Published online May 6, 2021. doi: 10.12998/wjcc.v9.i13.3102
Peer-review started: November 12, 2020
First decision: January 17, 2021
Revised: January 28, 2021
Accepted: March 4, 2021
Article in press: March 4, 2021
Published online: May 6, 2021
Processing time: 161 Days and 11.1 Hours
Sarcomatoid carcinoma of the pancreas is extremely rare and has an extremely poor prognosis. Although a few cases of sarcomatoid carcinoma of pancreas have been reported, most are focused on a histopathological review. To the best of our knowledge, there are no reports documenting multimodality imaging characteristics and chronological changes with emphasis on radiologic features.
A 64-year-old woman was admitted to Chungnam National University Hospital with acute appendicitis. Contrast-enhanced computed tomography of the abdomen revealed a 2.6 cm × 2.8 cm multilobular cystic mass in the pancreatic tail. The pancreatic lesion showed suspected mural nodules and thin septa. Hence, mucinous cystic neoplasm of pancreas was considered. After 7 mo, the patient was readmitted for repeated epigastric abdominal pain and nausea. Follow-up contrast-enhanced computed tomography of the abdomen and magnetic resonance imaging revealed a marked enlargement (5.4 cm × 4 cm), with a predominant internal solid component. The mass showed low signal intensity on a T1-weighted image and heterogeneously intermediate high signal intensity on a T2-weighted image. It showed diffusion restriction and peripheral rim enhancement on an arterial phase image, and progressive enhancement on portal venous and delayed phase images. Distal pancreatectomy was performed. Based on the morphology and immunohistochemical staining of the specimen, pancreatic sarcomatoid carcinoma was diagnosed.
We present the computed tomography, magnetic resonance imaging, and positron emission tomography computed tomography findings, pathologic features, and chronological changes for preoperative diagnosis.
Core Tip: Sarcomatoid carcinoma of the pancreas is extremely rare and has a poor prognosis. Herein, we present the rapid disease progression with a focus on the chronological multimodality imaging findings. We also summarize the radiologic findings including preoperative diagnosis based on the appended computed tomography and magnetic resonance imaging scans. The main imaging finding of pancreatic sarcomatoid carcinoma is a multilobular, cystic and solid mass showing rapid growth.
- Citation: Lim HJ, Kang HS, Lee JE, Min JH, Shin KS, You SK, Kim KH. Sarcomatoid carcinoma of the pancreas — multimodality imaging findings with serial imaging follow-up: A case report and review of literature. World J Clin Cases 2021; 9(13): 3102-3113
- URL: https://www.wjgnet.com/2307-8960/full/v9/i13/3102.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i13.3102
Pancreatic sarcomatoid carcinoma (PSC) is an extremely rare but highly malignant variant of pancreatic carcinoma, classified as sarcomatoid in undifferentiated carcinoma according to the World Health Organization classification[1,2]. PSC is histologically composed of a mixture of carcinomatous and sarcomatous elements; however, the pathogenesis of PSC remains unknown. Following the first reported case of PSC in 1951, approximately 40 cases have been reported, with a mean patient age of 67 years[3,4]. To date, PSC has not been fully described and no current treatment guidelines exist, with most reported patients having undergone surgical resection and adjuvant chemotherapy. However, the prognosis of PSC is dismal, with a reported median survival rate of 6 mo[4]. Moreover, characteristic radiological findings for PSC have not been established. Therefore, an early and accurate diagnosis is important in determining the course of treatment given PSC has a poor prognosis and shows rapid progression. Here, we report clinical and imaging findings in relation to PSC in a 64-year-old woman, with an emphasis on chronological changes in multimodality imaging findings using computed tomography (CT) and magnetic resonance imaging (MRI), as well as a corresponding literature review of 24 cases with appropriate images.
A 64-year-old woman was admitted to our hospital with repeated epigastric abdominal pain on the right side and nausea.
The patient’s symptoms started 7 d ago.
The patient underwent appendectomy 7 mo ago, and she had a medical history of hypertension and diabetes mellitus.
The patient denied any personal history of alcohol and cigarette consumption. Her family history has nothing notable.
On physical examination, the abdomen was soft and tender in the epigastric area.
Laboratory analysis revealed that carbohydrate antigen 19-9 (CA19-9) (6.39 U/mL) and carcinoembryonic antigen (CEA) (1.98 ng/mL) levels were within the normal range (CA 19-9 < 27 U/mL; CEA < 4.7 ng/mL). Liver function tests and complete blood count, except for a slightly increased white blood cell (12000/µL) count, were within the normal range.
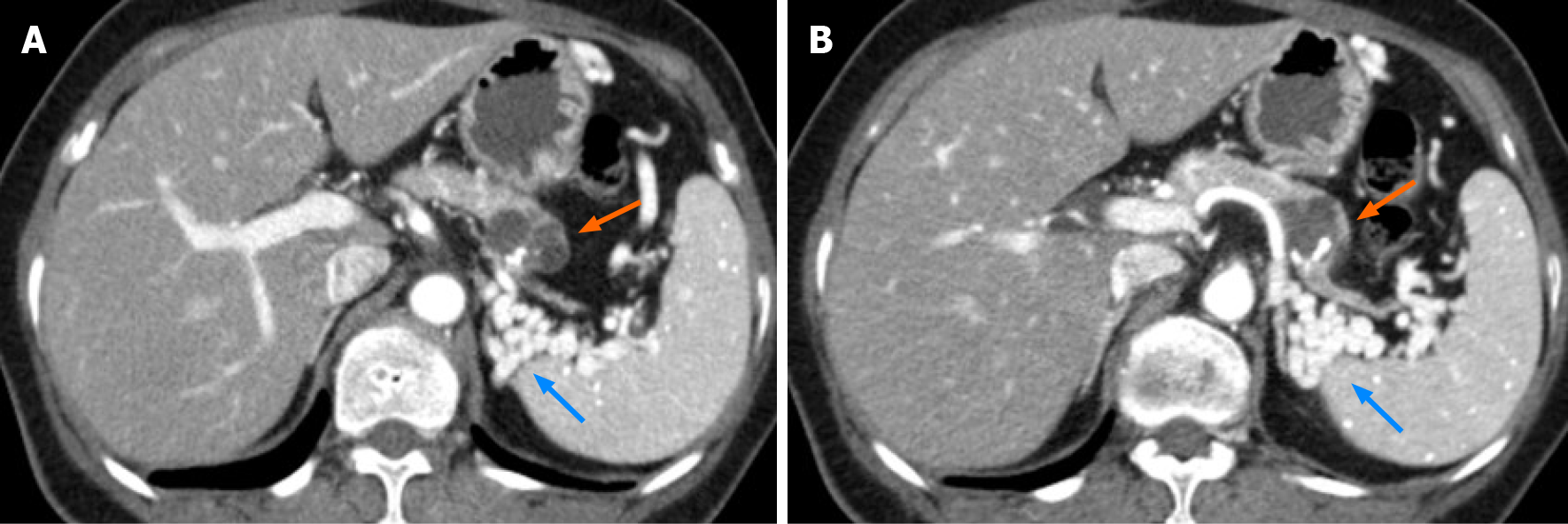
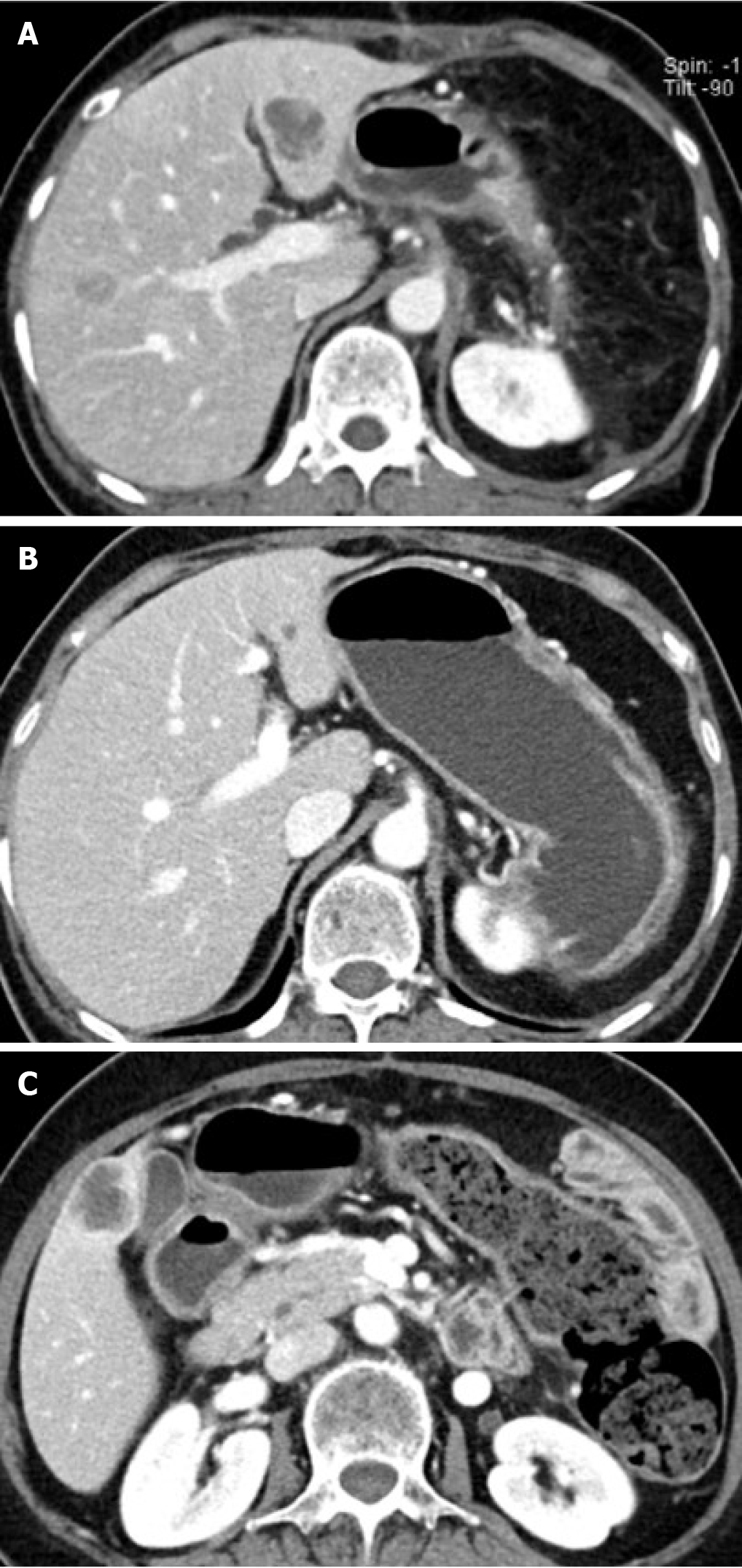
At the time of the diagnosis of acute appendicitis, contrast-enhanced CT of the abdomen incidentally revealed a 2.6 cm × 2.8 cm multilobulated cystic mass with thin septa, and eccentric coarse calcification in the pancreatic tail (Figure 1). The pancreatic mass showed an indefinite eccentric mural thickening, and the main pancreatic duct was not dilated. Pancreatic tail showed severe parenchymal atrophy and it was difficult to delineate the splenic vein at the splenic hilum, which was replaced by tortuous splenorenal collaterals. We formulated a diagnosis of mucinous cystic neoplasm (MCN) or intraductal papillary mucinous neoplasm. The patient had been informed that the pancreatic lesion could be potentially malignant and that further evaluation was necessary. However, after appendectomy, she voluntarily refused further evaluation and treatment and was discharged from the hospital.
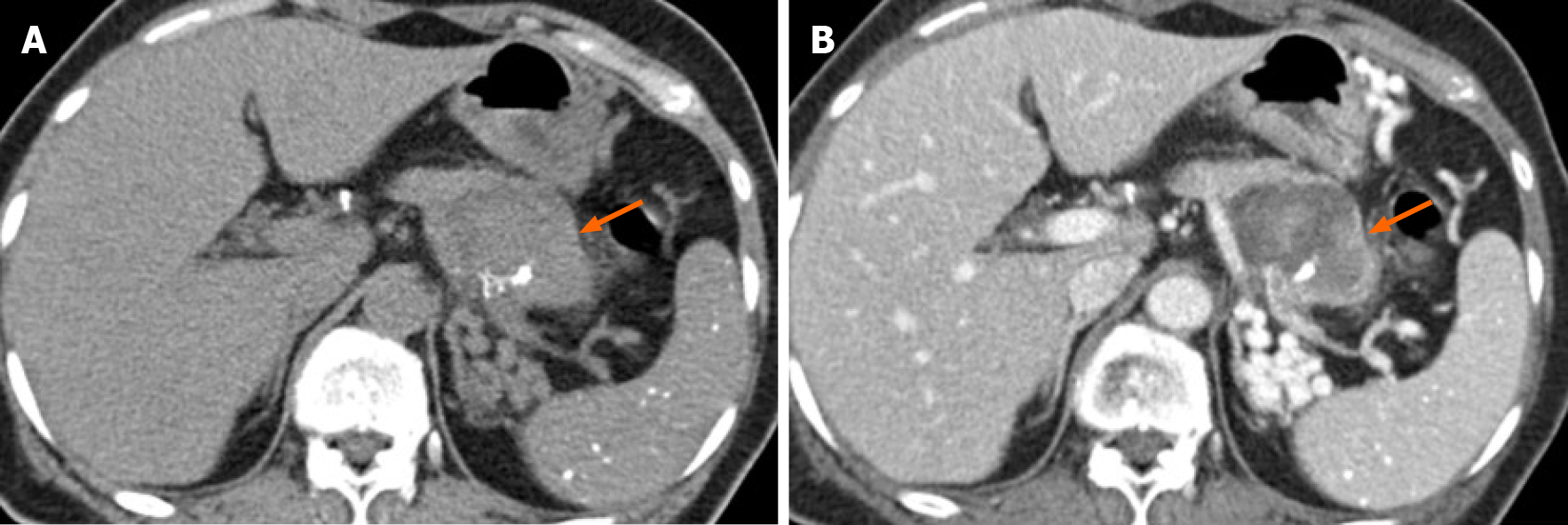
After 7 mo, the patient was hospitalized through ER (Emergency Department), for nausea and whole abdominal pain; contrast-enhanced CT of the abdomen was performed that showed an enlarged pancreatic cystic mass (5.4 cm × 4 cm) in the pancreatic tail with the appearance of an intra-cystic heterogeneously enhanced solid component (Figure 2). This solid mass almost completely occupied the existing cystic mass, and only a small cystic portion remained in the peripheral portion.
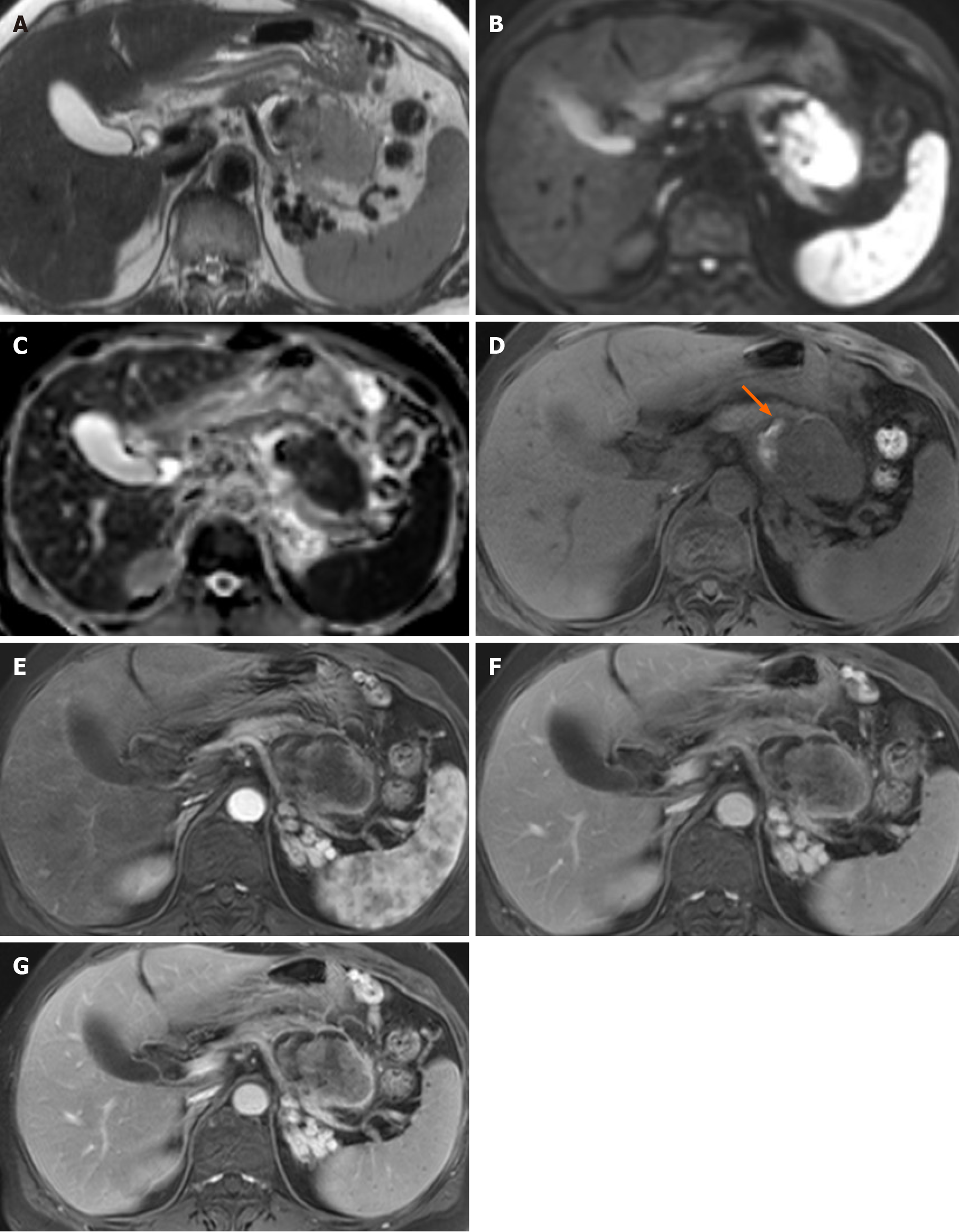
MRI of the pancreas was performed for further evaluation. An axial T2-weighted image showed a solid portion with an intermediate-to-high signal intensity (SI) within the cystic mass in the pancreatic tail (Figure 3). Small amount of intra-tumoral hemorrhage, which showed high SI on a T1-weighted image and low SI on the T2-weighted image, was noted. The solid portion of the mass showed diffusion restriction. Axial contrast-enhanced dynamic T1-weighted images demonstrated significant peripheral, progressive enhancement of the solid portion.
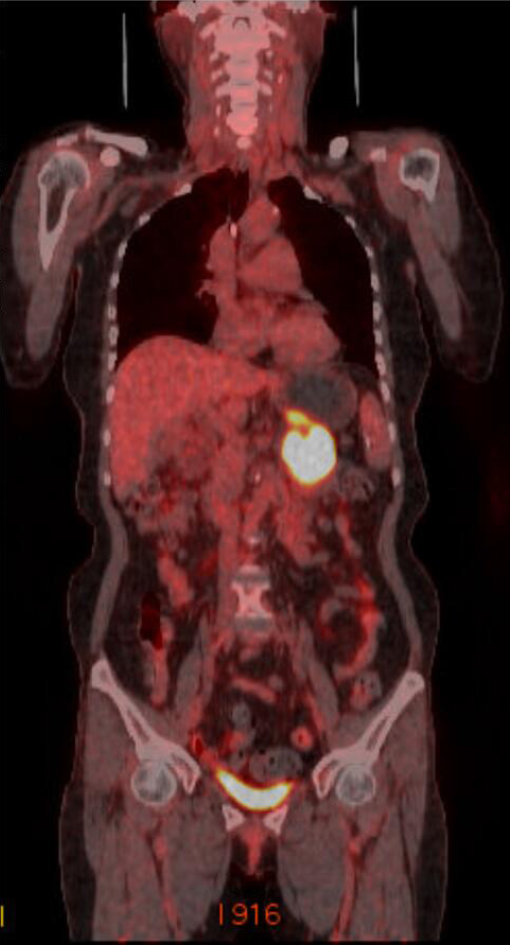
18F-Fluorodeoxyglucose positron emission tomography-CT (PET-CT) was performed to evaluate distant metastasis. PET-CT showed intense uptake of 18F-fluorodeoxyglucose (Figure 4). There was no evidence of distant metastasis. The multimodality imaging findings indicated the possibility of pancreatic mucinous cystadenocarcinoma.
Radical resection of the pancreatic tumor was planned. At the time of surgery, the mass appeared to extend directly into the transverse mesocolon. The patient underwent distal pancreatectomy with splenectomy and colonic segmental resection.
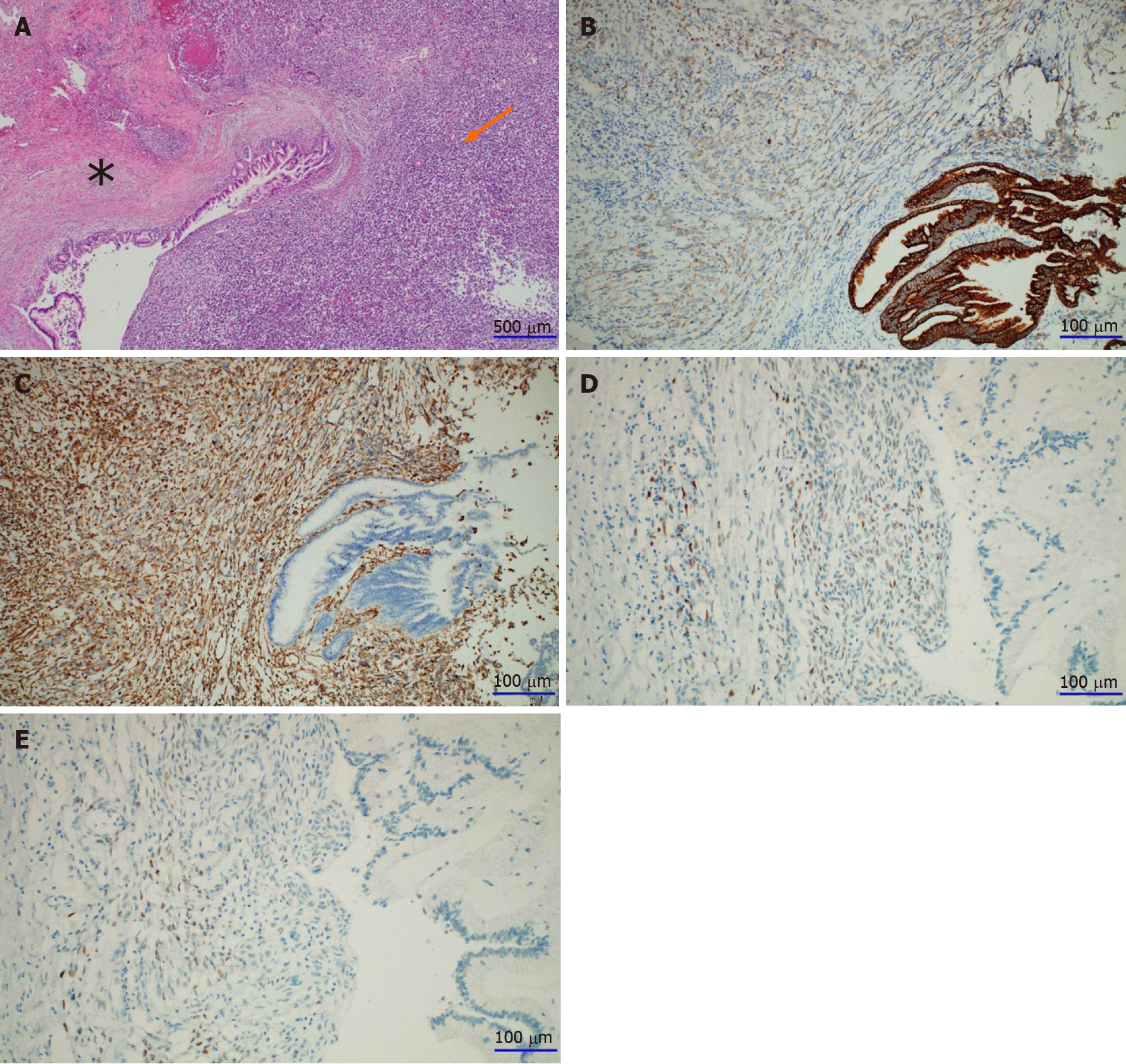
A gross section of the pancreatic mass revealed nearly total replacement by a solid mass with a dilated cystic portion inside. The mass showed necrotic foci in 20% of the total volume. The tumor extended to the transverse mesocolon; however, there were no tumor cells in the colon and spleen. Microscopically, the cystic portion of the lesion was lined with high-grade dysplastic columnar epithelium with underlying ovarian-type stroma, which was positive for estrogen and progesterone receptors on immunohistochemistry (Figure 5). The part of the solid portion located near the cystic portion consisted of undifferentiated pleomorphic malignant cells, which were strongly positive for vimentin, weakly positive for pan-cytokeratin, and negative for the other antibodies such as CD56, chromogranin, and synaptophysin.
Finally, depending on the morphology and the immunohistochemical staining pattern, the patient was diagnosed with PSC associated with MCN.
Postoperative adjuvant chemotherapy was administered as six cycles of gemcitabine 1000 mg/m2 combined with abraxane 75 mg/m2.
Her postoperative course was uneventful. At 1 mo follow-up, the patient was found to have multiple small hepatic metastases despite chemotherapy (Figure 6). At 4-mo follow-up, a CT scan demonstrated a decrease in the size of the hepatic metastasis. However, a new hepatic metastatic lesion had developed by the 7 mo follow-up. Furthermore, peritoneal seeding nodules in the right perihepatic space were noted. The patient died 10 mo after the surgery.
PSC is an extremely rare exocrine neoplasm that is histologically characterized by a mixture of carcinomatous and sarcomatous elements[1,5]. The pathogenesis of sarcomatoid carcinoma is still unknown; however, despite controversies, the following three main hypotheses have been proposed: (1) conversion: The sarcomatous component is derived from the carcinomatous components through metaplastic transformation, (2) combination: Both components undergo early divergence from a common stem cell, and (3) collision: Independent growth of the carcinoma and sarcoma adjacent to each other[1,6-9]. The current study supports that most carcino-sarcomas are combination or conversion tumors[1,7-9].
From the summary of the 24 reported cases of PSC (Table 1), we found that PSC was common in the middle-aged and elderly patients, with a mean age of 61 years (range, 24-85 years). The male-to-female ratio was 1:3. The most common symptoms were abdominal pain, nausea, and vomiting. The serum CA19-9 level was elevated in 12 of 17 patients, whereas it was within normal range in five cases, similar to our case. The CEA level was within the normal range.
| No. | Ref. | Age/sex | Clinical manifestation | CA19-9/CEA | Site | Size | Imaging findings | Pancreatic duct dilatation | Preoperative diagnosis | Treatment | Cause of death | Survival (mo) |
| 1 | Wenig et al[14], 1997 | 67/M | Abdominal pain | NA | Tail | 19 cm × 14 cm × 8 cm | Complex, partially cystic lesion | (-) | Pseudocyst | Distal pancreatectomy with splenectomy | Extensive intra-abdominal involvement | 15 |
| 2 | Pan and Wang[21], 2007 | 70/F | Anemia, weight loss | NA | Body, tail | 10.4 cm × 8.3 cm × 6.6 cm | Cystic-solid mass | (-) | NA | Distal pancreatectomy with splenectomy | NA | > 4 |
| 3 | Gelos et al[22],2008 | 61/F | Anemia | NA | Head | 7 cm × 6 cm × 3.5 cm | Mass | NA | NA | PD | Peritoneal carcinomatosis | 11 |
| 4 | Nakano et al[23],2008 | 82/F | Anorexia | 231/15 | Head | 18 cm × 11 cm × 10 cm | Cystic-solid mass | (+) | NA | PD with transverse colonic segmental resection | Sepsis | 13 d |
| 5 | Okamura et al[24],2010 | 64/F | Incidentally found | 87/2.7 | Tail | 2 cm | Cystic-solid mass | (+) | IPMN | Distal pancreatectomy | NA | > 12 |
| 6 | Shen et al[25], 2010 | 72/F | Abdominal pain, nausea, vomiting | Normal | Head | 5 cm × 4 cm × 4 cm | Cystic-solid mass | (-) | NA | PD with left, hepatic lobe resection | Recurrence in the tail/ multiple liver metastasis | 2 |
| 7 | Kim et al[26], 2011 | 48/M | Incidentally found | 694.7/NA | Tail | 5.5 cm × 5 cm × 5 cm | Cystic-solid mass | (-) | Pancreatic cancer with pseudocyst | Distal pancreatectomy, splenectomy with colonic segmental resection | Multiple hepatic/peritoneal metastasis | 4 |
| 8 | Palaniappan and Bindhu[15], 2011 | 46/M | Dyspepsia, jaundice | 252/NA | Head | 3.4 cm × 3.4 cm × 1.5 cm | Hypodense, oval lesion | (+) | NA | PD | NA | > 28 |
| 9 | Zhu et al[16], 2012 | 53/F | Abdominal pain, jaundice | 89.08/NA | Head | 5 cm × 4 cm × 3 cm | Mass | (+) | NA | PD | NA | > 20 |
| 10 | Oymaci et al[27],2013 | 66/M | Abdominal pain, jaundice | NA | Head | 3.5 cm × 2.0 cm × 1.5 cm | Cystic mass with hyperdense mural nodule | (-) | NA | PD | Upper gastrointestinal bleeding | 20 d |
| 11 | Yao et al[28],2013 | 48/M | Epigastralgia, weight loss | 134/12 | Tail | 10 cm × 8 cm × 5 cm | Cystic-solid mass | (-) | Cystadenoma | Laparoscopic spleen-preserving left pancreatectomy | Recurrence | 3 |
| 12 | Kim et al[29],2014 | 77/M | Poorly controlled blood sugar | 160.57/NA | Body | 2.2 cm | Mass | (+) | Pancreatic cancer | Biopsy and chemotherapy | NA | NA |
| 13 | Katsourakis et al[17], 2015 | 70/M | Anemia | 169.67/NA | Head | 4.7 cm × 4.5 cm | Mass | (+) | NA | PD | NA | > 16 |
| 14 | Lai et al[30], 2015 | 55/M | Abdominal discomfort, weight loss | Normal | Body, tail | 14 cm | Heterogeneous hypodense mass | NA | NA | Distal pancreatectomy with splenectomy, and resection of the proximal jejunum, and transverse colon | NA | NA |
| 15 | Lee et al[31], 2015 | 24/F | Epigastric pain, weight loss | 7.7/7.1 | Tail | 4.7 cm × 3.5 cm | Cystic-solid mass | (-) | Solid pseudopapillary tumor | Distal pancreatectomy with splenectomy | NA | NA |
| 16 | Shi et al[10], 2015 | 74/F | Abdominal pain | 148.4/10.05 | Tail | 2.2 cm × 2 cm | Cystic-solid mass | (-) | Mucinous cystadenocarcinoma | Distal pancreatectomy with splenectomy | NA | NA |
| 17 | Jia et al[18], 2017 | 44/F | Abdominal discomfort | > 1200/NA | Head | 2.9 cm × 1.6 cm | Nodular hyperdense mass | (+) | NA | PD | NA | > 31 |
| 18 | Li et al[6], 2017 | 60/M | Steatorrhea, weight loss, abdominal mass | Elevated | Head | 10 cm × 9 cm × 9 cm | Cystic-solid mass | (+) | IPMN | Total pancreatectomy with splenectomy | NA | > 2 |
| 19 | Mszyco et al[13], 2017 | 85/M | Abdominal pain, weight loss | NA/NA | Head | 7.2 cm × 7.2 cm | Mixed solid and cystic mass, mild peripheral enhancement | (+) | NET, mucinous cystadenocarcinoma | PD | NA | NA |
| 20 | Ruess et al[7], 2017 | 73/F | Epigastric pain | 29.1/10.2 | Head | 4 | Abrupt change in caliber of the main pancreatic duct | (+) | Combined type IPMN with high-risk features | PD | Poor general condition | 4 |
| 21 | Xie et al[19], 2018 | 63/M | Epigastralgia, jaundice, weight loss | Normal | Head | 2.5 cm × 2 cm × 1.8 cm | Bile duct dilation and abrupt narrowing at the distal CBD | (+) | Distal bile duct cancer | PD | Hepatic metastasis | 18 |
| 22 | Liu et al[20], 2019 | 66/M | Jaundice | NA | Head | 4.1 cm × 3.3 cm × 2.2 cm | Irregularly shaped mass with an unclear boundary | (+) | NA | Bile duct jejunum anastomosis and radioactive seed implantation | NA | > 12 |
| 23 | Zhou et al[32], 2019 | 59/M | Jaundice | 14.6/NA | Head | 1.5 cm × 1.1 cm | Hypodense mass with slightly enhancement | (+) | NA | PD | Hepatic metastasis, peritoneal metastasis | 6 |
| 24 | Quinn et al[33], 2020 | 42/F | Epigastric pain | NA | Body, tail | 11.3 cm × 7.34 cm × 10.6 cm | Complex cystic, multiloculated | (-) | NA | Subtotal pancreatectomy, splenectomy, Lt. partial adrenalectomy, Lt. hemicolectomy | NA | > 15 |
Although most of the reports were based on pathologic findings, some included imaging features. PSC usually occurred in the head (14 of 24 cases) and tail (9 of 24 cases) of the pancreas. At the time of diagnosis, the tumor size was very large, with an average of 7 cm in the greatest dimension (range, 1.5-30 cm). Most PSCs showed a contrast-enhanced large irregular cystic and solid mass. Dilatation of the main pancreatic duct was common in cases where the mass was located in the pancreatic head. Our chronological image follow-up indicated a change in PCS size and morphology from a small cystic lesion with calcification at the tail of pancreas on the initial CT scans to an enlarged mixed solid cystic mass. It also showed a rapid growth pattern, from 2.8 cm to 5.4 cm in diameter within 6 mo, which was similar to a previously reported rapid growth pattern[10].
None of the patients were accurately diagnosed before surgery. The preoperative diagnosis varied, including pancreatic ductal adenocarcinoma, MCN, invasive intraductal papillary mucinous neoplasm, solid pseudopapillary tumor, and pseudocyst. It was difficult to distinguish PSC from other pancreatic cancers. In our case, we also initially diagnosed it as a MCN based on the presence of cystic and solid mass in the pancreatic tail as commonly seen in middle-aged women. In the follow-up observation, seeing that the solid portion had increased, it was judged that the existing MCN had undergone a malignant change; however, the change in size was observed too fast, which was a different finding compare to the existing MCN with malignant change.
The main differential diagnosis of PSC includes MCN with associated invasive carcinoma and pancreatic ductal adenocarcinoma with cystic change. Generally, MCN with associated invasive carcinoma has high levels of CEA and CA19-9, unlike what was found in our case[11]. The morphology of PSC such as multilocular cystic mass with thickened wall, calcification, papillary proliferations, vascular involvement, and hypervascularity is similar to that of other malignant MCN[11]. However, based on our case findings and those of Shi et al[10], PSC showed rapid growth and presence of a predominant solid mass, unlike the majority of MCNs, which are slow growing and asymptomatic[11]. Compared to ductal adenocarcinoma with cystic change, PSC has more vascularity[8]. Extra-pancreatic perineural and vascular invasion, invasion of the adjacent organ, and atrophy of the pancreatic parenchyma are less common in a PSC, unlike pancreatic adenocarcinoma with cystic changes[6,10]. However, PSCs metastasize easily to the liver and peritoneum, which is the main cause of death. Thus, short-term follow-up at regular intervals is needed to understand the patient’s prognosis in case of suspicious lesions in the pancreatic cystic lesion.
Strategies to improve treatment options and prognosis for PSC remain limited due to its rapid progression and rare occurrence. We found that PSC also initially appears similar to other pancreatic cystic tumors in terms of chronologic changes and multimodality imaging findings. Given that no management guidelines for PSC exist, it may be necessary to refer to worrisome features and/or high-risk stigmata of the lesion, as detailed in the American College of Radiology 2017 guideline[12]. Therefore, evaluation of cystic pancreatic lesions using multimodality imaging is crucial, and serial follow-up imaging studies are helpful for treatment planning. CT is the primary imaging modality for evaluating pancreatic solid and cystic lesions but has some limitations. Endoscopic ultrasonography, MRI, especially T2-sequencing, is more useful for evaluating key features due to its superior soft-tissue contrast resolution.
Most patients with PSC have a poor prognosis, with an average survival of 6 mo after surgery[13]. In our case, hepatic metastases had occurred just after 1 mo of surgical resection, and despite 6 cycles of chemotherapy, the patient deceased 10 mo after the surgery. Despite the development of chemotherapy, PSC shows multiple metastases and disease recurrence. Fortunately, recent case reports have described a long-term survival of more than one year following surgery[14-20]. Therefore, despite challenges in obtaining a definitive preoperative diagnosis, an accurate and early diagnosis is important for active and appropriate treatment.
In conclusion, we present a rare case of PSC with emphasis on its radiologic features. Although it is extremely rare, PSC must be kept in mind as a differential diagnosis for rapidly growing solid and cystic tumors of pancreas due to its aggressive nature and poor prognosis.
The authors would like to acknowledge Boyoung Kim from the Department of Radiology, Chungnam National University Hospital, for her invaluable assistance in preparing the literature review.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Gokce E, Kovacevic B, Ren K S-Editor: Gao CC L-Editor: A P-Editor: Liu JH
| 1. | Li J, Wei T, Zhang J, Wei S, Chen Q, Chen BW, Zhou Y, Wen L, Qin H, Bai X, Liang T. Carcinosarcoma of the pancreas: comprehensive clinicopathological and molecular characterization. HPB (Oxford). 2020;22:1590-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | World Health Organization. WHO classification of tumours: digestive system tumors. 5th ed. Genève, Switzerland: World Health Organization, 2019: 328-329. |
| 3. | Van JD, Snoeks T. Carcinosarcoma of the body of the pancreas. Acta Gastro-enterologica Belgica. 1951;14:106-113. |
| 4. | Alhatem A, Quinn PL, Xia W, Chokshi RJ. Pancreatic Carcinosarcoma Clinical Outcome Analysis of the National Cancer Institute Database. J Surg Res. 2021;259:62-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Virmani V, Khandelwal A, Sethi V, Fraser-Hill M, Fasih N, Kielar A. Neoplastic stomach lesions and their mimickers: spectrum of imaging manifestations. Cancer Imaging. 2012;12:269-278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Li BQ, Liu QF, Chang XY, Hu Y, Chen J, Guo JC. Pancreatic carcinosarcoma mimics malignant intraductal papillary mucinous neoplasm: A rare case report and literature review. Medicine (Baltimore). 2017;96:e6961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Ruess DA, Kayser C, Neubauer J, Fichtner-Feigl S, Hopt UT, Wittel UA. Carcinosarcoma of the Pancreas: Case Report With Comprehensive Literature Review. Pancreas. 2017;46:1225-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Thompson L, Chang B, Barsky SH. Monoclonal origins of malignant mixed tumors (carcinosarcomas). Evidence for a divergent histogenesis. Am J Surg Pathol. 1996;20:277-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 247] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 9. | Cantrell LA, Blank SV, Duska LR. Uterine carcinosarcoma: A review of the literature. Gynecol Oncol. 2015;137:581-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 162] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 10. | Shi HY, Xie J, Miao F. Pancreatic carcinosarcoma: first literature report on computed tomography imaging. World J Gastroenterol. 2015;21:1357-1361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Testini M, Gurrado A, Lissidini G, Venezia P, Greco L, Piccinni G. Management of mucinous cystic neoplasms of the pancreas. World J Gastroenterol. 2010;16:5682-5692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Megibow AJ, Baker ME, Morgan DE, Kamel IR, Sahani DV, Newman E, Brugge WR, Berland LL, Pandharipande PV. Management of Incidental Pancreatic Cysts: A White Paper of the ACR Incidental Findings Committee. J Am Coll Radiol. 2017;14:911-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 216] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 13. | Mszyco S, Teng L, Annunziata J, Hartman MS. Pancreatic Carcinosarcoma: A Case Report Highlighting Computed Tomography Characteristics. Curr Probl Diagn Radiol. 2017;46:342-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Wenig BM, Albores-Saavedra J, Buetow PC, Heffess CS. Pancreatic mucinous cystic neoplasm with sarcomatous stroma: a report of three cases. Am J Surg Pathol. 1997;21:70-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Palaniappan J, Bindhu S. Carcinosarcoma of the pancreas: report of a case with a concise review of the literature. J Clin Diagn Res. 2011;5:621-624. |
| 16. | Zhu WY, Liu TG, Zhu H. Long-term recurrence-free survival in a patient with pancreatic carcinosarcoma: a case report with a literature review. Med Oncol. 2012;29:140-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Katsourakis A, Svoronos C, Chatzitheoklitos E, Hadjis I, Alatsakis M, Mirelis C, Sovatzidis A, Noussios G. Carcinosarcoma of the pancreas: how a common blood disorder can hide an extremely rare tumour. JOP. 2015;16:310-312. [DOI] [Full Text] |
| 18. | Jia Z, Zhang K, Huang R, Zhou X, Jiang L. Pancreatic carcinosarcoma with rare long-term survival: Case report and review of the literature. Medicine (Baltimore). 2017;96:e5966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Xie Y, Xiang Y, Zhang D, Yao X, Sheng J, Yang Y, Zhang X. Sarcomatoid carcinoma of the pancreas: A case report and review of the literature. Mol Med Rep. 2018;18:4716-4724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Liu Y, Hao H, Guo X, Xu J, Kang L, Zheng G, Zhao H. Rare pancreatic carcinosarcoma in a patient with medical history of esophageal cancer: A case report and literature review. Medicine (Baltimore). 2019;98:e15238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Pan ZG, Wang B. Anaplastic carcinoma of the pancreas associated with a mucinous cystic adenocarcinoma. A case report and review of the literature. JOP. 2007;8:775-782. [PubMed] |
| 22. | Gelos M, Behringer D, Philippou S, Mann B. Pancreatic carcinosarcoma. Case report of multimodal therapy and review of the literature. JOP. 2008;9:50-55. [PubMed] |
| 23. | Nakano T, Sonobe H, Usui T, Yamanaka K, Ishizuka T, Nishimura E, Hanazaki K. Immunohistochemistry and K-ras sequence of pancreatic carcinosarcoma. Pathol Int. 2008;58:672-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Okamura J, Sekine S, Nara S, Ojima H, Shimada K, Kanai Y, Hiraoka N. Intraductal carcinosarcoma with a heterologous mesenchymal component originating in intraductal papillary-mucinous carcinoma (IPMC) of the pancreas with both carcinoma and osteosarcoma cells arising from IPMC cells. J Clin Pathol. 2010;63:266-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Shen ZL, Wang S, Ye YJ, Wang YL, Sun KK, Yang XD, Jiang KW. Carcinosarcoma of pancreas with liver metastasis combined with gastrointestinal stromal tumour of the stomach: is there a good prognosis with the complete resection? Eur J Cancer Care (Engl). 2010;19:118-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Kim HS, Joo SH, Yang DM, Lee SH, Choi SH, Lim SJ. Carcinosarcoma of the pancreas: a unique case with emphasis on metaplastic transformation and the presence of undifferentiated pleomorphic high-grade sarcoma. J Gastrointestin Liver Dis. 2011;20:197-200. [PubMed] |
| 27. | Oymaci E, Argon A, Coşkun A, Uçar AD, Carti E, Erkan N, Yildirim M. Pancreatic carcinosarcoma: case report of a rare type of pancreatic neoplasia. JOP. 2013;14:212-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 28. | Yao J, Qian JJ, Zhu CR, Bai DS, Miao Y. Laparoscopic left pancreatectomy for pancreatic sarcomatoid carcinoma: A case report and review of the literature. Oncol Lett. 2013;6:568-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Kim HS, Kim JI, Jeong M, Seo JH, Kim IK, Cheung DY, Kim TJ, Kang CS. Pancreatic adenocarcinosarcoma of monoclonal origin: a case report. World J Gastroenterol. 2014;20:12682-12686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Lai CW, Chen CW, Lee YH, Chen JH. Sarcomatoid carcinoma of the pancreas. Ciji Yixue Zazhi. 2015;27:46-47. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 31. | Lee J, Hyun JJ, Lee HS. A Rare Cause of Abdominal Pain by Pancreatic Mass in a Young Female Patient. Carcinosarcoma of the Pancreas. Gastroenterology. 2015;149:e3-e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Zhou DK, Gao BQ, Zhang W, Qian XH, Ying LX, Wang WL. Sarcomatoid carcinoma of the pancreas: A case report. World J Clin Cases. 2019;7:236-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Quinn PL, Ohioma D, Jones AMK, Ahlawat SK, Chokshi RJ. Treatment of Rare and Aggressive Pancreatic Carcinosarcoma. ACG Case Rep J. 2020;7:e00379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |