Published online May 6, 2021. doi: 10.12998/wjcc.v9.i13.3140
Peer-review started: December 17, 2020
First decision: January 24, 2021
Revised: February 7, 2021
Accepted: March 4, 2021
Article in press: March 4, 2021
Published online: May 6, 2021
Processing time: 125 Days and 22.8 Hours
Rearrangements of the anaplastic lymphoma kinase (ALK) gene (ALK-positive) represent an oncogenic driver in approximately 3%-5% of non-small-lung cancer (NSCLC) patients. Sarcoidosis is a multisystem disease, and its reported incidence in Asia is 1 or less per 100000 people per year. The co-occurrence of sarcoidosis and ALK-positive NSCLC is rare, and ALK-positive lung cancer is likely to spread quickly. Therefore, the co-occurrence of sarcoidosis is more easily misdiagnosed as metastatic lung cancer by radiological examination.
A 50-year-old man had a nodule in the left superior lobe, many small nodules in left superior and right lungs, and enlarged bilateral hilar, mediastinal, and right supraclavicular lymph nodes. Computed tomography-guided pulmonary biopsy of the nodule in the left superior lobe revealed echinoderm microtubule-associated protein-like 4 gene-ALK positive NSCLC with concomitant non
Our experience illustrates that pathological evidence is needed to confirm metastatic disease, especially when some suspected metastatic lesions are negative for malignancy.
Core Tip: The co-occurrence of sarcoidosis and echinoderm microtubule-associated protein-like 4 anaplastic lymphoma kinase (ALK)-positive non-small-cell lung cancer is rare. Here, we present one case of sarcoidosis mimicking metastases in an echi
- Citation: Chen X, Wang J, Han WL, Zhao K, Chen Z, Zhou JY, Shen YH. Sarcoidosis mimicking metastases in an echinoderm microtubule-associated protein-like 4 anaplastic lymphoma kinase positive non-small-lung cancer patient: A case report. World J Clin Cases 2021; 9(13): 3140-3146
- URL: https://www.wjgnet.com/2307-8960/full/v9/i13/3140.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i13.3140
Rearrangements of the anaplastic lymphoma kinase (ALK) gene (ALK-positive) represent an oncogenic driver in approximately 3%-5% of non-small-cell lung cancer (NSCLC) patients[1,2]. The most common ALK partner is the echinoderm microtubule-associated protein-like 4 gene (EML4) and generates EML4-ALK fusion transcripts[3]. ALK tyrosine kinase inhibitors, including crizotinib, ceritinib, and alectinib, are the standard first-line treatment for advanced ALK positive NSCLC patients.
Sarcoidosis is a multisystem disease characterized by persistent granulomatous inflammation, affecting the intrathoracic region and lungs in over 90% of cases[4]. The reported incidence of sarcoidosis in Asia is 1 or less per 100000 people per year[5,6]. The co-occurrence of sarcoidosis and ALK positive NSCLC is rare. Here, we present one interesting case of sarcoidosis mimicking metastases in an EML4-ALK positive NSCLC patient.
A 50-year-old man was admitted to our hospital for chest tightness and a productive cough.
The patient reported that the chest tightness and productive cough have lasted 2 wk.
The patient had no history of past illness.
The patient was a former heavy smoker and a former heavy drinker.
There were no physical findings to note.
TSPOT-tuberculosis test was negative. His tumor marker levels were as follows: carcinoembryonic antigen 2.1 (0.0-5.0 ng/mL), carbohydrate antigen 199 2.3 (0.0-37.0 ng/mL), cancer antigen 125 115.9 (0.0-35.0 ng/mL), neuron-specific enolase 15.8 (0-30.0 ng/mL), C-terminus of cytokeratin 19 7.0 (0-7.0 ng/mL), squamous cell carcinoma antigen 0.9 (0.0-1.5 ng/mL), and cancer antigen 724 20.8 (0.0-16.4 ng/mL).
