Published online May 26, 2021. doi: 10.12998/wjcc.v9.i15.3649
Peer-review started: October 25, 2020
First decision: January 17, 2021
Revised: January 27, 2021
Accepted: March 15, 2021
Article in press: March 15, 2021
Published online: May 26, 2021
Processing time: 197 Days and 22.7 Hours
Brucellosis is a contagious bacterial disease caused by Brucella species, which is a leading zoonotic disease worldwide. Most patients with brucellosis have a clear infection source; however, our case had a rare presentation of secondary haemophagocytic lymphohistiocytosis without any epidemiological history.
A 50-year-old man was admitted to our hospital with a fever of unknown origin. After laboratory examinations, such as blood culture and bone marrow biopsy, the patient was diagnosed with brucellosis and secondary haemophagocytic lymphohistiocytosis. After antibiotic therapy, the patient was afebrile, and his haemogram recovered to normal, after which he was discharged.
Brucellosis cannot be excluded in patients with clinically unexplained fever, even in those without epidemiologic history.
Core Tip: Most patients of brucellosis present with a clear infection source. However, our patient showed a rare case presentation of haemophagocytic lymphohistiocytosis with no clear infection source and unremarkable medical history. Our findings suggest that brucellosis cannot be excluded in patients with clinically unexplained fever, even in those without epidemiologic history. To prevent timely exacerbation of the disease, before obtaining the aetiology test results, the administered antibiotics should cover rare pathogens, such as Brucella.
- Citation: Tian LH, Dong ZG, Chen XY, Huang LJ, Xiao PP. Brucellosis of unknown origin with haemophagocytic syndrome: A case report. World J Clin Cases 2021; 9(15): 3649-3654
- URL: https://www.wjgnet.com/2307-8960/full/v9/i15/3649.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i15.3649
Brucellosis is a zoonotic infectious disease caused by Brucella species[1]. Brucellosis has various clinical manifestations, the most common being fever, followed by weakness, hyperhidrosis, myalgias, and arthralgias[2,3]. Most patients come into contact with infected animals or ingest infected meat or unpasteurised milk. However, unknown origin brucellosis is relatively rare. Here, we report a case of brucellosis with secondary haemophagocytic lymphohistiocytosis without epidemiologic history.
A 50-year-old man was admitted to our hospital with a history of fever.
The patient’s symptoms were fever and weakness for 6 d and diarrhoea for half a day. His body temperature reached 39.2 °C; he had no cough or other symptoms.
The patient had no remarkable medical history.
The patient had unremarkable personal and family history.
There were no remarkable findings on physical examination except the patient’s temperature was 39.5 °C.
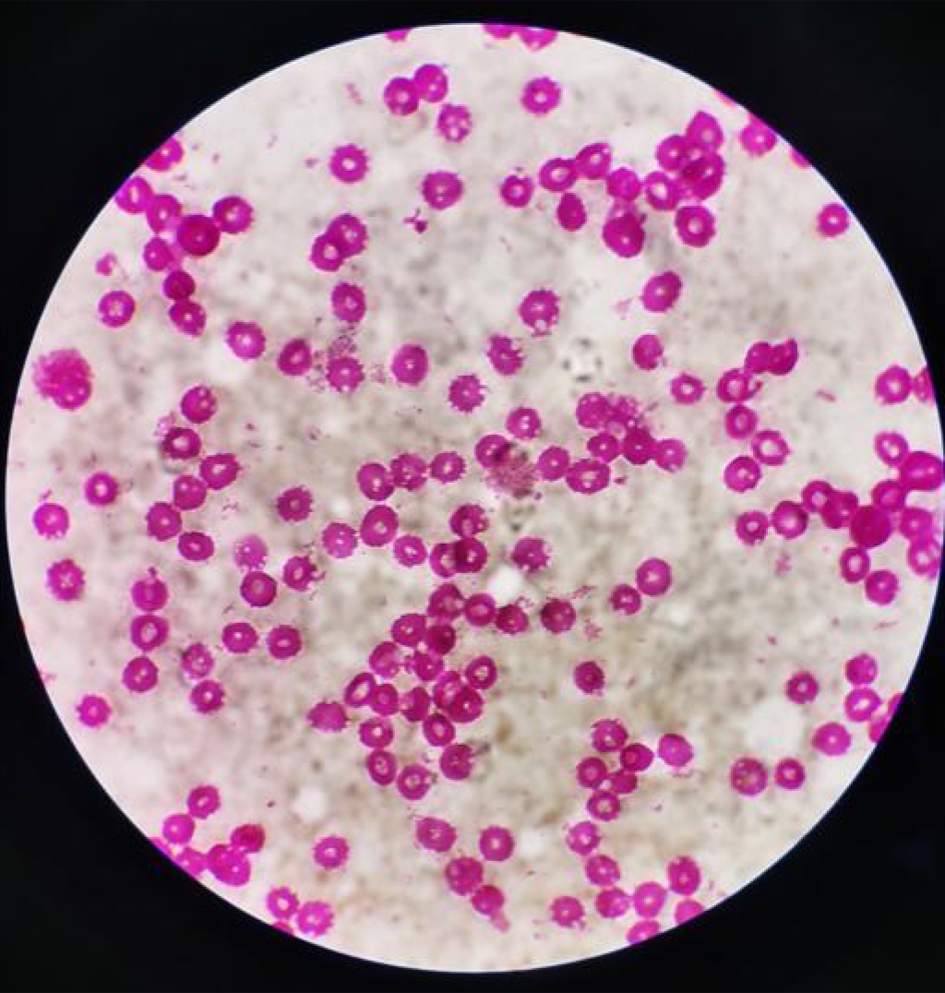
Laboratory analyses were conducted for blood, blood culture, biochemical tests, coagulation, and levels of serum C-reactive protein, serum procalcitonin, and ferritin. Laboratory data showed pancytopenia with a white blood cell count of 2.67 × 109 cells/L, haemoglobin levels of 14.7 g/dL, and a platelet count of 83 × 109 cells/L (Table 1). The blood culture on the sixth day of incubation grew Brucella melitensis (Figure 1). The level of soluble CD25 was 3256 U/mL, and abdominal ultrasonography revealed no splenomegaly or hepatomegaly. High resolution computed tomography of the chest showed no abnormalities.
| Test | Day 1 | Day 4 | Day 7 | Day 10 | Reference range |
| WBC, 109/L | 2.67 | 2.35 | 3.92 | 3.98 | 3.5–9.5 |
| HGB, g/L | 147 | 139 | 143 | 145 | 130–175 |
| PLT, 109/L | 83 | 57 | 99 | 181 | 125–350 |
| CRP, mg/L | 30.76 | 18.25 | 4.87 | 0.79 | 0–10 |
| PCT, ng/mL | 0.385 | - | - | - | 3.5–9.5 |
| Ferritin, ng/mL | 825.7 | > 1500 | 1401.2 | 895 | 23.9–336.2 |
| AST, U/L | 84.1 | 98.1 | 98.3 | 102.5 | 15–40 |
| ALT, U/L | 64.9 | 70.9 | 75.5 | 95.3 | 9–50 |
| APTT, s | 36.4 | 42.1 | 36.3 | 36.2 | 25.1–36.5 |
| PT, s | 11.3 | 11.8 | 11.8 | 11.8 | 9.4–12.5 |
| Fib, g/L | 3.17 | 2.69 | 3.17 | 2.73 | 2.0–4.8 |
| FDP-D-dimer, ng/mL | 1560 | 4190 | 696 | 284 | 0–222 |
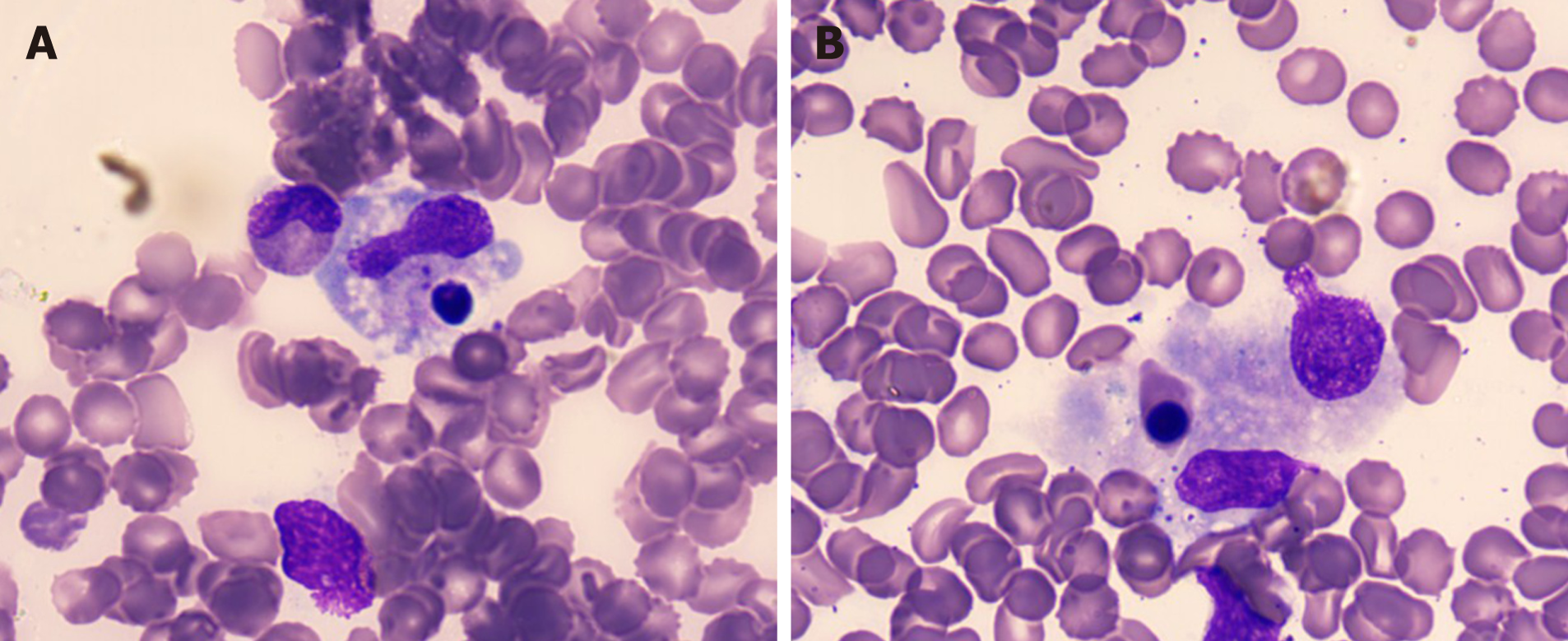
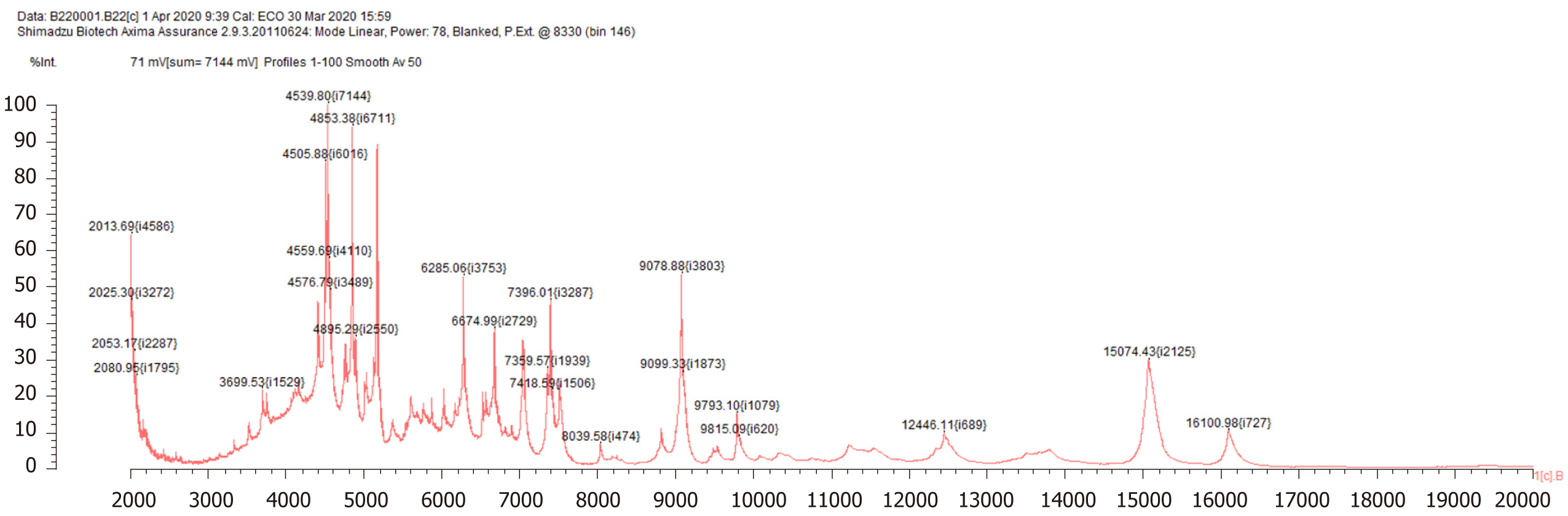
Bone marrow biopsy showed slight hypocellularity with an increase in macrophages exhibiting haemophagocytosis (Figure 2). Brucella was detected by mass spectrometry (Figure 3).
The final diagnosis of the presented case was brucellosis with haemophagocytic lymphohistiocytosis.
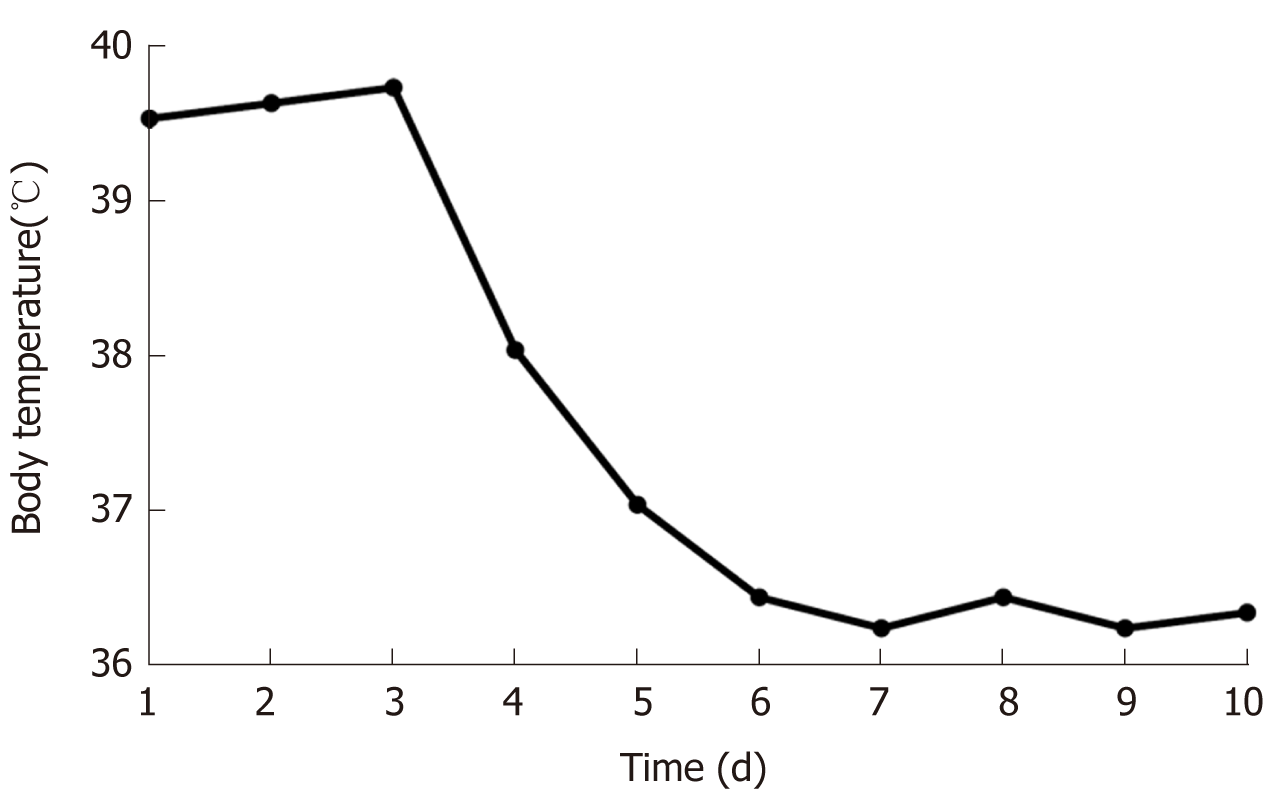
Treatment for the infection and conventional supportive therapy were administered after admission. After oral doxycycline (100 mg/dose, twice a day) and intravenous cefoperazone/sulbactam (3000 mg/dose, twice a day) for 4 d, the fever disappeared, and the body temperature was normal (Figure 4).
The laboratory data of day 10 showed recovery. The patient was subsequently discharged from our hospital.
Brucellosis is a zoonotic disease caused by bacteria of the Brucella species[4,5]. Diagnostic criteria include the epidemiologic history, clinical manifestations, and Brucella detection[6]. In this case, we identified Brucella by blood culture and mass spectrometry. This was a case of brucellosis with secondary haemophagocytic lymphohistiocytosis. After treatment with anti-brucella drugs, the haemogram became normal.
Generally, brucellosis develops after exposure to infected animals or contaminated products such as milk[7-9]. It should be noted that this patient lacked any history of exposure to these predefined epidemiologic factors. Interestingly, more and more unexplained infected cases have occurred. Recently, two cases of infective endocarditis in injection drug users without zoonotic exposure have been reported[10]. Zange et al[11] reported a patient with brucellosis who had no travel history, no exposure to unpasteurised dairy products, no animal contact, and no insect bites. The patient’s ingested meat samples also showed negative results in polymerase chain reaction testing and microbiological cultures for Brucella species. Hence, from these reports, it is recommended to pay attention to unexplained infected individuals in whom an accurate diagnosis may have been missed. The patient in this present case denied any exposure to animals or contaminated products. Before the detection of Brucella, the absence of epidemiologic factors contributed to misdiagnosis, especially before the results of aetiological examination. Thus, in all unexplained, infectious febrile patients admitted to hospitals, it is necessary to use broad-spectrum antibiotics. Moreover, it is also warranted to consider rare pathogenic bacteria, such as Brucella.
Other concerns in this case report were the indicators of inflammation. Brucella is a gram-negative bacterium, and the detection of C-reactive protein, procalcitonin, and ferritin contribute to the severity of the inflammation. However, we noticed that C-reactive protein and ferritin levels showed a typical rising at the time of onset, although procalcitonin levels were below normal during hospitalisation. Obviously, procalcitonin levels did not correlate with the severity of illness. Incidentally, changes in procalcitonin levels may not be reflective of the severity of brucellosis.
Brucellosis cannot be excluded in patients with clinically unexplained fever, even in those without epidemiologic history. Before obtaining the aetiology test results, the administered antibiotics should cover rare pathogens, such as Brucella, which could prevent the timely exacerbation of the disease.
We thank our patient who agreed to the publication of this case report and provided information.
Manuscript source: Unsolicited manuscript
Specialty type: Infectious diseases
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Olt S S-Editor: Zhang L L-Editor: Filipodia P-Editor: Zhang YL
| 1. | Franco MP, Mulder M, Gilman RH, Smits HL. Human brucellosis. Lancet Infect Dis. 2007;7:775-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 711] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 2. | de Figueiredo P, Ficht TA, Rice-Ficht A, Rossetti CA, Adams LG. Pathogenesis and immunobiology of brucellosis: review of Brucella-host interactions. Am J Pathol. 2015;185:1505-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 314] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 3. | Dean AS, Crump L, Greter H, Hattendorf J, Schelling E, Zinsstag J. Clinical manifestations of human brucellosis: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2012;6:e1929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 350] [Cited by in RCA: 310] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 4. | Seleem MN, Boyle SM, Sriranganathan N. Brucellosis: a re-emerging zoonosis. Vet Microbiol. 2010;140:392-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 439] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 5. | Deng Y, Liu X, Duan K, Peng Q. Research Progress on Brucellosis. Curr Med Chem. 2019;26:5598-5608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 6. | Ulu-Kilic A, Metan G, Alp E. Clinical presentations and diagnosis of brucellosis. Recent Pat Antiinfect Drug Discov. 2013;8:34-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Dhanashekar R, Akkinepalli S, Nellutla A. Milk-borne infections. An analysis of their potential effect on the milk industry. Germs. 2012;2:101-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | de Glanville WA, Conde-Álvarez R, Moriyón I, Njeru J, Díaz R, Cook EAJ, Morin M, Bronsvoort BMC, Thomas LF, Kariuki S, Fèvre EM. Poor performance of the rapid test for human brucellosis in health facilities in Kenya. PLoS Negl Trop Dis. 2017;11:e0005508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Dadar M, Shahali Y, Whatmore AM. Human brucellosis caused by raw dairy products: A review on the occurrence, major risk factors and prevention. Int J Food Microbiol. 2019;292:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 108] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 10. | Cafardi JM, Haas D, Lamarre T, Feinberg J. Brucella Endocarditis in Persons Who Inject Drugs. Open Forum Infect Dis. 2020;7:ofaa063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Zange S, Schneider K, Georgi E, Scholz HC, Antwerpen MH, Walter MC, Zoeller L, von Buttlar H, Borde JP. A headache with surprising outcome: first case of brucellosis caused by Brucella suis biovar 1 in Germany. Infection. 2019;47:863-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |