Published online May 6, 2021. doi: 10.12998/wjcc.v9.i13.3147
Peer-review started: November 24, 2020
First decision: January 24, 2021
Revised: January 29, 2021
Accepted: March 5, 2021
Article in press: March 5, 2021
Published online: May 6, 2021
Processing time: 143 Days and 5 Hours
Giant cell tumors (GCT) are most commonly seen in the distal femur. These tumors are uncommon in the small bones of the hand and feet, and a very few cases have been reported. A giant cell tumor of the talus is rarely seen clinically and could be a challenge to physicians.
We report a rare case of GCT of the talus in one patient who underwent a new reconstructive surgery technique using a three-dimensional (3D) printing talar prosthesis. The prosthesis shape was designed by tomographic image processing and segmentation using technology to match the intact side by mirror symmetry with 3D post-processing technologies. The patient recovered nearly full range of motion of the ankle after 6 mo. The visual analogue scale and American Orthopaedic Foot and Ankle Society scores were 1 and 89 points, respectively.
We demonstrated that 3D printing of a talar prosthesis is a beneficial option for GCT of the talus.
Core Tip: Three-dimensional printing technology has been widely used in orthopedics. The purpose of this study is to evaluate the clinical results of the treatment of giant cell tumor of talus bone using a three-dimensional printing personalized talus prosthesis designed by our team. The casting process of this prosthesis is also discussed. Compared with other customized three-dimensional printing talus prostheses, our talus prosthesis is personalized and accurately constructed according to the anatomical data of the patient's normal foot.
- Citation: Yang QD, Mu MD, Tao X, Tang KL. Three-dimensional printed talar prosthesis with biological function for giant cell tumor of the talus: A case report and review of the literature. World J Clin Cases 2021; 9(13): 3147-3156
- URL: https://www.wjgnet.com/2307-8960/full/v9/i13/3147.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i13.3147
Malignant tumors around the foot and ankle are rare, accounting for less than 1%[1] of all malignant tumors. However, 22%-39.2% of tumors occurring in these locations are malignant[2]. Tumors most commonly occur at the metatarsals and calcaneus followed by the phalanges and talus.
Giant cell tumors (GCTs), also called osteoclastomas, account for approximately 5% of bone tumors and 20% of benign bone tumors[3,4]. The highest incidence rate of GCTs occurs between the ages of 20 and 40 years with a peak at the age of 30. The incidence rate before the age of 10 is only approximately 1%[5]. These tumors often occur in individuals aged 30-40 years old and rarely occur in individuals under 20 years of age. For giant cell tumor of bone, it is important to completely remove tumor cells to prevent recurrence.
For bone tumors of the talus, currently available treatment methods include partial talus resection[6] and fusion surgery[7]. The more widely accepted surgical method is fusion surgery, including talocrural arthrodesis and shortening arthrodesis between the calcaneus and the tibia[8,9]. However, the loss of ankle joint function caused by fusion surgery is a problem that we cannot ignore. Fusion and other surgical methods seem to be difficult to deal with significantly large talar tumors. In particular, some studies have reported that complications, such as loss of motion of the ankle and adjacent joints and shortening of the affected limb after fusion surgery, are unacceptable to young patients[9].
With the development of three-dimensional (3D) printing technology, this methodology has been widely used in clinical practice and has yielded good re
A 22-year-old patient visited our hospital because of pain in the left ankle for half a year.
The pain was persistent, became aggravated during walking and weight bearing, and was relieved during rest. The pain was associated with limited mobility of the ankle joint.
The patient had a free previous medical history.
