Published online May 6, 2021. doi: 10.12998/wjcc.v9.i13.3219
Peer-review started: January 9, 2021
First decision: January 24, 2021
Revised: February 4, 2021
Accepted: March 9, 2021
Article in press: March 9, 2021
Published online: May 6, 2021
Processing time: 102 Days and 22.7 Hours
Acute severe ulcerative colitis (ASUC) is a complication of ulcerative colitis associated with high levels of circulating tumor necrosis factor alpha, due to the intense inflammation and faster stool clearance of anti-tumor necrosis factor drugs. Dose-intensified infliximab treatment can be beneficial and is associated with lower rates of colectomy. The aim of the study was to present a case of a patient with ASUC and megacolon, treated with hydrocortisone and accelerated scheme of infliximab that was monitored by drug trough level.
A 22-year-old female patient diagnosed with ulcerative colitis, presented with diarrhea, rectal bleeding, abdominal pain, vomiting, and distended abdomen. During investigation, a positive toxin for Clostridium difficile and colonic dilatation of 7 cm consistent with megacolon were observed. She was treated with oral vancomycin for pseudomembranous colitis and intravenous hydrocortisone for severe colitis, which led to the resolution of megacolon. Due to the persistent severe colitis symptoms, infliximab 5 mg/kg was prescribed, monitored by drug trough level (8.8 μg/mL) and fecal calprotectin of 921 μg/g (< 30 μg/g). Based on the low infliximab trough level after one week from the first infliximab dose, the patient received a second infusion at week 1, consistent with the accelerated regimen (infusions at weeks 0, 1, 2 and 6). We achieved a positive clinical and endoscopic response after 6 mo of therapy, without the need for a colectomy.
Infliximab accelerated infusions can be beneficial in ASUC unresponsive to the treatment with intravenous corticosteroids. Longitudinal studies are necessary to define the best therapeutic drug monitoring and treatment regimen for these patients.
Core Tip: Acute severe ulcerative colitis (ASUC) is associated with high circulating levels of tumor necrosis factor-alpha, due to intense inflammation and faster stool clearance of the anti-tumor necrosis factor drug. Consequently, these patients may need higher doses or more frequent administrations of infliximab. A young patient with a recent diagnosis of ulcerative colitis presenting with ASUC associated with megacolon, was successfully treated with intravenous corticosteroids and an accelerated infliximab regimen, based on the serum levels of the medication. Despite the favorable outcome in the case reported, longitudinal studies are necessary to define the best therapeutic drug monitoring and treatment regimen for these patients.
- Citation: Garate ALSV, Rocha TB, Almeida LR, Quera R, Barros JR, Baima JP, Saad-Hossne R, Sassaki LY. Treatment of acute severe ulcerative colitis using accelerated infliximab regimen based on infliximab trough level: A case report. World J Clin Cases 2021; 9(13): 3219-3226
- URL: https://www.wjgnet.com/2307-8960/full/v9/i13/3219.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i13.3219
Ulcerative colitis (UC) is characterized by a chronic inflammation of the colon and rectum and the therapeutic management depends on its extent and severity[1]. Approximately 15% of the patients experience an episode of severe exacerbation as a medical emergency which may require hospitalization[2,3], defined as acute severe UC (ASUC). Cyclosporine, a calcineurin inhibitor, and infliximab, an anti-tumor necrosis factor-alpha (TNF-α) agent, have been effective in the management of ASUC as a rescue therapy in patients unresponsive to the initial treatment with corticosteroids[3,4].
ASUC is a complication of UC associated with high circulating levels of TNF-α, due to the intense inflammation and faster stool clearance of the anti-TNF drugs[3,5]. Consequently, these patients may need higher doses and/or more frequent infliximab administrations to maintain the therapeutic levels[3]. Studies indicate that the dose intensification improves the prognosis, reducing the chance of colectomy in 1 year of follow-up[4]. Despite that, not enough data exists defining the ideal serum therapeutic level related to higher rates of clinical response, mucosal healing, and colectomy-free survival in ASUC patients. Interesting, the present study reported a patient with ASUC associated with megacolon, treated with intravenous corticosteroids and an accelerated infliximab regimen, based on the serum levels of the medication, highlighting the rarity of the case and the importance of evaluating this new therapeutic tool in the management of patients with ASUC.
A 22-year-old female Caucasian patient was diagnosed with UC 3 mo ago, complaining of bloody diarrhea, abdominal pain and weight loss, and discontinued mesalamine due to the gastric intolerance. The patient underwent a colonoscopy 2 wk before the admission to the hospital, which revealed lesions consistent with UC of moderate endoscopic activity (Mayo endoscopic score 2).
She was admitted to the emergency department in due to frequent liquid and bloody stool and intense abdominal pain for 2 mo, with worsening of the symptoms during the last week, in poor condition with nausea, vomiting, and weight loss (10 kg) and without the improvement from the previous use of antibiotics.
At hospital admission (day 1 of hospital admission), the patient presented in poor condition, dehydrated, tachycardic (110 beat/min), blood pressure 100/60 mmHg, temperature > 37.8 °C, with distended and diffusely painful abdomen, and rebound tenderness.
Laboratory tests showed inflammatory process (C-reactive protein 20.3 mg/dL) and anemia (hematocrit 25.8%, hemoglobin 8.1 g/dL) at admission (Table 1).
| Day 1 (at hospital admission) | Day 4 | Day 8 | Day 15 | Day 21 | Day 51 | After 6 mo | |
| Partial Mayo score (points) | 9 | 7 | 7 | 7 | 3 | 2 | 0 |
| Mayo endoscopic score (points) | 3 | - | - | - | - | - | 0 |
| Hematocrit/hemoglobin, (%)/(g/dL) | 25.8/8.11 | 27.1/8.61 | 35.6/11.9 | 34.4/10.8 | 32.3/10.6 | 28.9/9.4 | 40.2/13.8 |
| C-reactive protein (hs-CRP) (< 1.0mg/dL) | 20.3 | 6.0 | 2.9 | 3.7 | 2.7 | - | 0.0 |
| Albumin (g/dL) | - | 1.9 | 1.9 | 2.1 | 2.1 | 3.5 | 4.4 |
| Fecal calprotectin (< 30 μg/g) | - | - | - | 921 | - | 166 | - |
| Infliximab trough level (μg/mL) | - | - | - | 8.8 | > 20 | 9.1 | - |
| Medical treatment | Hydrocortisone 300 mg/d + oral vancomycin 250 mg qid | Infliximab 5 mg/kg (first infusion) + hydrocortisone300 mg/d | Infliximab 5 mg/kg (second infusion) + hydrocortisone 100 mg/d. Discharge with azathioprine + prednisone 40 mg | Infliximab 5 mg/kg + azathioprine (third infusion) + corticosteroid tapering | Infliximab 5 mg/kg + azathioprine (fourth infusion) + prednisone 20 mg (dose tapering) | Infliximab 5 mg/kg + azathioprine (maintenance treatment) |
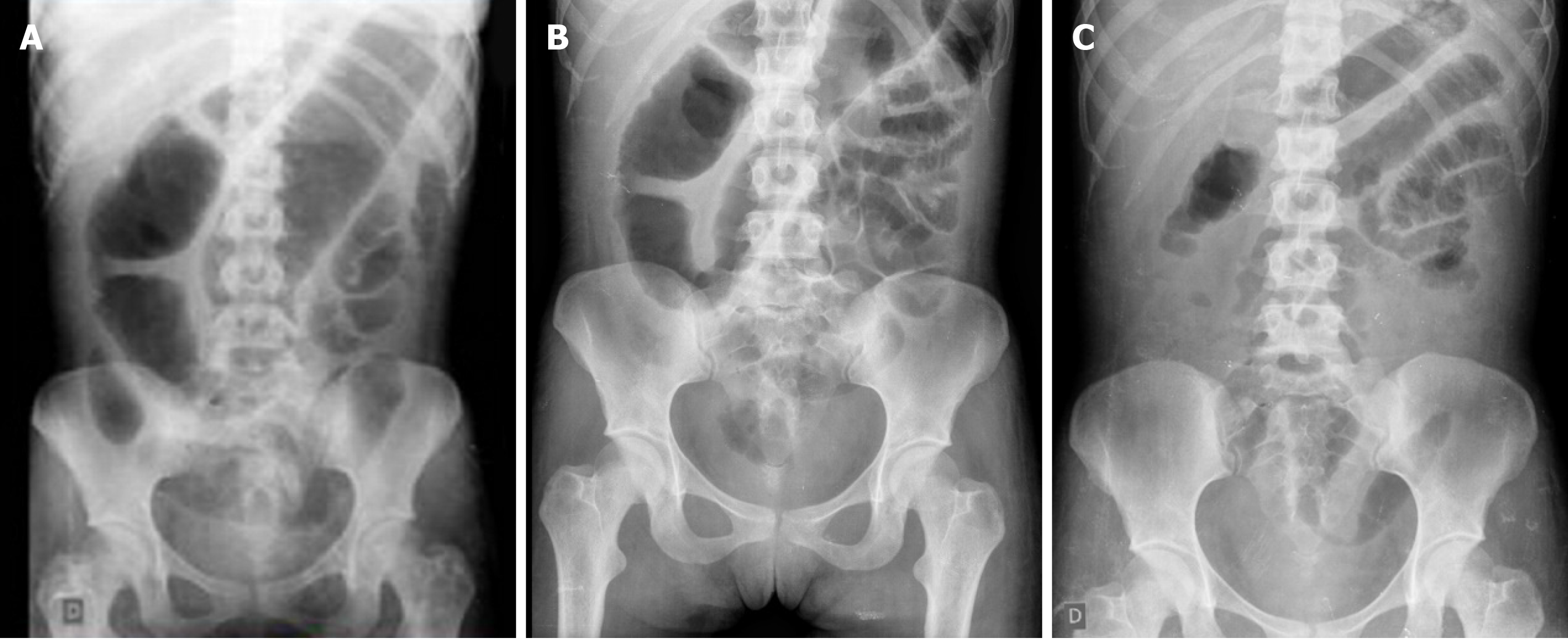
Abdominal X-ray revealed colonic dilation of 7 cm, consistent with megacolon (Figure 1).
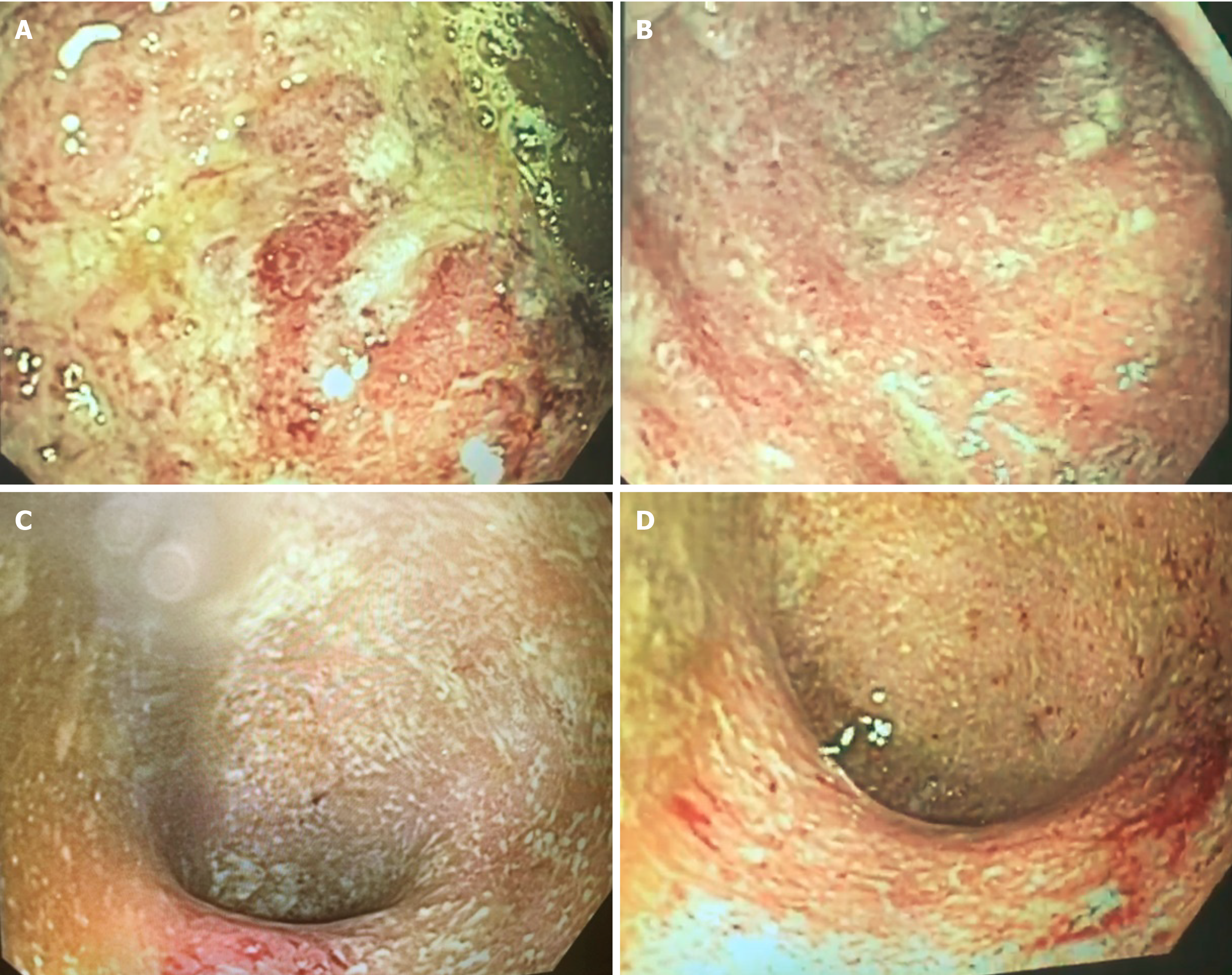
Clostridium difficile (C. difficile) A and B toxin was positive, and the treatment with oral vancomycin 250 mg qid was initiated. However, the patient presented with worsening of diarrhea and rectal bleeding (> 10 episodes/d), increased abdominal distension, and fever. A flexible sigmoidoscopy was performed (day 4 of hospital admission) and inserted up to 25 cm with no insufflation, showing ulcers covered by fibrin, mucosal friability, edema, and intense enanthem with spontaneous bleeding in sigmoid and rectum, consistent with UC of severe activity (Mayo endoscopic score 3) (Figure 2). Histopathological evaluation showed chronic colitis in intense activity with structural abnormalities of the mucosa, presence of crypt micro-abscesses and plasmacytosis, consistent with severe inflammatory activity without the evidence of C. difficile or cytomegalovirus infection.
The final diagnosis was ASUC with a complication of C. difficile infection and megacolon.
Hydrocortisone 300 mg IV per day was started on day 4 with resolution of the abdominal distension on X-ray (Figure 1), in addition to thromboembolic prophylaxis with heparin. Laboratory exams showed C-reactive protein of 6.0 mg/dL (Table 1). However, the patients’ diarrhea and rectal bleeding persisted, and infliximab (5 mg/kg) was indicated (day 8 of hospital admission) for the treatment of ASUC.
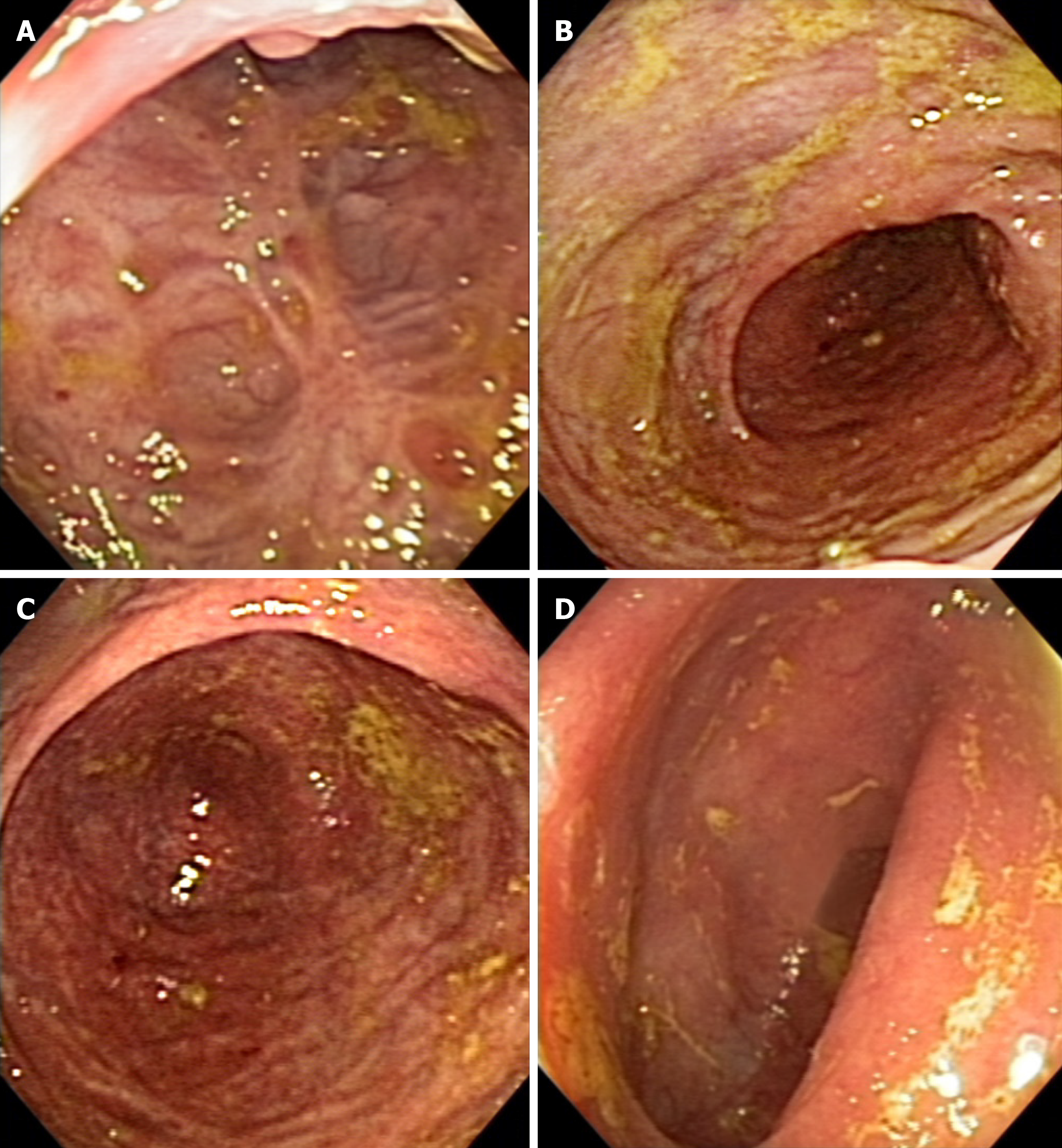
After 1 wk from the first dose of infliximab (day 15 of hospital admission), infliximab serum level was 8.8 μg/mL and fecal calprotectin was 921 μg/g (< 30 μg/g) (Table 1). Due to the low infliximab trough level and the high fecal calprotectin reflecting the inflammatory process, the patient received a second infusion, based on the accelerated infliximab regimen. The patient significantly improved, with the reduction in stool frequency and cessation of rectal bleeding and abdominal pain. The patient was discharged receiving azathioprine 2 mg/kg and prednisone 40 mg/d (Table 1). After 1 wk of the second infusion of infliximab, the patients’ infliximab serum level was > 20 μg/mL, C-reactive protein was 2.7 mg/dL (Table 1), and she received the third infusion of infliximab, and corticosteroid tapering was started. After 30 d, she returned for the fourth infusion of infliximab, in clinical remission, reporting stool frequency of once per day and no abdominal pain or distension, and with infliximab serum level of 9.1 μg/mL (Table 1). The patient received regular treatment with azathioprine and infliximab 5 mg/kg every 8 wk for the maintenance of clinical and endoscopic remission (Figure 3).
ASUC and toxic megacolon are potentially serious and fatal complications of the UC which must be rapidly recognized and effectively treated to improve the mucosal lesions and prognosis of the patient. We reported the case of a young patient with a recent diagnosis of UC presenting two complications, successfully treated with intravenous corticosteroid and accelerated infliximab regimen, based on the serum levels of the medication. Monitoring drug level is as important tool in the therapeutic arsenal of inflammatory bowel disease and, particularly in the case reported, it was essential for the success of the treatment.
The treatment of ASUC includes clinical support such as electrolyte disorder correction, nutritional therapy, and medications, but sometimes surgery might be necessary for refractory cases. Corticosteroids remain the first-line therapy[5], but patients who do not respond to the high doses of intravenous corticosteroids and with stool frequency higher than eight times a day and C-reactive protein > 45 mg/L often require surgical intervention[1,2]. Cyclosporine and, more recently, infliximab, have emerged as an advance in the management of severe UC, and have been used as a rescue therapy for corticosteroid non-responders[5]. Both are effective and comparable in the initial response and can reduce the need of early colectomy[3,5], but many clinicians choose infliximab due to the absence of renal toxicity and facility of administration[4,6], which was also done in our case.
Since ASUC is associated with high circulating levels of TNF-α[3] and fecal loss of the drug from an inflamed colon[7], patients may benefit from the dose intensification. It can be done by the dose optimization for induction therapy prescribing 10 mg/kg and/or 3 infusions in 20 d, at weeks 0, 1, and 2, aiming to reduce the risk of colectomy during the induction period[3], or in 1 year[4]. Dose intensification is beneficial in at least 50% of the patients and can reduce the rate of early colectomy by up to 80%, although these data need to be confirmed in the prospective studies[4].
On the other hand, a systematic review of short and long-term efficacy outcomes which included a total of 705 patients (308 received intensified infliximab therapy) showed no difference in the short or long-term colectomy rates in hospitalized ASUC patients[8]. Overall, the quality of data from the selected studies is poor and some of the factors, as the difference in disease severity, timing of the infliximab initiation and median interval to the second infusion, besides the use of variable doses and schedules for infliximab therapy, could have interfered with the results[8]. Likewise, a recent published retrospective study found that initial induction dosing strategy did not change the short-term or long-term colectomy rates, but a subgroup of patients who presented with more severe disease could benefit from intensified infliximab therapy, without any increase of complication rates[9]. A retrospective cohort study conducted by Chao et al[10] also showed that a high-dose infliximab induction (10 mg/kg) was not superior to the standard infliximab induction (5 mg/kg) in the colectomy rate reduction at 1, 3, or 24 mo. This question might be answered after the conclusion of the PREDICT-UC study, which is evaluating different infliximab induction strategies for the ASUC[11].
One possibility for the discrepancy between study results could be the absence of drug monitoring during the treatment. Despite the knowledge about the increased drug clearance and drug fecal loss through the inflamed mucosa of the colon[4,6], the recommended drug trough level for treatment of ASUC is not established. The serum concentration of infliximab < 16.5 μg/mL at week 2 was an independent predictor for colectomy [hazard ratio (HR): 5.6; 95% confidence interval (CI): 1.1-27.8; P = 0.034], in addition to other factors such as the presence of severe colitis (HR: 24; 95%CI: 2.5-231; P = 0.006), C-reactive protein > 5 mg/L (HR: 11; 95%CI: 2.1-58.8; P = 0.005) and albumin < 40 g/L (HR: 9.5; 95%CI: 1.3-71.4; P = 0.026) in a study that evaluated 99 infliximab primary non-responders[12]. Similarly, a Hungarian study showed that the serum level of the infliximab biosimilar, CTP-13, at week 2 was associated with week 14 clinical response (11.5 μg/mL) and remission (15.3 μg/mL) as well as at week 30 with clinical response (11.5 μg/mL) and remission (14.5 μg/mL)[13]. In the present case, the patient had a serum infliximab concentration of 8.8 μg/mL at week 1 of the treatment; therefore, below the recommended dosage of the aforementioned studies, so, a second infusion of infliximab was prescribed at this moment, based on the accelerated regimen. Longitudinal studies with determined outcomes are essential to define the ideal drug serum level in the patients with ASUC.
Toxic megacolon is characterized by the dilation of colon associated with systemic symptoms. It is usually related to UC; however, it can also be secondary to the infections, such as C. difficile, ischemic colitis, volvulus, diverticulitis, and obstruction due to the colon cancer[2]. The use of intravenous corticosteroids associated with early colectomy reduced the mortality of patients and, in the referral centers, mortality varies around 3% or even less[2]. Toxic megacolon management comprises treatment of the underlying disease and supportive care. In our case, the C. difficile infection associated with ASUC could be the underlying cause of the megacolon. The patient improved after the management of the infection and the intravenous corticosteroid therapy. Patient’s monitoring must be continuous and performed by a multidisciplinary team to avoid intestinal perforation and systemic complications. In the face of any sign of complication, surgery should be indicated.
This study reported a case of a young patient, diagnosed with ASUC associated with megacolon and C. difficile infection. The patient improved from the megacolon due to the prescription of antibiotics for C. difficile infection and intravenous hydrocortisone, but without any benefit for other symptoms. We opted for the accelerated infliximab treatment, based on the patient's clinical condition and serum level of medication. The patient achieved clinical improvement and endoscopic remission of UC. Despite the success of the reported case, many questions remain unanswered and longitudinal studies are necessary to establish the best treatment regimen and therapeutic drug level for these patients. Furthermore, recent studies have emerged showing a potential treatment using tofacitinib, an oral small synthetic Janus kinase inhibitor, in ASUC, especially in biologic treatment experienced patients[14].
The study has some limitations, such as being based in only 1 case, and the lack of calprotectin at all moments to assess the intestinal inflammation. Despite this, the reported case shows an example of success in the management of ASUC monitored by the serum dosage of the medication and future patients can benefit from this therapeutic strategy.
Infliximab accelerated infusions can be beneficial in ASUC patients unresponsive to the treatment with intravenous corticosteroids. Monitoring the drug levels in these cases is essential to guide the frequency of infusions. Longitudinal studies are necessary to define the best therapeutic drug monitoring and treatment regimen for these patients.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ozair A, Yang TY S-Editor: Gao CC L-Editor: A P-Editor: Xing YX
| 1. | Magro F, Gionchetti P, Eliakim R, Ardizzone S, Armuzzi A, Barreiro-de Acosta M, Burisch J, Gecse KB, Hart AL, Hindryckx P, Langner C, Limdi JK, Pellino G, Zagórowicz E, Raine T, Harbord M, Rieder F; European Crohn’s and Colitis Organisation [ECCO]. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J Crohns Colitis. 2017;11:649-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1446] [Cited by in RCA: 1292] [Article Influence: 161.5] [Reference Citation Analysis (0)] |
| 2. | Daperno M, Sostegni R, Rocca R, Rigazio C, Scaglione N, Castellino F, Ercole E, Pera A. Review article: medical treatment of severe ulcerative colitis. Aliment Pharmacol Ther. 2002;16 Suppl 4:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Gibson DJ, Heetun ZS, Redmond CE, Nanda KS, Keegan D, Byrne K, Mulcahy HE, Cullen G, Doherty GA. An accelerated infliximab induction regimen reduces the need for early colectomy in patients with acute severe ulcerative colitis. Clin Gastroenterol Hepatol 2015; 13: 330-335. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 200] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 4. | Hindryckx P, Novak G, Vande Casteele N, Laukens D, Parker C, Shackelton LM, Narula N, Khanna R, Dulai P, Levesque BG, Sandborn WJ, D'Haens G, Feagan BG, Jairath V. Review article: dose optimisation of infliximab for acute severe ulcerative colitis. Aliment Pharmacol Ther. 2017;45:617-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 5. | Dulai PS, Jairath V. Acute severe ulcerative colitis: latest evidence and therapeutic implications. Ther Adv Chronic Dis. 2018;9:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 6. | Rosen MJ, Minar P, Vinks AA. Review article: applying pharmacokinetics to optimise dosing of anti-TNF biologics in acute severe ulcerative colitis. Aliment Pharmacol Ther. 2015;41:1094-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 7. | Brandse JF, van den Brink GR, Wildenberg ME, van der Kleij D, Rispens T, Jansen JM, Mathôt RA, Ponsioen CY, Löwenberg M, D'Haens GR. Loss of Infliximab Into Feces Is Associated With Lack of Response to Therapy in Patients With Severe Ulcerative Colitis. Gastroenterology 2015; 149: 350-5. e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 314] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 8. | Sebastian S, Myers S, Nadir S, Subramanian S. Systematic Review: Efficacy and Safety of Accelerated Induction Regimes in Infliximab Rescue Therapy for Hospitalized Patients with Acute Severe Colitis. Dig Dis Sci. 2019;64:1119-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 9. | Sebastian S, Myers S, Argyriou K, Martin G, Los L, Fiske J, Ranjan R, Cooper B, Goodoory V, Ching HL, Jayasooriya N, Brooks J, Dhar A, Shenoy AH, Limdi JK, Butterworth J, Allen PB, Samuel S, Moran GW, Shenderey R, Parkes G, Lobo A, Kennedy NA, Subramanian S, Raine T. Infliximab induction regimens in steroid-refractory acute severe colitis: a multicentre retrospective cohort study with propensity score analysis. Aliment Pharmacol Ther. 2019;50:675-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Chao CY, Al Khoury A, Aruljothy A, Restellini S, Wyse J, Afif W, Bitton A, Lakatos PL, Bessissow T. High-Dose Infliximab Rescue Therapy for Hospitalized Acute Severe Ulcerative Colitis Does Not Improve Colectomy-Free Survival. Dig Dis Sci. 2019;64:518-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | De Cruz P. Optimising Infliximab induction therapy for acute severe ulcerative colitis (PREDICT-UC). [Accessed Apr 17, 2020]. In: ClinicalTrials.gov [Internet]. Melbourne (Victoria): Austin Health. ClinicalTrials.gov. Identifier: NCT02770040 Available from: https://clinicaltrials.gov/ct2/show/NCT02770040. |
| 12. | Papamichael K, Rivals-Lerebours O, Billiet T, Vande Casteele N, Gils A, Ferrante M, Van Assche G, Rutgeerts PJ, Mantzaris GJ, Peyrin-Biroulet L, Vermeire S. Long-Term Outcome of Patients with Ulcerative Colitis and Primary Non-response to Infliximab. J Crohns Colitis. 2016;10:1015-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 13. | Gonczi L, Vegh Z, Golovics PA, Rutka M, Gecse KB, Bor R, Farkas K, Szamosi T, Bene L, Gasztonyi B, Kristóf T, Lakatos L, Miheller P, Palatka K, Papp M, Patai Á, Salamon Á, Tóth GT, Vincze Á, Biro E, Lovasz BD, Kurti Z, Szepes Z, Molnár T, Lakatos PL. Prediction of Short- and Medium-term Efficacy of Biosimilar Infliximab Therapy. Do Trough Levels and Antidrug Antibody Levels or Clinical And Biochemical Markers Play the More Important Role? J Crohns Colitis. 2017;11:697-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Kotwani P, Terdiman J, Lewin S. Tofacitinib for Rescue Therapy in Acute Severe Ulcerative Colitis: A Real-world Experience. J Crohns Colitis. 2020;14:1026-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |