mild spongiosis with parakeratosis containing neutrophilic crusts (Figure 1D-F).
Case 2: Skin biopsy of the pustular area on the left dorsal foot revealed psoriasiform epidermal hyperplasia with neutrophil aggregation within the superficial epidermis (Figure 2D-F).
Case 1: The patient underwent infliximab treatment for almost 3 years and had a stable response to the drug. She gradually developed moderate anti-nuclear antibody titers. As she presented flared psoriatic lesions and generalized pustulosis after the 18th infliximab treatment without any evidence of infection, a diagnosis of paradoxical psoriasis induced by infliximab was made.
Case 2: The patient underwent 11 courses of infliximab treatment, and had a good response of intestinal symptoms. As he presented generalized pustulosis during the infliximab treatment, a diagnosis of paradoxical psoriasis induced by infliximab was made.
Case 1: The patient was treated with secukinumab (300 mg at baseline, 1, 2, 3, and 4 wk, and monthly afterward) in place of infliximab, as the latter yielded unsatisfactory results. Although pustule and joint symptoms improved significantly after the second injection, psoriatic erythema and scales relapsed after the sixth dose. The rash became hypertrophied and confluent (Figure 1G-I). Therefore, injection of secukinumab was increased to twice monthly, with 300 mg/injection. Three injections later, the rash did not subside as expected. Oral colchicine (0.5 mg, bid) was supplemented, and the color, thickness, and scales of the lesions improved (Figure 1J-L).
Case 2: Infliximab was terminated and 300 mg/d cyclosporine was given orally. The pustules were controlled during cyclosporine treatment. However, high blood pressure (150/100 mmHg) was found after 2 mo of treatment with cyclosporine, which was replaced by acitretin (20-40 mg/d). However, the pustules recurred. Subsequently, cyclosporine was applied again, but with less efficacy. In addition, intestinal symptoms relapsed. Colonoscopy showed new multiple ulcers in the colon, so ustekinumab (45 mg at baseline, 4 wk, and every 12 wk afterward) was given, and cyclosporine was tapered.
Case 1: At the last follow-up, the patient had some recalcitrant lesions on her legs and arms without pustulosis, and joint pain was relieved.
Case 2: During the last telephone follow-up, there were still occasional appearances of two-three pustules on the patient’s palms. The symptoms of Crohn's disease had disappeared (Figure 2G-I).
Diagnosis of paradoxical psoriasis is considered with the development or worsening of psoriasis during treatment with TNF antagonists. Among all types of paradoxical psoriasis, generalized pustular psoriasis is the most severe. Whenever paradoxical psoriasis is suspected, skin biopsy as well as viral and bacterial cultures should be carried out to exclude other skin diseases. Table 3 shows common differential characteristics of pustular psoriasis[6-8]. Lesions in paradoxical psoriasis are usually relieved after the termination of TNF-α antagonist treatment. In addition, common medications for psoriasis are also effective. If the patient does not respond well to those treatments, discontinuation of TNF-α antagonists and switching to other biological agents should be performed[9].
| Presentation | Histopathology | Etiology and pathoimmunology | |
| Acute generalized pustular psoriasis | Widespread formation of sterile pustules with erythema on the trunk and limbs. Pustules often expand into lakes of pus. Relapsed course | Overall epidermal architecture similar to plaque psoriasis. Formation of intra-epidermal neutrophilic abscesses, with marked dermal infiltrate composed of neutrophils, monocytes, and T-lymphocytes | Infection, stress, corticosteroid (treatment withdrawal). IL36RN mutation[6] |
| Palmoplantar pustulosis | Scattered clusters of pinhead-size sterile pustules on the palms and soles. Chronic course | As GPP | Genetic, roles of nicotine and contact allergens, certain medications and stress[7] |
| Acute exanthematous generalized pustular eruption | Polymorphous eruption more prominent than psoriasis, short duration, and no subsequent relapsing course | Necrotic keratinocytes and eosinophils are common | Drugs, notably anti-infectious chemotherapy, also non-steroidal anti-inflammatory drugs[8] |
Both patients in this study had extensive lesions and pustules, so we initially stopped infliximab treatment. Case 1 was switched to another biological agent, secukinumab, because it has a good treatment effect on both psoriasis vulgaris and pustular psoriasis. Unexpectedly, the rash was exacerbated after 8 wk of secukinumab treatment. Increasing the dose of secukinumab was not effective, so colchicine was supplemented. Addition of colchicine yielded a steady improvement of the rash. Case 2 had no previous skin diseases or a family history of psoriasis. After infliximab was stopped, cyclosporine and acitretin were applied successively, but the pustules persisted or worsened and Crohn's disease relapsed. After the patient was started on ustekinumab, intestinal symptoms and cutaneous pustules were both improved. At the 1.5-year follow-up visit, the patient reported persistent, rare occurrences of new pustules.
Although the prognosis of the majority of cases is good, 63.6% of patients with generalized pustules can improve but often fail to achieve complete resolution[5]. Both cases described here further support that pustular presentation in paradoxical psoriasis is an adverse prognostic factor: both patients presented with extensive pustules, which necessitated trying out different systemic agents, resulting in a longer disease course with persistent disease. In case 2, multiple trials of different systemic medications could not prevent new lesions from developing.
At present, the etiology of paradoxical psoriasis is unknown. Imbalance of the cytokines interferon (IFN)-α) and TNF-α may be involved. Compared with psoriasis vulgaris, increased proportion of CD123+ plasmacytoid dendritic cells (pDC) and IFN-α overexpression were found in paradoxical psoriasis[10]. Usually, when the skin suffers a mechanical injury, an infection, autoimmune reaction, or a tumor, pDCs migrate from the peripheral blood to the skin tissue and are stimulated to produce a large amount of IFN-α.
We noted that both cases described above developed moderate-to-high anti-nuclear antibody titers during infliximab treatment, and the amounts of the blood IL-8 and/or TNF-α were also elevated. We speculated that exposure to autoantigens induced the activation of pDC and production of cytokines such as IL-8, IFN-α and TNF-α that subsequently caused the occurrence of paradoxical pustular psoriasis. Some investigators have found that the positivity rates of anti-nuclear antibodies are higher in cases with paradoxical psoriasis than those without rash[11].
Based on these cases, treatment of severe paradoxical psoriasis is challenging. The development of anti-nuclear antibodies may be a risk factor for paradoxical psoriasis, with generalized pustulosis indicating poor prognosis.
Our 2 cases highlight the paradoxical psoriasis caused by infliximab, which is a very rare drug side effect of biologics. We found that anti-nuclear antibody titer seems to be a risk factor for paradoxical psoriasis. In addition, extensive pustular presentation might be a negative prognostic indicator and portend a protracted clinical course refractory to therapy.
We would like to express our gratitude to our patients for their support and information.
Manuscript source: Unsolicited manuscript
Specialty type: Dermatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gupta SK S-Editor: Zhang H L-Editor: Filipodia P-Editor: Zhang YL
| 1. | Verea MM, Del Pozo J, Yebra-Pimentel MT, Porta A, Fonseca E. Psoriasiform eruption induced by infliximab. Ann Pharmacother. 2004;38:54-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Ko JM, Gottlieb AB, Kerbleski JF. Induction and exacerbation of psoriasis with TNF-blockade therapy: a review and analysis of 127 cases. J Dermatolog Treat. 2009;20:100-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 215] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 3. | Collamer AN, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: clinical features and possible immunopathogenesis. Semin Arthritis Rheum. 2010;40:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 185] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 4. | Brown G, Wang E, Leon A, Huynh M, Wehner M, Matro R, Linos E, Liao W, Haemel A. Tumor necrosis factor-α inhibitor-induced psoriasis: Systematic review of clinical features, histopathological findings, and management experience. J Am Acad Dermatol. 2017;76:334-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 5. | Joyau C, Veyrac G, Dixneuf V, Jolliet P. Anti-tumour necrosis factor alpha therapy and increased risk of de novo psoriasis: is it really a paradoxical side effect? Clin Exp Rheumatol. 2012;30:700-706. [PubMed] |
| 6. | Gooderham MJ, Van Voorhees AS, Lebwohl MG. An update on generalized pustular psoriasis. Expert Rev Clin Immunol. 2019;15:907-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 144] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 7. | Putra-Szczepaniak M, Maj J, Jankowska-Konsur A, Czarnecka A, Hyncewicz-Gwóźdź A. Palmoplantar pustulosis: Factors causing and influencing the course of the disease. Adv Clin Exp Med. 2020;29:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Shalom G, Davidovici BB, Horev A, Halevy S. Acute generalized exanthematous pustulosis and psoriasis: what can be learned from comorbidities. G Ital Dermatol Venereol. 2019;154:413-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Collamer AN, Guerrero KT, Henning JS, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: a literature review and potential mechanisms of action. Arthritis Rheum. 2008;59:996-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 220] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 10. | Conrad C, Di Domizio J, Mylonas A, Belkhodja C, Demaria O, Navarini AA, Lapointe AK, French LE, Vernez M, Gilliet M. TNF blockade induces a dysregulated type I interferon response without autoimmunity in paradoxical psoriasis. Nat Commun. 2018;9:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 201] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 11. | Cleynen I, Vermeire S. Paradoxical inflammation induced by anti-TNF agents in patients with IBD. Nat Rev Gastroenterol Hepatol. 2012;9:496-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |