Published online May 26, 2021. doi: 10.12998/wjcc.v9.i15.3631
Peer-review started: October 17, 2020
First decision: November 3, 2020
Revised: December 16, 2020
Accepted: February 24, 2021
Article in press: February 24, 2021
Published online: May 26, 2021
Processing time: 205 Days and 23.6 Hours
Functioning farnesoid X receptor (FXR; encoded by NR1H4) is key to normal bile acid homeostasis. Biallelic mutations in NR1H4 are reported in a few children with intrahepatic cholestasis. We describe a boy with progressive familial intrahepatic cholestasis and homozygous mutation in NR1H4.
A boy had severe neonatal cholestasis with moderate hypercholanemia and persistently elevated alpha-fetoprotein. Despite medical treatment, coagulopathy was uncontrollable, prompting liver transplantation at age 8 mo with incidental splenectomy. The patient experienced catch-up growth with good liver function and did not develop allograft steatosis. However, 1 year after transplant, he died from an acute infection, considered secondary to immunosuppression and asplenia. A homozygous protein-truncating mutation, c.547C > T, p.(Arg183Ter), was subsequently identified in NR1H4, and both parents were shown to be heterozygous carriers. Absence of FXR and of bile salt export pump expression was confirmed by immunostaining of explanted liver.
Severe cholestasis with persistently high alpha-fetoprotein and modest elevation of serum bile acid levels may suggest FXR deficiency. Some patients with FXR deficiency may not develop allograft steatosis and may respond well to liver transplantation.
Core Tip: Despite the central role farnesoid X receptor (FXR) plays in bile acid metabolism, only a few children with cholestasis and biallelic FXR deficiency have been reported, and that only recently. Using banked DNA from patients without previous successful genetic diagnosis, we have identified a child with a homozygous mutation predicted to truncate FXR prematurely. We describe his disease course before and after liver transplantation, accompanied by immunohistochemical studies. This report adds meaningfully to the available information regarding disease course and outcomes in patients with severe FXR deficiency. It highlights biochemical findings that may be characteristic of FXR deficiency.
- Citation: Czubkowski P, Thompson RJ, Jankowska I, Knisely AS, Finegold M, Parsons P, Cielecka-Kuszyk J, Strautnieks S, Pawłowska J, Bull LN. Progressive familial intrahepatic cholestasis — farnesoid X receptor deficiency due to NR1H4 mutation: A case report. World J Clin Cases 2021; 9(15): 3631-3636
- URL: https://www.wjgnet.com/2307-8960/full/v9/i15/3631.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i15.3631
A key function of the farnesoid X receptor (FXR) is maintenance of physiologic bile acid (BA) pool size. FXR activation by BA within terminal-ileum enterocytes triggers production of fibroblast growth factor 19, which in turn suppresses hepatocellular synthesis of BA. In addition, FXR protects against intrahepatocytic BA accumulation by increasing bile salt export pump (BSEP) expression and by suppressing both uptake of BA from plasma via Na+-taurocholate cotransporting polypeptide and synthesis of BA via sterol-27 hydroxylase[1].
NR1H4 encodes FXR. Biallelic mutation in NR1H4 is a rare cause of intrahepatic cholestasis. Eight such children have been reported, three of whom underwent liver transplantation (LT)[2-4]. We describe a patient with FXR deficiency, due to biallelic mutation in NR1H4, who presented with neonatal cholestasis that progressed to end-stage liver disease successfully treated by LT.
Neonate presented with rapidly progressing cholestatic jaundice.
Jaundice was observed from the second postnatal day. Phototherapy was given. At age 5 wk, deepening jaundice and pale stools were noted. He was hospitalized at age 6 wk and transferred to Children’s Memorial Health Institute at age 7 wk.
The patient had an otherwise unremarkable medical history.
A boy was born at term, weighing 4.1 kg (93rd percentile), to parents without known consanguinity. Pregnancy and spontaneous vaginal delivery were uncomplicated. A brother aged 5 years was healthy; however, two maternal uncles had died as neonates for unknown reasons.
At transfer, he was jaundiced but otherwise well [5.8 kg (77th percentile)]. Motor development was normal. Stools varied from pale to bright yellow. The liver and spleen were palpable, respectively 3 cm and 2 cm below the costal margin. A left iridial coloboma and a right inguinal hernia were found.
Conjugated hyperbilirubinemia accompanied elevated serum transaminase activity (Table 1). Serum BA and alpha-fetoprotein (AFP) levels were elevated. Serum gamma-glutamyl transpeptidase activity was normal. Hypocoagulability did not respond to vitamin K. Persistent hypoglycemia required intravenous glucose. Investigation for metabolic disorders yielded no diagnosis.
| Serum analyte | Reference range | Age 7 wk (presentation) | Age 22 wk (before liver transplantation) |
| Hematocrit (%) | 33.0-39.0 | 29.2 | 29.6 |
| Hemoglobin (g/dL) | 10.5-13.5 | 10.1 | 10.1 |
| White-cell count (per mm3) | 6.0-17.5 | 9.1 | 11.4 |
| Platelet count (per mm3) | 150-400 | 184 | 154 |
| Total bilirubin (mg/dL) | 0.0-1.0 | 9.4 | 23.8 |
| Direct bilirubin (mg/dL) | 0.0-0.4 | 7.3 | 14.0 |
| ALT (U/L) | 10-55 | 170 | 224 |
| AST (U/L) | 10-55 | 260 | 456 |
| GGT (U/L) | 10-80 | 35 | 41 |
| Alkaline phosphatase (U/L) | 15-350 | 331 | 356 |
| Bile acids (mol/L) | 0-11 | 69 | 157 |
| Cholesterol (mg/dL) | 80-170 | 228 | 220 |
| Triglycerides (mg/dL) | 50-150 | 136 | 137 |
| Creatine kinase (U/L) | 60-400 | 30 | |
| Activated partial-thromboplastin time (s) | 21-33 | 49 | 59 |
| Prothrombin time (s) | 10-13 | 17 | 20 |
| International normalized ratio | < 1.2 | 1.50 | 2.03 |
| D-dimer | < 500 | 138 | 274 |
| Fibrinogen | 150-400 | 200 | 174 |
| Factor V (%) | 70-140 | 70.4 | - |
| Factor VII (%) | 70-120 | 36.9 | - |
| Ammonia (g/dL) | 12-48 | 178 | 243 |
| Glucose (mg%) | 70-110 | 55 | 30 |
| Creatinine (mg/dL) | 0.3-1.0 | 0.2 | 0.2 |
| Protein (g/L) | 60-83 | 45 | 55 |
| Albumin (g/L) | 33-50 | 25 | 34 |
| Globulin (g/L) | 26-41 | 7 | 14 |
| Immunoglobulin G (mg/dL) | 231-1411 | 713 | - |
| Immunoglobulin A (mg/dL) | 0-83 | 21 | - |
| Immunoglobulin M (mg/dL) | 0-145 | 79 | - |
| 1Alpha-fetoprotein (IU/mL) | 358000 | 230000 | |
| Vitamin A (ng/mL) | 200-800 | 165 | - |
| Vitamin E (g/mL) | 3.8-16.0 | 8.4 | - |
| 25OHD3 (ng/mL) | 11-54 | 26 | - |
A normal gallbladder and non-dilated bile ducts were visible on sonography; the left lobe of the liver was enlarged. Splenomegaly was confirmed. After 3 d of phenobarbital, hepatobiliary scintigraphy found decreased and patchy isotope uptake by the liver and substantially decreased, but detectable, excretion into the bowel. A type 2 atrial septal defect (assessed as clinically unimportant) and a butterfly vertebra were noted.
Percutaneous liver-biopsy (age 9 wk) found moderate fibrosis, mild inflammation and ductular proliferation; immunostaining for BSEP was unavailable. At age 12 wk, open cholangiography was performed and found a normal biliary tree; wedge liver biopsy revealed changes like those seen previously.
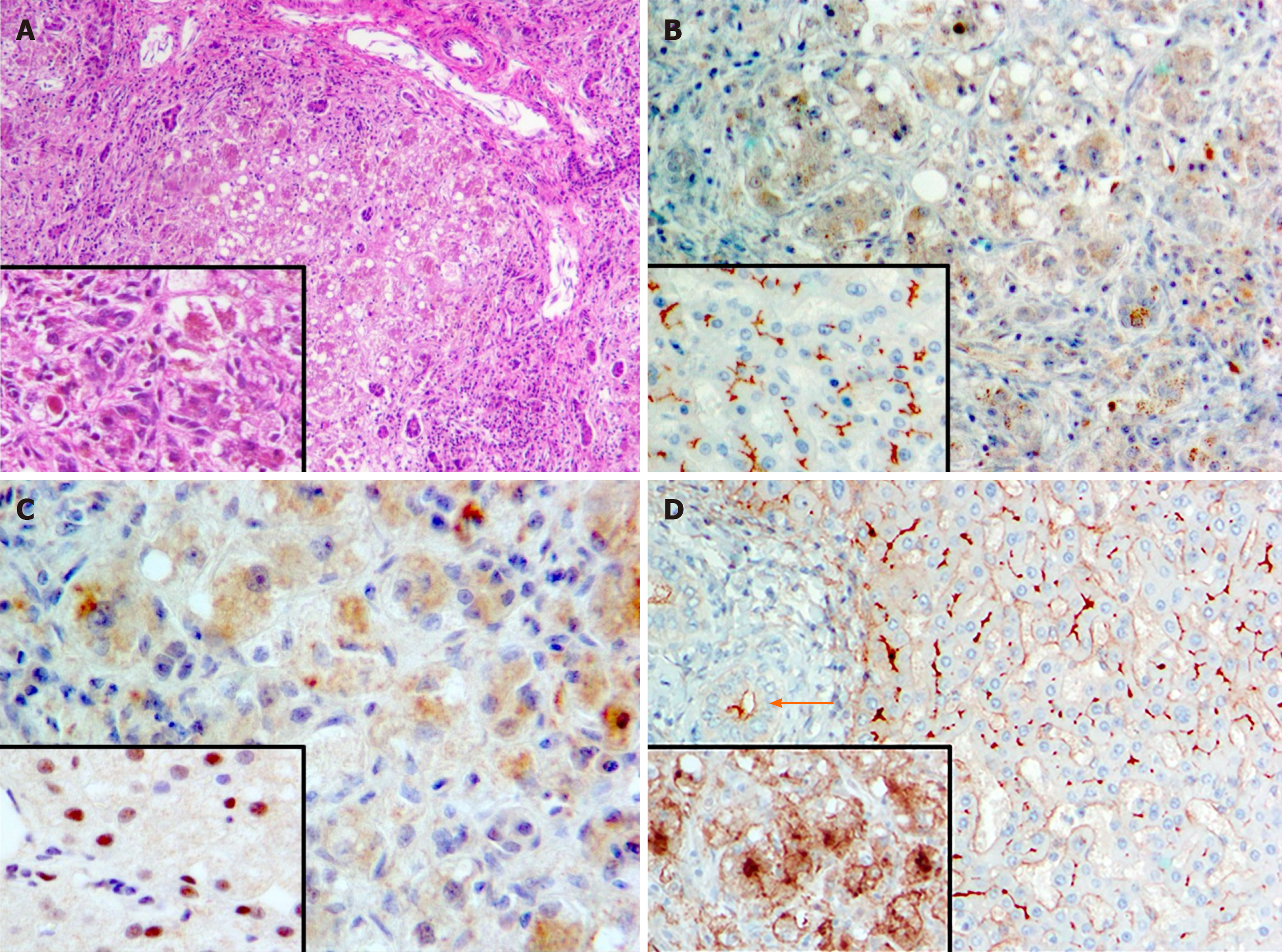
Archived patient DNA underwent sequencing of a panel of cholestasis-associated genes (ATP8B1, ABCB11, ABCB4, TJP2, JAG1, NOTCH2, BAAT) with no diagnostic variants found. These results were confirmed when patient DNA then underwent whole-exome sequencing. However, homozygosity was found for c.547C > T, p.(Arg183Ter), a nonsense mutation in NR1H4. Sanger sequencing confirmed homozygosity for this variant in the patient and heterozygosity in each parent. Immunostaining revealed absence of BSEP (Figure 1B) and of nuclear marking for FXR (Figure 1C). Gamma-glutamyl transpeptidase expression was present but very abnormal (Figure 1D).
Vitamin supplementation and ursodeoxycholic acid (20 mg/kg per d) were initiated. Icterus and hypercholanemia worsened and the patient required repeated intravenous vitamin K. At age 8 mo [8.4 kg (40th percentile)], uncontrollable coagulopathy prompted maternal-donor LT, with splenectomy due to substantial splenomegaly. Histopathologic evaluation revealed marked hepatocellular and canalicular cholestasis with steatosis and micronodular cirrhosis (Figure 1A). Dysplasia and malignancy were not found.
There were no surgical issues after LT. Immunosuppression included tacrolimus and corticosteroids, with trimethoprim/sulfamethoxazole prophylaxis. Vaccination against meningococcus infection was unavailable. His course after LT was unremarkable for 12 mo, apart from transaminitis (cytomegalovirus infection, resolved with ganciclovir). His weight recovered [75th percentile (age 19 mo) 11 mo after LT]. At age 20 mo, vomiting and fever required hospital admission elsewhere. He was assessed as in good condition, but within 12 h he died with disseminated intravascular coagulation and multiorgan failure. No micro-organisms were cultured. Necropsy found hemorrhagic adrenal necrosis. The liver was unremarkable. The heart contained a few foci of remote intramyocardial arterial thrombosis, with dystrophic mineralization.
The fundamental role of FXR in BA homeostasis is consistent with the severity of liver disease associated with its absence. Our patient had substantially impaired liver function from presentation at Children’s Memorial Health Institute onward (hypoglycemia; coagulopathy unresponsive to parenteral vitamin K). These disease manifestations overlap with those in the children in whom FXR deficiency was first reported[2]. Coagulopathy is common in cholestasis. Initially, however, it usually responds to vitamin K. In FXR deficiency, vitamin K does not correct the clotting, which seems disproportionate to the severity of liver disease. This may reflect direct involvement of FXR in regulating-clotting factor production[5]. Serum BA elevations in our patient and in one previously described FXR-deficient patient for whom serum BA were reported[2] were modest compared with those in patients with similarly severe cholestasis and BSEP deficiency. This difference may reflect failure of FXR-mediated down-regulation of Na+-taurocholate cotransporting polypeptide in FXR deficiency, with consequent unabated hepatocellular uptake of BA[6].
High AFP levels are found in hepatocellular carcinoma and, during early infancy, in severe early-onset cholestasis. As seen in our patient and others, persistently elevated AFP values in the absence of hepatocellular carcinoma may be a feature of NR1H4 disease[2-4]. This may complicate monitoring for hepatocellular carcinoma development.
Two siblings who underwent LT for FXR deficiency developed mild allograft steatosis, ascribed provisionally to disrupted ileal control of enterohepatic BA homeostasis[2]. The third transplanted patient had stable graft function, and no steatosis in post-transplant liver biopsies in the first 2 years. Our patient had no allograft steatosis, good graft function and no evidence of gastrointestinal disease. These observations suggest heterogeneity in compensation for extrahepatic FXR deficiency. We consider our patient’s death (sepsis, adrenal haemorrhage; predisposed by immunosuppression and lack of spleen) to be independent of NR1H4 mutation.
FXR deficiency due to NR1H4 mutation is a rare cause of neonatal cholestasis. Severe cholestasis with persistently high AFP and modestly elevated serum BA levels may suggest FXR deficiency.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: Polish Society of Gastroenterology.
Specialty type: Medicine, research and experimental
Country/Territory of origin: Poland
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mrzljak A S-Editor: Gao CC L-Editor: Filipodia P-Editor: Li JH
| 1. | Claudel T, Sturm E, Kuipers F, Staels B. The farnesoid X receptor: a novel drug target? Expert Opin Investig Drugs. 2004;13:1135-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Gomez-Ospina N, Potter CJ, Xiao R, Manickam K, Kim MS, Kim KH, Shneider BL, Picarsic JL, Jacobson TA, Zhang J, He W, Liu P, Knisely AS, Finegold MJ, Muzny DM, Boerwinkle E, Lupski JR, Plon SE, Gibbs RA, Eng CM, Yang Y, Washington GC, Porteus MH, Berquist WE, Kambham N, Singh RJ, Xia F, Enns GM, Moore DD. Mutations in the nuclear bile acid receptor FXR cause progressive familial intrahepatic cholestasis. Nat Commun. 2016;7:10713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 200] [Article Influence: 22.2] [Reference Citation Analysis (2)] |
| 3. | Chen HL, Li HY, Wu JF, Wu SH, Chen HL, Yang YH, Hsu YH, Liou BY, Chang MH, Ni YH. Panel-Based Next-Generation Sequencing for the Diagnosis of Cholestatic Genetic Liver Diseases: Clinical Utility and Challenges. J Pediatr 2019; 205: 153-159. e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 4. | Himes RW, Mojarrad M, Eslahi A, Finegold MJ, Maroofian R, Moore DD. NR1H4-related Progressive Familial Intrahepatic Cholestasis 5: Further Evidence for Rapidly Progressive Liver Failure. J Pediatr Gastroenterol Nutr. 2020;70:e111-e113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Zhao A, Lew JL, Huang L, Yu J, Zhang T, Hrywna Y, Thompson JR, de Pedro N, Blevins RA, Peláez F, Wright SD, Cui J. Human kininogen gene is transactivated by the farnesoid X receptor. J Biol Chem. 2003;278:28765-28770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Denson LA, Sturm E, Echevarria W, Zimmerman TL, Makishima M, Mangelsdorf DJ, Karpen SJ. The orphan nuclear receptor, shp, mediates bile acid-induced inhibition of the rat bile acid transporter, ntcp. Gastroenterology. 2001;121:140-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 335] [Article Influence: 14.0] [Reference Citation Analysis (0)] |