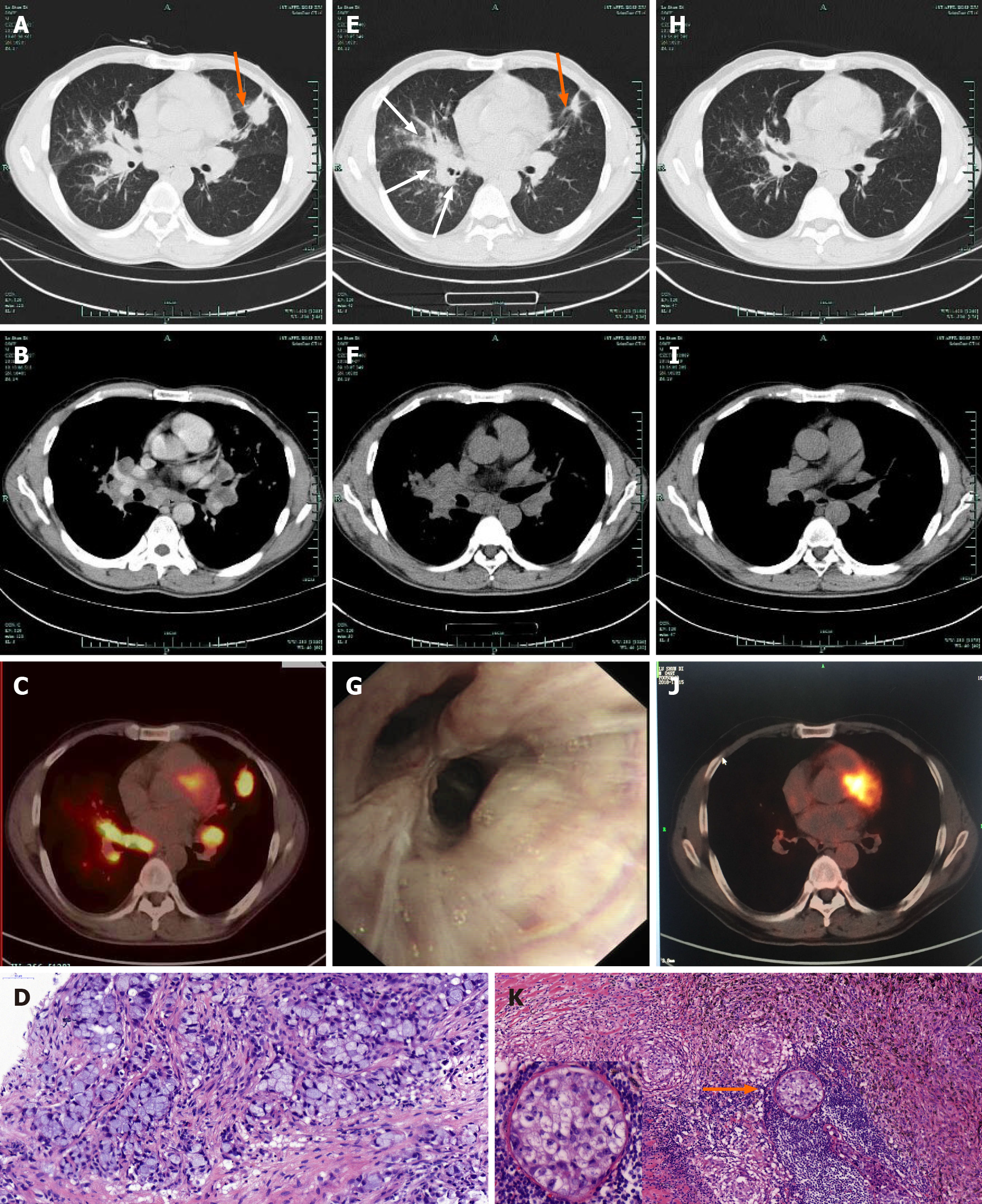
Chest computed tomography (CT) showed a 2.3 cm × 2.7 cm nodule in the left superior lobe, many small nodules in left superior and right lungs, and enlarged bilateral hilar and mediastinal lymph nodes (Figure 1A and B). Fluorine-18 fluoro
The left upper lung nodule was diagnosed as adenocarcinoma with concomitant noncaseating granulomas by CT-guided biopsy (Figure 1D). The diagnosis was clinical stage IVa (cT3N3M1b) adenocarcinoma. EML4-ALK fusion was detected using the amplification refractory mutation system method. Thus, the patient began receiving crizotinib 250 mg twice daily. CT was repeated 30 d later and revealed that the left upper lung nodule decreased dramatically in size; however, the lesions in the right lung progressed, and the mediastinal lymph nodes did not show a significant reduction except for the lymph nodes in station 5 (Figure 1E and F).
Therefore, this patient was admitted to our hospital for further evaluation. An elevated level of angiotensin-converting enzyme (ACE) (194 U/L, normal value, 24-139 U/L) was noted. Purified protein derivative skin tests and serum calcium levels were normal. The right supraclavicular lymph node was diagnosed with noncaseating granulomatous inflammation with negative acid-fast bacillus staining. Bronchoscope showed multiple nodular infiltrations in the right middle lobe (Figure 1G), and the pathological diagnosis was granulomas. The endobronchial ultrasound images showed enlarged lymph nodes in stations 4R, 7, and 11L, but no tumor cells were identified by endobronchial ultrasound-guided transbronchial needle aspiration.
Therefore, he was diagnosed as lung adenocarcinoma accompanied by sarcoidosis (stage II). As the disease progressed within 1 mo, he was administered oral methyl
The final diagnosis was stage IIIA (cT1N2M0) lung adenocarcinoma accompanied by sarcoidosis (stage II).
The multidisciplinary team therefore considered that the patient was suitable for surgery, and the patient underwent radical resection of left superior pulmonary carcinoma by video-assisted thoracic surgery. The intraoperative findings showed a hard nodule approximately 2 cm in diameter on the left superior lobe and enlarged hilar and mediastinal lymph nodes.
The pathologic findings revealed a small cluster of heteromorphic cells in the nodule and that the remaining lung tissues had granulomatous inflammation. One of eight parabronchial lymph nodes and one of one lymph node in station 5 contained metastases with concomitant granulomatous inflammation (Figure 1K). The pathologic diagnosis was stage IIIA (ypT1N2M0) lung adenocarcinoma accompanied by pulmo
This patient refused chemotherapy and radiotherapy after surgery. He continued to receive crizotinib and methylprednisolone. Methylprednisolone was gradually tapered to a maintenance dose of 4 mg daily. The serum ACE activity was reexamined 9 mo after the diagnosis of sarcoidosis and the level was normal. To date, he has been followed-up for 24 mo and remains recurrence-free.
This is one case of sarcoidosis mimicking metastases in an EML4-ALK positive NSCLC patient. ALK-positive patients tend to be younger, have little to no smoking history, and have histologic characteristics of adenocarcinoma[7]. Sarcoidosis is more common in young adults, African Americans, and women[4]. The exact etiology of sarcoidosis remains largely unknown. However, both environmental and genetic factors, including specific occupations and exposures, human leukocyte antigen class II, and cytokine polymorphisms, are likely to define the risk of developing the disease[4,8]. Furthermore, a history of ever smoking instead is protective against sarcoidosis[8]. Therefore, both ALK-positive NSCLC and sarcoidosis tend to occur in younger patients, but no other common susceptibility factors exist between the two diseases.
Sarcoidosis can develop as local sarcoid reactions that are induced by lung cancer[9] or medical treatments including chemotherapy[10], tyrosine kinase inhibitors[11], or immune check-point inhibitors[12]. In this case, the primary lung cancer lesion was in the left upper lobe, but granulomas were found even in the nonneoplastic right lung and supraclavicular lymph node. Moreover, the serum ACE level was high. These findings suggest that the granulomas were more likely to be coincidental, true sarcoidosis rather than sarcoid reactions in respond to lung cancer.
Apart from affecting the lungs and lymph nodes, both sarcoidosis and lung cancer can affect bone. Bone involvement is moderately rare in sarcoidosis, occurring in 3%-13% of patients[13]. Earlier studies demonstrated that the small bones of the hands and feet are typically affected[13]. However, axial bone involvement including spine and pelvis was found to be more frequent with the usage of magnetic resonance imaging and PET-CT scanning[14,15]. In contrast, bone metastases are a common complication in lung cancer[16], and sacrum involvement is frequently observed[17]. In this case, the lesions in the sacrum were initially assessed as a sign of extensive metastasis based on the PET-CT findings, and the pathological findings of concomitant granulomas obtained by CT-guided biopsy were ignored by us at first. Moreover, the use of PET/CT led to a false-positive result in this case due to the absorption of fluorine-18 FDG by the inflammatory lesions associated with sarcoidosis[18,19]. As such, this case illustrates that when found in cancer patients, sarcoidosis can easily be misdiagnosed as metastatic lesions.
Herein, we described a case of sarcoidosis mimicking metastases in an EML4-ALK positive NSCLC patient. From this case, we can learn that sarcoidosis is easily misdiagnosed as metastatic lesions of lung cancer by radiological examinations and that pathological evidence is needed to confirm metastatic disease, especially when some suspected metastatic lesions are negative for malignancy. Moreover, further examinations are needed when posttreatment follow-up evaluation results are unexpected.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jiang D S-Editor: Zhang H L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Camidge DR, Doebele RC. Treating ALK-positive lung cancer--early successes and future challenges. Nat Rev Clin Oncol. 2012;9:268-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 196] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 2. | Wang J, Cai Y, Dong Y, Nong J, Zhou L, Liu G, Su D, Li X, Wu S, Chen X, Qin N, Zeng X, Zhang H, Zhang Z, Zhang S. Clinical characteristics and outcomes of patients with primary lung adenocarcinoma harboring ALK rearrangements detected by FISH, IHC, and RT-PCR. PLoS One. 2014;9:e101551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina K, Hatanaka H, Bando M, Ohno S, Ishikawa Y, Aburatani H, Niki T, Sohara Y, Sugiyama Y, Mano H. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3816] [Cited by in RCA: 4098] [Article Influence: 227.7] [Reference Citation Analysis (0)] |
| 4. | Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357:2153-2165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1469] [Cited by in RCA: 1428] [Article Influence: 79.3] [Reference Citation Analysis (0)] |
| 5. | Morimoto T, Azuma A, Abe S, Usuki J, Kudoh S, Sugisaki K, Oritsu M, Nukiwa T. Epidemiology of sarcoidosis in Japan. Eur Respir J. 2008;31:372-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 255] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 6. | Jeon MH, Kang T, Yoo SH, Swan HS, Kim HJ, Ahn HS. The incidence, comorbidity and mortality of sarcoidosis in Korea, 2008-2015: a nationwide population-based study. Sarcoidosis Vasc Diffuse Lung Dis. 2020;37:24-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 7. | Shaw AT, Yeap BY, Mino-Kenudson M, Digumarthy SR, Costa DB, Heist RS, Solomon B, Stubbs H, Admane S, McDermott U, Settleman J, Kobayashi S, Mark EJ, Rodig SJ, Chirieac LR, Kwak EL, Lynch TJ, Iafrate AJ. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27:4247-4253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1345] [Cited by in RCA: 1511] [Article Influence: 94.4] [Reference Citation Analysis (0)] |
| 8. | Newman LS, Rose CS, Bresnitz EA, Rossman MD, Barnard J, Frederick M, Terrin ML, Weinberger SE, Moller DR, McLennan G, Hunninghake G, DePalo L, Baughman RP, Iannuzzi MC, Judson MA, Knatterud GL, Thompson BW, Teirstein AS, Yeager H Jr, Johns CJ, Rabin DL, Rybicki BA, Cherniack R; ACCESS Research Group. A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am J Respir Crit Care Med. 2004;170:1324-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 442] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 9. | Sugio K, Inoue T, Yokoyama H, Ishida T, Nakano S, Sugimachi K. Sarcoid reactions in regional lymph nodes of primary lung cancer. Respiration. 1993;60:133-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Umezu H, Chida M, Inoue T, Araki O, Tamura M, Tatewaki M, Fukushima Y, Honma K. Sarcoidosis development during induction chemotherapy for lung cancer mimicked progressive disease. Gen Thorac Cardiovasc Surg. 2010;58:434-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Facchinetti F, Gnetti L, Balestra V, Silva M, Silini EM, Ventura L, Majori M, Bordi P, Tiseo M. Sarcoid-like reaction mimicking disease progression in an ALK-positive lung cancer patient receiving lorlatinib. Invest New Drugs. 2019;37:360-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Paydas S. Pulmonary sarcoidosis induced by the anti-PD-1 monoclonal antibody pembrolizumab or post-immunotherapy granulomatous reaction: which is more appropriate terminology? Ann Oncol. 2016;27:1650-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Wilcox A, Bharadwaj P, Sharma OP. Bone sarcoidosis. Curr Opin Rheumatol. 2000;12:321-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 102] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Zhou Y, Lower EE, Li H, Farhey Y, Baughman RP. Clinical characteristics of patients with bone sarcoidosis. Semin Arthritis Rheum. 2017;47:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 15. | Sparks JA, McSparron JI, Shah N, Aliabadi P, Paulson V, Fanta CH, Coblyn JS. Osseous sarcoidosis: clinical characteristics, treatment, and outcomes--experience from a large, academic hospital. Semin Arthritis Rheum. 2014;44:371-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 16. | Riihimäki M, Hemminki A, Fallah M, Thomsen H, Sundquist K, Sundquist J, Hemminki K. Metastatic sites and survival in lung cancer. Lung Cancer. 2014;86:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 599] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 17. | Tsuya A, Kurata T, Tamura K, Fukuoka M. Skeletal metastases in non-small cell lung cancer: a retrospective study. Lung Cancer. 2007;57:229-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 198] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 18. | Hellwig D, Graeter TP, Ukena D, Georg T, Kirsch CM, Schäfers HJ. Value of F-18-fluorodeoxyglucose positron emission tomography after induction therapy of locally advanced bronchogenic carcinoma. J Thorac Cardiovasc Surg. 2004;128:892-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Kaira K, Oriuchi N, Otani Y, Yanagitani N, Sunaga N, Hisada T, Ishizuka T, Endo K, Mori M. Diagnostic usefulness of fluorine-18-alpha-methyltyrosine positron emission tomography in combination with 18F-fluorodeoxyglucose in sarcoidosis patients. Chest. 2007;131:1019-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |