Published online May 6, 2021. doi: 10.12998/wjcc.v9.i13.3170
Peer-review started: December 6, 2020
First decision: December 21, 2020
Revised: February 6, 2021
Accepted: February 26, 2021
Article in press: February 26, 2021
Published online: May 6, 2021
Processing time: 136 Days and 23.5 Hours
Perioperative stroke is a rare but devastating complication. The risk factors for massive cerebral stroke in surgical patients include older age, male sex, prior cerebrovascular disease, hypertension, renal failure, smoking, diabetes mellitus, and atrial fibrillation.
We describe two cases of perioperative massive cerebral stroke following thoracic surgery and one case following bronchoscopy. Neurologic symptoms, including changes in mental status and hemiplegia, occurred within 10 h after surgery in the three patients. All three patients died after the surgery.
Perioperative massive cerebral stroke may be more likely to occur in thoracic surgical patients if there are pre-existing factors including previous stroke, hypotension, and hypoxemia. Sufficient pain control after surgery and timely neurology consultation and management are helpful for the diagnosis and control of stroke in high-risk patients.
Core Tip: Perioperative stroke is a rare but devastating complication; however, the risk factors for perioperative stroke remain unclear. Two cases following thoracic surgery and one case following bronchoscope presented with perioperative massive cerebral stroke are documented in this case series. The risk factors for perioperative massive cerebral stroke in thoracic patients include previous stroke, hypotension, and hypoxemia. Sufficient pain control after surgery and timely neurology consultation and management are helpful for diagnosis and control of stroke in high risk patients.
- Citation: Jian MY, Liang F, Liu HY, Han RQ. Perioperative massive cerebral stroke in thoracic patients: Report of three cases. World J Clin Cases 2021; 9(13): 3170-3176
- URL: https://www.wjgnet.com/2307-8960/full/v9/i13/3170.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i13.3170
Perioperative stroke is a rare but devastating complication. Surgical patients are vulnerable to stroke due to alterations in the coagulation system resulting from stress responses to surgery. The risk factors for perioperative stroke include older age, male sex, prior cerebrovascular disease, hypertension, renal failure, smoking, diabetes mellitus, and atrial fibrillation. General anesthesia during surgery may also influence the risk of perioperative stroke[1]. The incidence of perioperative acute stroke after pulmonary lobectomy or pneumonectomy is approximately 0.4%-0.6%[2,3]. However, the risk factors for massive cerebral stroke in thoracic surgical patients remain unclear.
Here, we report perioperative massive cerebral stroke in two cases following thoracic surgery and one case following bronchoscopy. Written informed consent was obtained from the patients’ families for the publication of this case series and the accompanying images.
Case 1: A 65-year-old man presented with a chief complaint of shortness of breath lasting for 2 mo.
Case 2: A 58-year-old man presented with a chief complaint of difficulty swallowing lasting for 2 wk.
Case 3: A 61-year-old man presented with a chief complaint of coughing for 1 wk.
Case 1: There were no other symptoms.
Case 2: The patient presented with difficulty swallowing lasting for 2 wk, and there were no complaints of fever or any other symptoms.
Case 3: There were no other symptoms.
Case 1: The patient had a medical history of hypertension, diabetes mellitus, cerebral infarction, and stenoses of the bilateral carotid arteries, left subclavian artery, and bilateral iliac arteries. The patient had bilateral carotid stents and a left subclavian artery stent. His baseline blood pressure was approximately 130/80 mmHg.
Case 2: The patient had a medical history of cerebral infarction and myocardial infarction. Left vertebral stents and coronary stents were implanted. His baseline blood pressure was approximately 120/90 mmHg.
Case 3: The patient had a medical history of cerebral infarction for 3 mo and stenoses of the carotid artery and basilar artery. His baseline blood pressure was approximately 120/80 mmHg.
Imaging examinations
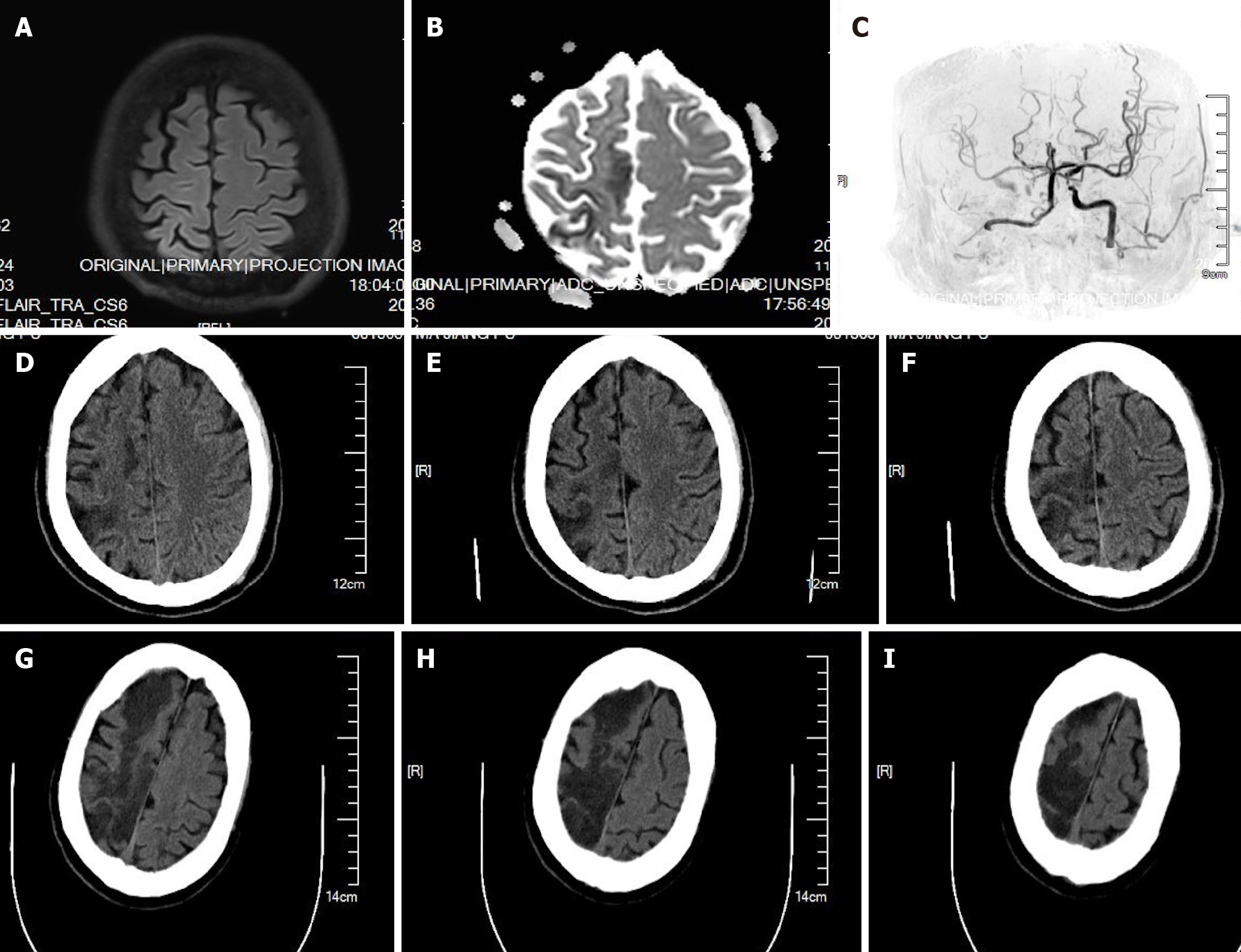
Case 1: Magnetic resonance imaging (MRI) (Figure 1A and B) and angiography (Figure 1C) performed 4 h after surgery showed right cerebral infarction, occlusion of the right internal carotid artery (ICA), and severe stenosis of the left ICA; Figure 1D and F shows computed tomography (CT) scans performed 1 d after surgery, which showed right cerebral infarction; Figure 1G-I showed CT scans performed 4 d after surgery, which showed right cerebral infarction progression.
Case 1: The patient was diagnosed with lung cancer in the right lower lobe.
Case 2: The patient was diagnosed with middle esophageal leiomyoma.
Case 3: The patient was diagnosed with multiple lung masses, an adrenal gland mass, and bone metastasis.
Case 1: Video-assisted thoracic surgery was performed for lung cancer in the right lower lobe. After anesthesia induction, a double-lumen tube was placed, and maintenance of anesthesia was performed with a combination of 0.5 minimum alveolar concentration (MAC) sevoflurane, propofol 3 mg/kg/h, and remifentanil 0.1 mcg/kg/min infusions. The depth of anesthesia was adjusted to maintain the blood pressure and saturation. The surgery commenced and progressed unremarkably. During the surgery, the patient experienced a 20-min period with a blood pressure of 90/60 mmHg, which was corrected by rapid crystalloid and dopamine infusion. His blood pressure was maintained at approximately 120/90 mmHg until the end of surgery. One-lung ventilation (OLV) was performed for 1 h, and a 5-min period with a saturation of 85% occurred, which was then corrected by alveolar recruitment maneuvers and a higher inspired oxygen fraction.
Case 2: The patient underwent thoracic surgery for middle esophageal leiomyoma. After anesthesia induction, a double-lumen tube was placed, and maintenance of anesthesia was performed with a combination of 0.5 MAC sevoflurane, propofol 3 mg/kg/h, and remifentanil 0.1 mcg/kg/min infusions. The depth of anesthesia was adjusted to maintain the blood pressure and saturation. OLV was performed for 2 h, and the lowest SPO2 was 92%, which was then corrected by a higher inspired oxygen fraction. There were no adverse events, such as intraoperative hypotension, hypertension, or hypoxia, throughout the operation.
Case 3: The patient underwent endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) for lymph node biopsy. After anesthesia induction, a laryngeal mask was inserted. The maintenance of anesthesia was performed with a combination of propofol 4 mg/kg/h and remifentanil 0.05 μg/kg/min infusions. There were no adverse events, such as intraoperative hypotension, hypertension, or hypoxia, throughout the operation.
Case 1: Three hours after the surgery, the patient suddenly presented left upper and lower limb weakness and unequal pupils (left:right 2.0:4.0 mm). An emergency CT scan showed right cerebral infarction. MRI and angiography showed right cerebral infarction, occlusion of the right ICA, and severe stenosis of the left ICA (Figure 1). Blood pressure management, intravenous fluids, aspirin, and atorvastatin were given. Recombinant tissue plasminogen activator (rt-PA) was not administered due to the risk of postoperative bleeding. Bilateral massive cerebral infarction was observed on a CT scan 12 d after the surgery. The patient died 14 d after the surgery.
Case 2: Ten hours after the surgery, the patient developed sudden left hemiplegia and unconsciousness. A CT scan showed right cerebral massive infraction. Blood pressure management, intravenous fluids, and atorvastatin were performed. Thirty-four hours after the surgery, right decompressive craniectomy was performed for brain herni
Case 3: The patient presented with a consciousness disturbance and lethargy at the postanesthetic care unit. An emergency CT scan showed left cerebral infarction. Then, CT angiography was performed, and left insula and frontotemporal parietal lobe infarction was found. Rt-PA was not administered. Blood pressure management, intravenous fluids, and atorvastatin were performed. Twenty-four hours after the surgery, bilateral massive cerebral infarction was observed on a CT scan. The patient died 3 d after the surgery.
Perioperative stroke is an important source of morbidity and mortality associated with noncardiac, nonvascular surgery, especially in elderly patients. Perioperative stroke has a mortality rate that ranges from 26% to as high as 87% in patients who have had a previous stroke compared to stroke in the general population, which has a mortality rate of 12.6%[2-5]. Here, we report three cases of massive cerebral stroke that occurred postoperatively in the thoracic department. Neurologic symptoms, including changes in mental status and hemiplegia, occurred within 10 h after surgery in the three patients; none of the patients received intra-arterial thrombolysis, and one patient underwent decompressive craniectomy. All three patients died after the surgery.
Intracranial atherosclerotic stenosis is a major cause of ischemic stroke, and all three patients in this case series had a medical history of intracranial atherosclerotic stenosis and cerebral infarction. Patients with pre-existing cerebrovascular disease demonstrate reduced cerebrovascular reserve and impaired cerebrovascular autoregulation, whereby vascular dilation is maximized distal to the sites of anatomical occlusion. Additional deleterious events such as hypotension and hypocapnia/hypercapnia throughout such vascular beds during surgery may further predispose these patients to hypoxic-ischemic injury[6]. In addition, patients with cerebral arterial stenosis may demonstrate increased oxygen extraction, compromised cerebral blood flow, and inadequate cerebral perfusion. These physiological vulnerabilities may lead to perioperative stroke in high-risk patients. Therefore, the Society for Neuroscience in Anesthesiology and Critical Care has suggested delaying elective surgical cases for at least 9 mo after a prior stroke in their newest guideline[6,7]. In this case series, case 3 had a previous stroke 3 mo before the surgery. If surgery needs to take place sooner, the patient’s blood pressure should be meticulously monitored during and after the surgery, and anesthesiologists should monitor the possibility of cerebral ischemia using transcranial Doppler, cerebral oximetry, or neurophysiology such as electro
Hypoperfusion is believed to be the most common cause of perioperative stroke for high-risk patients. Case 1 in this case series experienced hypotension during surgery. However, because of the rarity of stroke, the lack of standardized definitions for baseline blood pressure and intraoperative hypotension, and the time period during which hypotension may be most deleterious (i.e., intraoperative vs postoperative), some cohort studies have shown that hypotension during surgery is not a risk factor for perioperative stroke[2,9]. In general, the maintenance of mean or systolic blood pressures within 20% of baseline during surgery is an adequate target for cerebral perfusion pressure[8]. Hypotension will augment the injury produced by embolism or other causes, and this may be especially important in the postoperative period, during which monitoring is not nearly as attentive as in the operating room[10].
Intraoperative hypoxemia is a common complication during OLV for thoracic surgery. This is commonly associated with profound pathophysiological changes in OLV. Case 1 in this series had suffered from hypoxemia for 5 min. Protective lung ventilation strategies, including recruitment maneuvers and ventilation with sufficient positive end-expiratory pressure, may be helpful for the improvement of hypoxia. Tidal volumes of no more than 6 mL/kg should be set. Hyperventilation should be avoided, and the remaining normocarbic levels may prevent any further risk of cerebrovascular compromise[11]. Since hypoxic pulmonary vasoconstriction is inhibited by volatile anesthetics, some anesthesiologists preferentially select total intravenous anesthesia to avoid hypoxemia due to intrapulmonary blood shunting[12]. Shelley et al[13] reported an incidence of 2.3% of unplanned intensive care unit (ICU) admission among 7431 cases of lung resection performed in 16 United Kingdom thoracic surgical centers. Multivariate analysis indicated that unplanned ICU admissions were less frequent in patients receiving intravenous anesthesia than in those receiving inhalational anesthesia. However, the notion that anesthetic techniques may modulate perioperative stroke risk in certain patient populations certainly deserves further investigation[9].
Regional techniques such as thoracic epidural anesthesia or paravertebral blockade may be an advantageous anesthetic strategy for thoracic surgery[14]. Multimodal opiate-sparing regimens can reduce the dosage of analgesics and the concentrations of volatile anesthetics, avoid depressed cardiac output, and decrease blood pressure during surgery. Furthermore, it can provide superior analgesia during the first three postoperative days. Insufficient pain control may increase the risk of stroke during the postoperative period due to depression of the sympathetic nerve activity and affect locomotor and respiratory muscle function[15,16].
EBUS-TBNA is believed to be a minimally invasive and highly safe procedure, and the evidence has shown that it is flexible in patients with malignant space-occupying brain lesions[17], but whether it is safe in patients with cerebrovascular disease, especially in those who have had a previous stroke, is unclear. General anesthesia was performed in case 3 to decrease the incidence of coughing, hypercapnia, and sympathetic stimulation. However, moderate or deep sedation may be acceptable for high-risk patients to avoid hemodynamic fluctuations during EBUS-TBNA[18].
Continued clinical recognition of stroke symptoms along with timely neuroimaging is paramount for high-risk patients. A multidisciplinary consultation involving neurology, the primary surgical service, interventional neuroradiology, and anesthe
Perioperative covert stroke is an acute brain infarction detected on MRI after surgery without any stroke symptoms. Most postoperative covert strokes are not diagnosed because they are not clinically apparent. The NeuroVISION study (Perioperative covert stroke in patients undergoing non-cardiac surgery) described that it was more common that covert stroke was associated with an increased risk of cognitive decline and overt stroke or transient ischemic attack 1 year after noncardiac surgery. Embolic events and perioperative hemodynamic variations may have contributed to postoperative covert stroke in this study[21]. Covert stroke and overt stroke both need more research to investigate the risk factors and to establish prevention and management strategies in the perioperative setting.
Perioperative massive cerebral stroke may be more likely to occur if there are pre-existing factors, including previous stroke, hypotension, and hypoxemia, in thoracic surgery patients. Sufficient pain control after surgery and timely neurology consul
Manuscript source: Unsolicited manuscript
Specialty type: Anesthesiology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bianco L, Gottschalk A S-Editor: Liu M L-Editor: Wang TQ P-Editor: Yuan YY
| 1. | Wong GY, Warner DO, Schroeder DR, Offord KP, Warner MA, Maxson PM, Whisnant JP. Risk of surgery and anesthesia for ischemic stroke. Anesthesiology. 2000;92:425-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Hsieh JK, Dalton JE, Yang D, Farag ES, Sessler DI, Kurz AM. The Association Between Mild Intraoperative Hypotension and Stroke in General Surgery Patients. Anesth Analg. 2016;123:933-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 3. | Bateman BT, Schumacher HC, Wang S, Shaefi S, Berman MF. Perioperative acute ischemic stroke in noncardiac and nonvascular surgery: incidence, risk factors, and outcomes. Anesthesiology. 2009;110:231-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 210] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 4. | Wilcox T, Smilowitz NR, Xia Y, Berger JS. Cardiovascular Risk Scores to Predict Perioperative Stroke in Noncardiac Surgery. Stroke. 2019;50:2002-2006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Mashour GA, Shanks AM, Kheterpal S. Perioperative stroke and associated mortality after noncardiac, nonneurologic surgery. Anesthesiology. 2011;114:1289-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 237] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 6. | Vlisides PE, Moore LE, Whalin MK, Robicsek SA, Gelb AW, Lele AV, Mashour GA. Perioperative Care of Patients at High Risk for Stroke During or After Non-cardiac, Non-neurological Surgery: 2020 Guidelines From the Society for Neuroscience in Anesthesiology and Critical Care. J Neurosurg Anesthesiol. 2020;32:210-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 7. | Jørgensen ME, Torp-Pedersen C, Gislason GH, Jensen PF, Berger SM, Christiansen CB, Overgaard C, Schmiegelow MD, Andersson C. Time elapsed after ischemic stroke and risk of adverse cardiovascular events and mortality following elective noncardiac surgery. JAMA. 2014;312:269-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 121] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 8. | Ng JL, Chan MT, Gelb AW. Perioperative stroke in noncardiac, nonneurosurgical surgery. Anesthesiology. 2011;115:879-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 137] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 9. | Vlisides P, Mashour GA. Perioperative stroke. Can J Anaesth. 2016;63:193-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 10. | Meng L, Li J, Flexman AM, Tong C, Zhou X, Gelb AW, Wang T, McDonagh DL. Perceptions of Perioperative Stroke Among Chinese Anesthesiologists: Starting a Long March to Eliminate This Underappreciated Complication. Anesth Analg. 2019;128:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Akça O. Optimizing the intraoperative management of carbon dioxide concentration. Curr Opin Anaesthesiol. 2006;19:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Schilling T, Kozian A, Senturk M, Huth C, Reinhold A, Hedenstierna G, Hachenberg T. Effects of volatile and intravenous anesthesia on the alveolar and systemic inflammatory response in thoracic surgical patients. Anesthesiology. 2011;115:65-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 13. | Shelley BG, McCall PJ, Glass A, Orzechowska I, Klein AA; Association of Cardiothoracic Anaesthesia and collaborators. Association between anaesthetic technique and unplanned admission to intensive care after thoracic lung resection surgery: the second Association of Cardiothoracic Anaesthesia and Critical Care (ACTACC) National Audit. Anaesthesia. 2019;74:1121-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Kozian A, Kretzschmar MA, Schilling T. Thoracic anesthesia in the elderly. Curr Opin Anaesthesiol. 2015;28:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Binbin Z, Yutao W, Chengwei Z. Postoperative Cerebral Embolism After video-assisted thoracoscopic left upper lobectomy : A Case Report and Literature Review. J Stroke Cerebrovasc Dis. 2019;28:e139-e142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Licker M. Anaesthetic management and unplanned admission to intensive care after thoracic surgery. Anaesthesia. 2019;74:1083-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Grosu HB, Morice RC, Sarkiss M, Bashoura L, Eapen GA, Jimenez CA, Faiz S, Lazarus DR, Casal RF, Ost DE. Safety of flexible bronchoscopy, rigid bronchoscopy, and endobronchial ultrasound-guided transbronchial needle aspiration in patients with malignant space-occupying brain lesions. Chest. 2015;147:1621-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Wahidi MM, Herth F, Yasufuku K, Shepherd RW, Yarmus L, Chawla M, Lamb C, Casey KR, Patel S, Silvestri GA, Feller-Kopman DJ. Technical Aspects of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration: CHEST Guideline and Expert Panel Report. Chest. 2016;149:816-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 292] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 19. | Saltman AP, Silver FL, Fang J, Stamplecoski M, Kapral MK. Care and Outcomes of Patients With In-Hospital Stroke. JAMA Neurol. 2015;72:749-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 20. | Dong Y, Cao W, Cheng X, Fang K, Zhang X, Gu Y, Leng B, Dong Q. Risk Factors and Stroke Characteristic in Patients with Postoperative Strokes. J Stroke Cerebrovasc Dis. 2017;26:1635-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | NeuroVISION Investigators. Perioperative covert stroke in patients undergoing non-cardiac surgery (NeuroVISION): a prospective cohort study. Lancet. 2019;394:1022-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 170] [Article Influence: 28.3] [Reference Citation Analysis (0)] |