The skin of the patient's foot was in good condition without swelling and ulceration. There was obvious tenderness in the ankle of the patient. Ankle range of motion was slightly limited
The patient's preoperative laboratory examination was unremarkable, and a patho
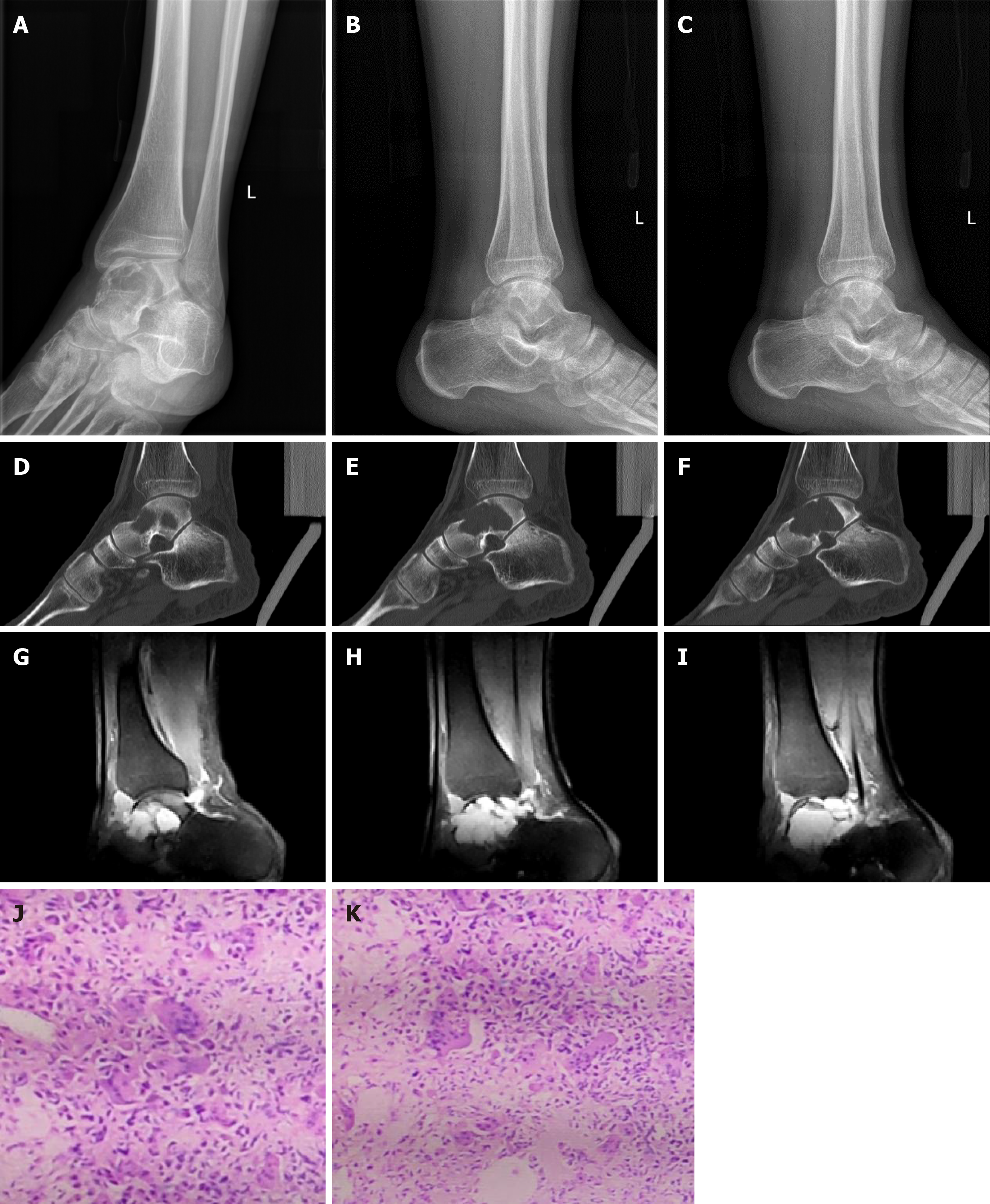
Computerized tomography (CT), magnetic resonance imaging (MRI), and whole-body bone scans were performed at our hospital, and the imaging findings suggested that the patient had a GCT of the talus bone, which was also demonstrated by pathological examination
Giant cell tumor of the left talus.
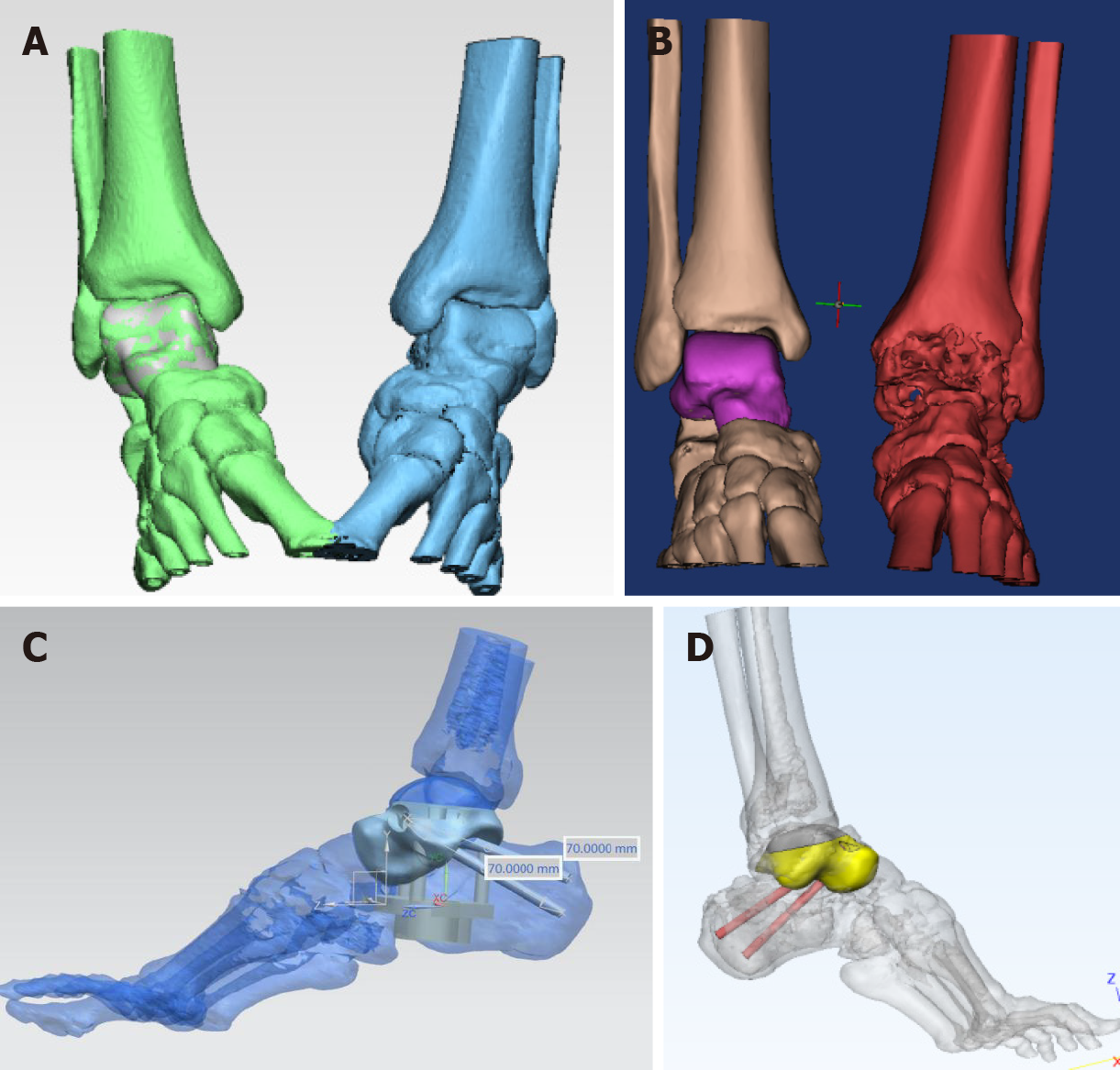
Complete data of the affected area were acquired by CT image processing and segmentation using 3D CT postprocessing technology (Figure 2A), and the 3D raw data of the affected side were obtained by reconstruction and matching performed by mirror and data registration technology (Figure 2B). Severe defects in the data of the necrotic talus were repaired using reverse repair technology so that non-defective raw data could be used for talar reconstruction. Then, the data of the tibiotalar and subtalar articular facets were analyzed and processed (Figure 2C). After collecting complete patient talus data, an electron beam 3D printer ARCAM Q10 (Sweden, GE) with a maximum print scanning speed of 8000 m/s and a layer thickness of 0.05 mm was used to print the talus prosthesis. Finally, accurate 3D reconstruction of the talar prosthesis was completed (Figure 2D).
The column of the talar prosthesis at the calcaneal side and the position of the cannulated screws for fixation of the subtalar joints were determined. Then, the talar prosthesis was located after drilling was performed in accordance with the test model. The 3D-printed structure was made porous on the sides of the subtalar joints. Full-range 3D printing was completed using the Arcam EBM Q10 system (United States). The specific casting process included mirror polishing of the tibial articular surface, polishing and trimming of the talus matrix, ultrasonic cleaning, fine cleaning, and drying. Finally, the articular surfaces of the talus and matrix were assembled and reviewed in the purification workshop and were packaged after sterilization. Titanium alloy powder was used as the talar structure material, and cobalt-chromium-molybdenum alloy powder was used as the articular facet material. The high-precision dovetail slot design process and screw channel fixation of the prosthetic tibiotalar articular facet were completed after assembly. The articular facet was subjected to bright polishing. Co-Cr-Mo material, which is more resistant to the friction between the talus and tibia, was used on the tibia side (Figure 3A and B). The articular surface of that subtalar joint was printed with micropores to increase surface roughness and to take advantage of the property of TI6-Al-4V alloy to promote bone growth (Figure 3C).
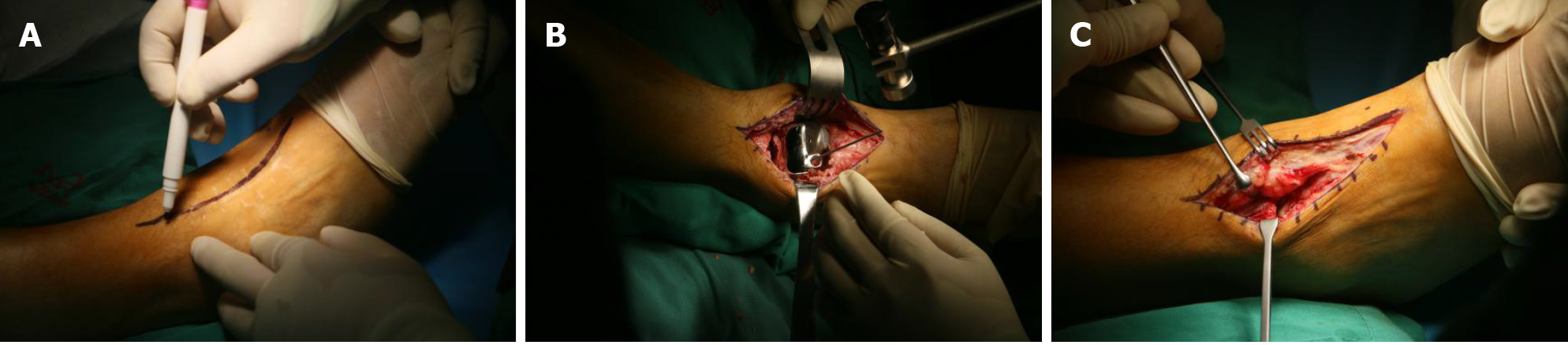
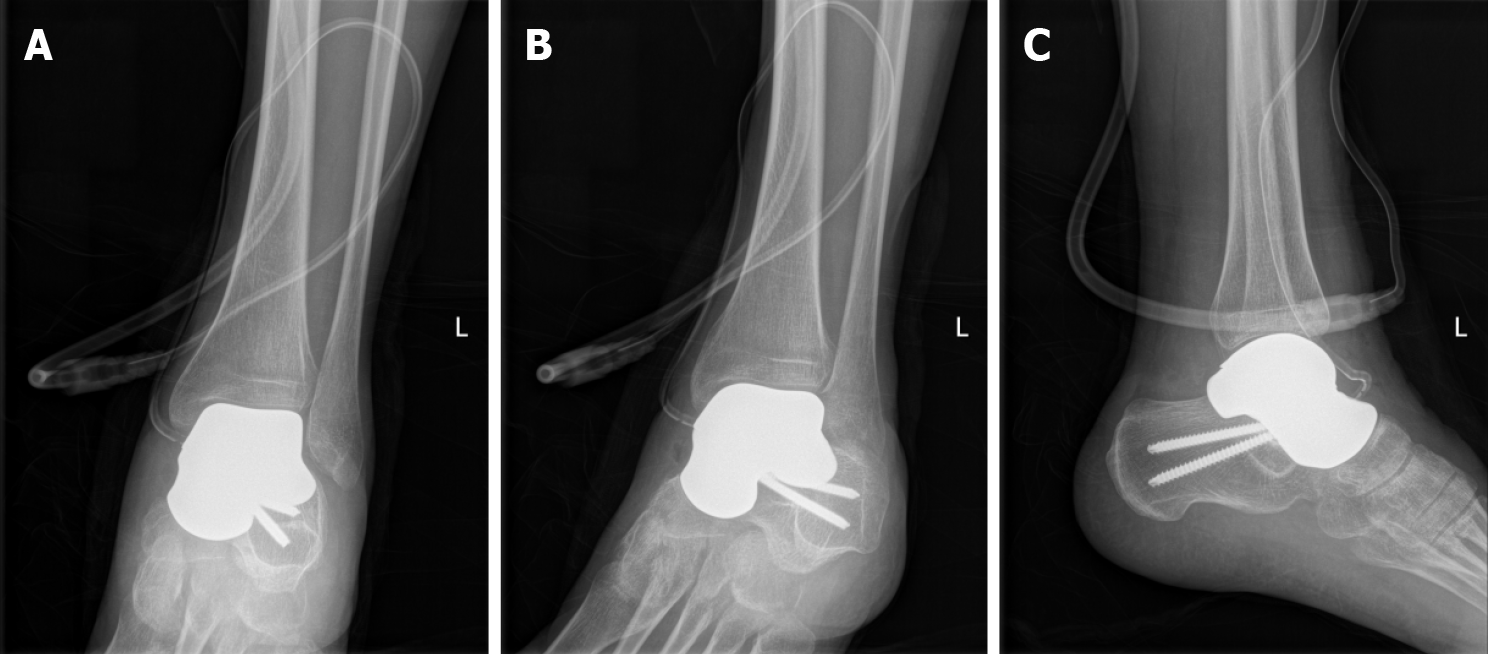
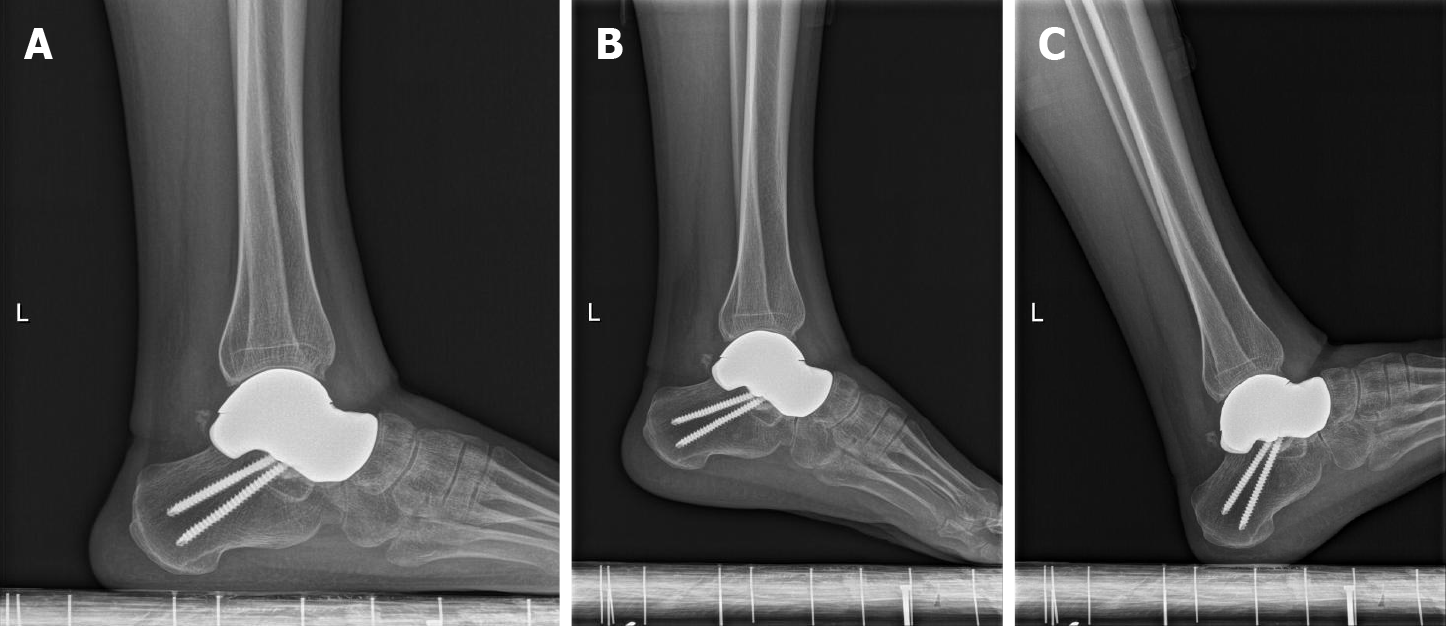
The innovative surgical technique was performed with the patient in a supine position under combined spinal epidural analgesia. A long incision in the middle of the ankle was made, and subcutaneous tissue was cut layer by layer, exposing the neck of the talus bone. The denatured and necrotic talus was broken with a narrow osteotome, and the talus was completely removed. After the hyperplastic synovium and cystic degeneration tissue were cleared, a pathological culture was obtained. The denatured subtalar articular cartilage was completely excised with a wide osteotome and remained fresh until the subchondral bone tissue was exposed. The articular cavity and bone fragments were washed with physiological saline and a large amount of iodophor. Then, the ankle joint was flexed. The 3D printed talus prosthesis was implanted into the articular cavity until it completely fit the subtalar joint, and the ankle joint was moved again so that it adapted completely to the ankle joint. The prosthesis was fixed to the calcaneus with two titanium screws. Under fluoroscopy, the prosthesis was observed to be in a good position, and the ankle joint was able to move. A drainage tube was inserted. Then, the skin and subcutaneous tissue were sutured layer by layer (Figure 4). Imaging data on the day after operation are presented in Figure 5.
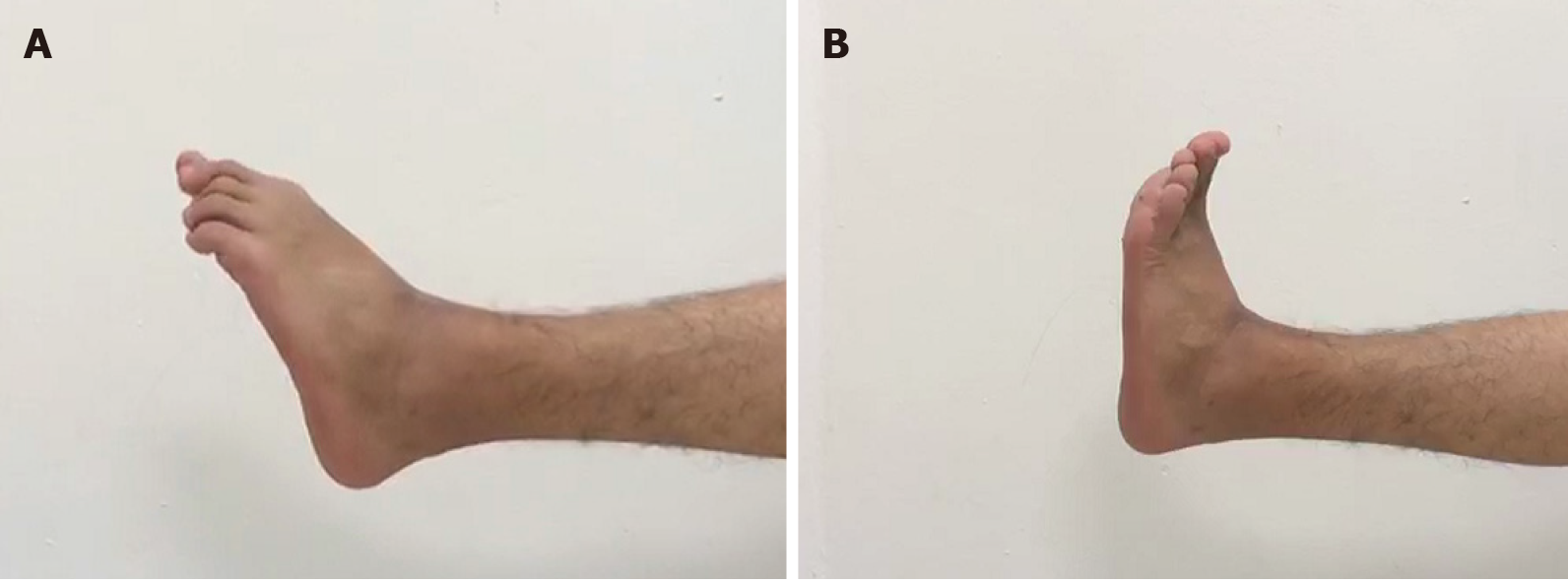
The patient was able to move his ankle with mild pain during sport activities 6 mo postoperatively and nearly full range of motion and grasp force of the ankle was achieved in 12 mo (Table 1). Weakness and numbness of the ankle were not observed (Figures 6 and 7).
| The affected ankle | The intact ankle | |
| Dorsal extension | 10 | 18 |
| Palmar flexion | 15 | 22 |
Degenerative arthritis and prosthetic dislocation were not detected on plain radiographs. The 3D printing talus prosthesis was placed in the original anatomic position. The VAS scores and AOFAS scores were 1 point and 89 points, respectively.
In addition, we measured the talar arc length, talar height, talar width, tibial alignment angle, talar tilt angle, Bohler's angle, and Meary's angle preoperatively and postoperatively. We found that talar height and Meary's angle were significantly changed (Table 2).
| Before surgery | At the last visit | |
| Talar arc length (mm) | 60 | 58 |
| Talar height (mm) | 30 | 36 |
| Talar width (mm) | 43 | 45 |
| Tibial alignment angle (°) | 84 | 86 |
| Talar tilt angle (°) | 2 | 3 |
| Bohler’s angle (°) | 43 | 45 |
| Meary’s angle (°) | 11 | 9 |
GCTs are benign tumors that have a tendency to exhibit local aggressiveness and have a high risk of recurrence. The most common sites are the distal end of the femur, upper end of the tibia, and lower end of the radius[15]. GCTs of the talus are rare in clinical practice and are challenging to treat. In the surgical management of malignancy of the talus, it is difficult to achieve both adequate surgical margins and functional reconstruction.
One treatment option is partial talus resection. Since the talus is located at the ankle, most conventional surgical approaches cannot completely expose the talus, and there is an area occluded in the field of vision. Moreover, GCTs of the talus often invade the whole talus, so it is difficult to clear them completely. Therefore, curettage for GCTs of the talus is associated with a high recurrence rate. The local recurrence rate can be reduced by additionally performing procedures, such as carbolic acid smearing and bone cement filling. However, the recurrence rate is still as high as 30%[8].
Another widely accepted option is arthrodesis. Dennison et al[9] reported a case of GCT of the talus treated by talus resection and tibial calcaneal fusion. There was no recurrence at 18 mo after the operation. However, the postoperative recovery process was long[16], and there was limited ankle function[17] due to arthrodesis.
Inspired by the recent success in treating stage IIIc Kienböck's disease with a 3D printed lunar prosthesis[18], our team successfully used 3D printing to generate a personalized talus prosthesis with biological function replacement technology to treat seven patients with severe talus collapse and necrosis. Moreover, the tumor in this case is too large to be treated using conventional treatment; therefore, we attempted to treat this case by 3D printing technology to completely solve the problem of easy recurrence of giant cell tumor of bone and restore the patient's walking function close to normal. In fact, most medical applications of 3D printing technology have been prostheses[19]. Three-dimensional printing technology serves as an innovative method of fabricating the complex shape and structure of the talus[20,21]. The talus imaging data were segmented and reconstructed in 3D based on CT and MRI data. Three-dimensional printing technology enables surgeons to design and manufacture anatomically matched implants for use in surgical operations[22]. In our study, the complete talus model was matched by comparing the healthy side and the affected side using mirror symmetry and multidimensional computer reconstruction techniques.
The operation was performed by an experienced foot and ankle surgeon who had performed more than 10000 operations. Our team also used 3D printed personalized prostheses with biological function for other cases who were suffering from irreversible ankle osteoarthritis. All of the patients exhibited good clinical effects as demonstrated by significant changes in AOFAS and VAS scores. The trend of imaging manifestation is similar to that noted in the study performed by Tracey et al[23] in 2019. Among these patients, the fixation methods adopted include subtalar joint and talonavicularis joint fixation and subtalar joint fixation only. In this case study, the fixation method used was subtalar joint fixation only. All prostheses are made of Ti-6Al-4V alloy. Given its superior biological properties (bone ingrowth characteristics), obvious bone ingrowth is observed in the postoperative imaging follow-up process both in the cases where the subtalar joint and the talonavicularis joint were previously fixed, and in this case where only the subtalar joint was fixed. Although the operation sacrificed the range of motion of the subtalar joint and the talonavicularis joint, the range of motion of the ankle joint was preserved to the greatest extent, and all patients were able to return to normal life.
Regarding talus prostheses for replacement operations, the first reported talus prosthesis replacement was completed and published by Harnroongroj and Vanadurongwan[24] in 1997. All of the eight patients whom they treated exhibited good prognoses within 11-15 years. However, in their subsequent studies, the long-term complication that occurred was loosening of the neck region of the talus prosthesis[25], which is related to the design of the first-generation prosthesis. The first research report on the second-generation talus prosthesis was completed by Taniguchi et al[26]. In total, 12 patients were treated with the second-generation prosthesis, and the results showed good clinical efficacy within 7 years[26]. To date, there have been some studies on the third generation of talus prostheses, and all of them have demonstrated good therapeutic effects[27-29] within a short-term follow-up period. Other case reports have also confirmed the clinical efficacy of the third-generation talus prostheses, and third-generation talus prostheses[30,31] are recommended.
Unlike other prostheses, this prosthesis has the following advantages: (1) All parts of the 3D printed structure are designed according to the functional anatomy of talus and Wolff's Law, which solves the problem regarding the anatomical and biome
Furthermore, this study has some limitations. First, the follow-up time was too short. The prognoses in terms of the prosthesis's clinical function should be assessed over a longer period. We will continue to follow-up this patient for a long-term follow-up study. Second, the 3D printed, personalized talus prosthesis replacement surgery was performed relatively late. To date, only seven patients in the world have undergone our surgery. The team is also performing relevant research on the biomechanical mechanism of prosthesis replacement. In summary, studies on many topics need to be conducted in the future.
We demonstrated that 3D printing of a talar prosthesis is a beneficial option for GCT of the talus.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lee TC S-Editor: Zhang L L-Editor: Wang TQ P-Editor: Yuan YY
| 1. | Bang JS, Adsul N, Lim JH, Jang IT. Extra-Osseous Ewing Sarcoma of Sciatic Nerve Masquerading as Benign Nerve Sheath Tumor and Presented as Lumbar Radiculopathy: Case Report and Review of Literature. World Neurosurg. 2018;115:89-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Azevedo CP, Casanova JM, Guerra MG, Santos AL, Portela MI, Tavares PF. Tumors of the foot and ankle: a single-institution experience. J Foot Ankle Surg. 2013;52:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Yayan J. Denosumab for Effective Tumor Size Reduction in Patients With Giant Cell Tumors of the Bone: A Systematic Review and Meta-Analysis. Cancer Control. 2020;27:1073274820934822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Hara H, Kawamoto T, Onishi Y, Fujioka H, Nishida K, Kuroda R, Kurosaka M, Akisue T. Reconstruction of the Midfoot Using a Free Vascularized Fibular Graft After En Bloc Excision for Giant Cell Tumor of the Tarsal Bones: A Case Report. J Foot Ankle Surg. 2016;55:838-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Li J, Zhou J, Liu Y, Sun X, Song W. Comprehensive treatment for multicentric giant cell tumors of the pelvis and spine using apatinib: A case report and literature review. J Cancer Res Ther. 2020;16:1020-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Kamoun K, Sellami T, Jlailia Z, Abid L, Jenzri M, Bouaziz M, Zouar O. Giant cell reparative granuloma of the hallux following enchondroma. Pan Afr Med J. 2015;22:363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Ch L, Th L. Giant Cell Tumor of the Peroneus Brevis Tendon Sheath. J Orthop Case Rep. 2015;5:68-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Melenevsky Y, Mackey RA, Abrahams RB, Thomson NB 3rd. Talar Fractures and Dislocations: A Radiologist's Guide to Timely Diagnosis and Classification. Radiographics. 2015;35:765-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Dennison MG, Pool RD, Simonis RB, Singh BS. Tibiocalcaneal fusion for avascular necrosis of the talus. J Bone Joint Surg Br. 2001;83:199-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 10. | Tetsworth K, Block S, Glatt V. Putting 3D modelling and 3D printing into practice: virtual surgery and preoperative planning to reconstruct complex post-traumatic skeletal deformities and defects. SICOT J. 2017;3:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 11. | Lal H, Patralekh MK. 3D printing and its applications in orthopaedic trauma: A technological marvel. J Clin Orthop Trauma. 2018;9:260-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 142] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 12. | Hamid KS, Parekh SG, Adams SB. Salvage of Severe Foot and Ankle Trauma With a 3D Printed Scaffold. Foot Ankle Int. 2016;37:433-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 13. | Kitaoka HB, Alexander IJ, Adelaar RS, Nunley JA, Myerson MS, Sanders M. Clinical rating systems for the ankle-hindfoot, midfoot, hallux, and lesser toes. Foot Ankle Int. 1994;15:349-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3199] [Cited by in RCA: 3115] [Article Influence: 100.5] [Reference Citation Analysis (0)] |
| 14. | Ibrahim T, Beiri A, Azzabi M, Best AJ, Taylor GJ, Menon DK. Reliability and validity of the subjective component of the American Orthopaedic Foot and Ankle Society clinical rating scales. J Foot Ankle Surg. 2007;46:65-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 493] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 15. | Bapat MR, Narlawar RS, Pimple MK, Bhosale PB. Giant cell tumour of talar body. J Postgrad Med. 2000;46:110-111. [PubMed] |
| 16. | Han Q, Liu Y, Chang F, Chen B, Zhong L, Wang J. Measurement of talar morphology in northeast Chinese population based on three-dimensional computed tomography. Medicine (Baltimore). 2019;98:e17142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Morash J, Walton DM, Glazebrook M. Ankle Arthrodesis Versus Total Ankle Arthroplasty. Foot Ankle Clin. 2017;22:251-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 18. | Xie MM, Tang KL, Yuan CS. 3D printing lunate prosthesis for stage IIIc Kienböck's disease: a case report. Arch Orthop Trauma Surg. 2018;138:447-451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Kuehn BM. Clinicians Embrace 3D Printers to Solve Unique Clinical Challenges. JAMA. 2016;315:333-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | MacDonald E, Wicker R. Multiprocess 3D printing for increasing component functionality. Science. 2016;353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 242] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 21. | Berry DB, You S, Warner J, Frank LR, Chen S, Ward SR. * A 3D Tissue-Printing Approach for Validation of Diffusion Tensor Imaging in Skeletal Muscle. Tissue Eng Part A. 2017;23:980-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Lee N. The Lancet Technology: 3D printing for instruments, models, and organs? Lancet. 2016;388:1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Tracey J, Arora D, Gross CE, Parekh SG. Custom 3D-Printed Total Talar Prostheses Restore Normal Joint Anatomy Throughout the Hindfoot. Foot Ankle Spec. 2019;12:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 24. | Harnroongroj T, Vanadurongwan V. The talar body prosthesis. J Bone Joint Surg Am. 1997;79:1313-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 81] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Harnroongroj T, Harnroongroj T. The Talar Body Prosthesis: Results at Ten to Thirty-six Years of Follow-up. J Bone Joint Surg Am. 2014;96:1211-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 26. | Taniguchi A, Tanaka Y. An Alumina Ceramic Total Talar Prosthesis for Avascular Necrosis of the Talus. Foot Ankle Clin. 2019;24:163-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Ando Y, Yasui T, Isawa K, Tanaka S, Tanaka Y, Takakura Y. Total Talar Replacement for Idiopathic Necrosis of the Talus: A Case Report. J Foot Ankle Surg. 2016;55:1292-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Tonogai I, Hamada D, Yamasaki Y, Wada K, Takasago T, Tsutsui T, Goto T, Sairyo K. Custom-Made Alumina Ceramic Total Talar Prosthesis for Idiopathic Aseptic Necrosis of the Talus: Report of Two Cases. Case Rep Orthop. 2017;2017:8290804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Stevens BW, Dolan CM, Anderson JG, Bukrey CD. Custom talar prosthesis after open talar extrusion in a pediatric patient. Foot Ankle Int. 2007;28:933-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Tsukamoto S, Tanaka Y, Maegawa N, Shinohara Y, Taniguchi A, Kumai T, Takakura Y. Total talar replacement following collapse of the talar body as a complication of total ankle arthroplasty: a case report. J Bone Joint Surg Am. 2010;92:2115-2120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 31. | Regauer M, Lange M, Soldan K, Peyerl S, Baumbach S, Böcker W, Polzer H. Development of an internally braced prosthesis for total talus replacement. World J Orthop. 2017;8:221-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Veronesi F, Torricelli P, Martini L, Tschon M, Giavaresi G, Bellini D, Casagranda V, Alemani F, Fini M. An alternativeex vivomethod to evaluate the osseointegration of Ti-6Al-4V alloy also combined with collagen. Biomed Mater. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |