Published online May 6, 2021. doi: 10.12998/wjcc.v9.i13.3070
Peer-review started: October 20, 2020
First decision: January 7, 2021
Revised: January 11, 2021
Accepted: February 22, 2021
Article in press: February 22, 2021
Published online: May 6, 2021
Processing time: 178 Days and 9.3 Hours
Vancomycin is often used as an anti-infective drug in patients receiving anti-tumor chemotherapy. There are concerns about its adverse drug reactions during treatment, such as nephrotoxicity, ototoxicity, hypersensitivity reactions, etc. However, potential convulsion related to high plasma concentrations of vancomycin in children receiving chemotherapy has not been reported.
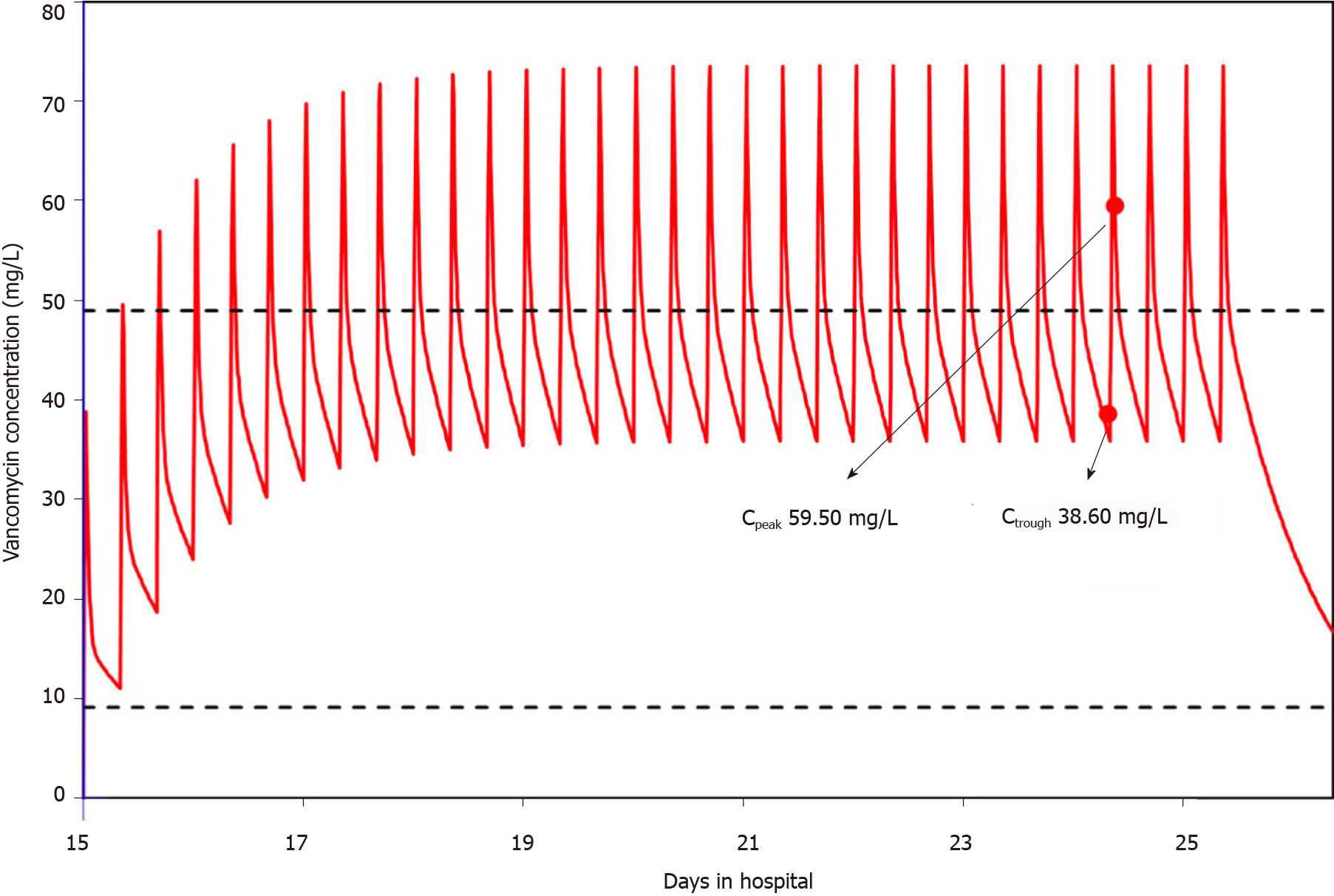
A 3.9-year-old pediatric patient with neuroblastoma receiving vancomycin to treat post-chemotherapy infection developed an unexpected convulsion. No other potential disease conditions could explain the occurrence of the convulsion. The subsequently measured overly high plasma concentrations of vancomycin could possibly provide a clue to the occurrence of this convulsion. The peak and trough plasma concentrations of vancomycin were 59.5 mg/L and 38.6 mg/L, respectively, which were much higher than the safe range. Simulation with the Bayesian approach using MwPharm software showed that the area under the concentration-time curve over 24 h was 1086.6 mg· h/L. Therefore, vancomycin was immediately stopped and teicoplanin was administered instead combined with meropenem and fluconazole as the anti-infective treatment strategy.
Unexpected convulsion occurring in a patient after chemotherapy is probably due to toxicity caused by abnormal pharmacokinetics of vancomycin. Overall evaluation and close therapeutic drug monitoring should be conducted to determine the underlying etiology and to take the necessary action as soon as possible.
Core Tip: Vancomycin is often used as an anti-infective drug in patients receiving anti-tumor chemotherapy. We present a rare case of sudden onset of convulsion related to vancomycin treatment in a pediatric patient with neuroblastoma. This case demonstrates that unexpected convulsion occurring in patients after chemotherapy is probably due to the toxicity caused by abnormal pharmacokinetics of vancomycin. Close therapeutic drug monitoring is recommended to ensure the plasma concentration of vancomycin is within the normal range. A model-based Bayesian estimation tool could be used to estimate the area under the concentration-time curve and help health care practitioners create an individualized dosing plan.
- Citation: Ye QF, Wang GF, Wang YX, Lu GP, Li ZP. Vancomycin-related convulsion in a pediatric patient with neuroblastoma: A case report and review of the literature. World J Clin Cases 2021; 9(13): 3070-3078
- URL: https://www.wjgnet.com/2307-8960/full/v9/i13/3070.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i13.3070
Neuroblastoma is an embryonal malignancy derived from primitive cells in the sympathetic nervous system and often appears in early childhood[1,2]. It can cause abnormal development of the adrenal medulla and paraspinal ganglia[3]. The molecular characteristics of neuroblastoma, as shown by extensive studies, include changes at the genome, epigenome, and transcriptome levels[1]. Neuroblastoma usually occurs sporadically, and its etiology is not exactly clear[4]. There is an association between a genetic variation in chromosome 6p22 and sporadic neuroblastoma[5]. Familial cases can be caused by ALK and PHOX2B germline mutations[6]. Neuro-blastoma is the second most common solid central nervous system tumor occurring in childhood worldwide[7]. It has a complex clinical course from metastatic spread to spontaneous regression without therapy which can occur at the primary or metastatic site[2]. Chemotherapy based on a biology- and response-based algorithm can help improve the outcome of patients[8-10]. The backbone of the induction chemo-therapeutic regimen commonly used to treat neuroblastoma includes cycles of cisplatin and etoposide alternating with vincristine (VCR), doxorubicin (DOX), and cyclo-phosphamide (CPM), as was developed at the Memorial Sloan-Kettering Cancer Center[11]. Later, the Children’s Oncology Group added topotecan (TOPO), which showed anti-neuroblastoma activity, to the induction regimen[12,13]. To prevent or treat potential infections caused by compromised immune function after chemotherapy, anti-infective drugs are often administered to patients with tumors[14]. Careful consideration of the anti-microbial strategy should be given to this special population as there is an increase in antimicrobial-resistant pathogens[14].
Vancomycin is a tricyclic glycopeptide antibiotic which inhibits the biosynthesis of the bacterial cell wall[15]. It was first used clinically in 1958, and was mainly used to treat gram-positive bacterial infection resistant to beta-lactam antibiotics, such as methicillin-resistant Staphylococcus aureus, coagulase-negative Staphylococci, etc.[15,16]. Vancomycin is poorly absorbed orally, and thus it is administered intravenously in clinical applications[17]. It is a hydrophilic molecule with plasma protein binding less than 50%[18] and is mainly excreted by the kidney (80% to 90%)[19]. Its penetration into cerebrospinal fluid (CSF) is poor with CSF-to-plasma ratios of 0.07-0.30, but has increased variation in patients with meningitis where the CSF-to-plasma ratios range from 0.06 to 0.81[16,20]. The anti-bacterial effect of vancomycin is time-dependent. The area under the concentration-time curve over 24 h to minimum inhibitory concentration ratio (AUC/MIC) is recognized as the index to evaluate its phar-macodynamic effect, with an AUC/MIC of ≥ 400 as the treatment target[21]. There are some adverse drug reactions (ADRs) related to vancomycin. Nephrotoxicity is the major concern associated with its use[21]. Other adverse effects include hypotension, tachycardia, phlebitis, ototoxicity, hypersensitivity reactions, exanthema, chills, fever, etc.[17]. Rare ADRs of the nervous system that can be caused by vancomycin are mononeuritis multiplex[22] and encephalopathy[23], which have been documented in two case reports, respectively. Vancomycin-induced hypertension along with transient blindness and generalized seizure were reported by Caglayan et al[24]. However, the plasma concentration of vancomycin was not measured in that case and it is unknown if this ADR was caused by abnormal pharmacokinetics of vancomycin.
Here, we report a rare case of sudden onset of convulsion related to vancomycin treatment in a pediatric patient with neuroblastoma.
A 3.9-year-old female patient with a body weight of 16 kg and a height of 104.0 cm, presented to the Department of Oncology at the Children’s Hospital of Fudan University to start Cycle 2 chemotherapy for neuroblastoma. During her presentation in the Department of Oncology, she had a sudden onset of convulsion in the left limbs on day 22 in hospital. The patient was transferred to the Pediatric Intensive Care Unit (PICU).
Four months ago, the patient had repeated fever with swelling, weakness and a limp in her right leg. Ultrasound and computed tomography (CT) indicated a space-occupying mass in the retroperitoneum, with routine blood examination showing C reactive protein > 160 mg/L (normal range < 8 mg/L) and hemoglobin 73 g/L (normal range 110-160 g/L). Biopsy of the mass confirmed poorly differentiated neuroblastoma with a medium mitosis-karyorrhexis index, MYCN-non-amplified and Shimada classification as unfavorable histology and Schwannian stroma-poor. Folliculus lymphaticus structure could be seen between tumor cells, indicating the possibility of lymph node metastasis. Nuclear medicine imaging showed multiple osteopathy and hence the possibility of bone metastasis. Using the International Neuroblastoma Staging System[25], the patient was classified as stage IV.
Induction chemotherapy for the high-risk group was administered to the patient, comprising CPM (230 mg) and TOPO (0.7 mg) for five days. One month later, the second induction chemotherapy consisting of CPM (290 mg) and TOPO (0.8 mg) was started and lasted for five days. The third chemotherapy regimen, which was given one month after the second regimen, included 1385 mg CPM for two days, 0.4 mg VCR for three days, and 16.5 mg DOX for three days. A combination of fluconazole, meropenem, and vancomycin was given to treat infection.
This hospitalization was for the purpose of receiving the fourth chemotherapy regimen.
The patient had no previous medical history.
The patient had no significant personal or family history.
During convulsion onset, the patient did not respond to sound. Physical examination revealed equally sized and shaped pupils with sensitive reflex to light. Frothing at the lips was noticed. Her left limbs had high muscular tension with continuous shaking. Her blood oxygen saturation was 100% and heart rate was 185-190 bpm. Three minutes after sedation, the convulsion gradually stopped.
On day 27, an electroencephalogram showed slower than normal blood flow in the middle cerebral artery, and mildly insufficient blood supply. Other electro-encephalogram parameters were within the normal range.
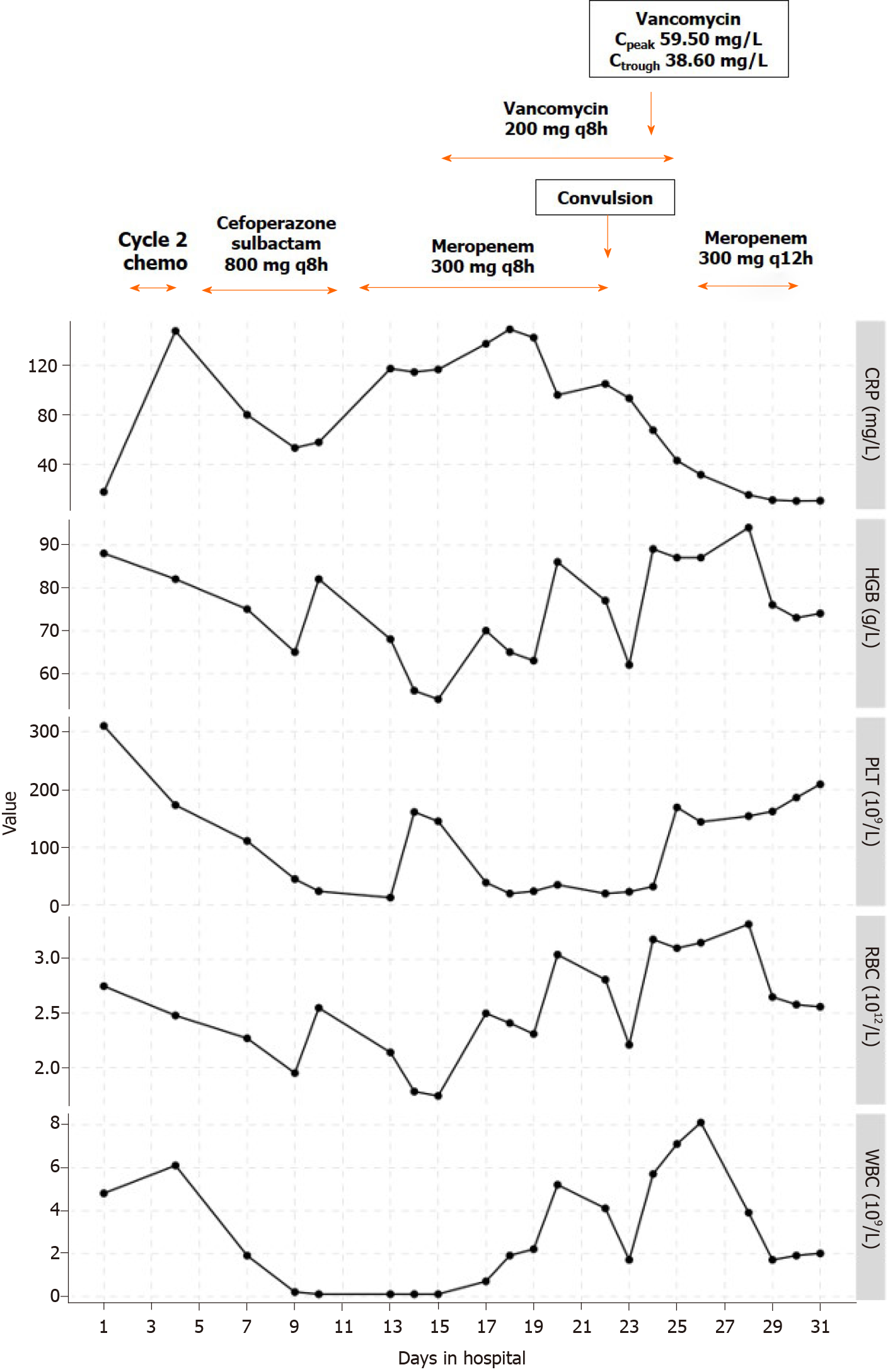
Before Cycle 2 of chemotherapy was started, laboratory tests showed C reactive protein 23 mg/L (normal range < 8 mg/L), hemoglobin 89 g/L (normal range 110-160 g/L), platelet count 265 × 109/L [normal range (100-400) × 109/L], red blood cell count 2.71 × 1012/L [normal range (4.0-5.5) × 1012/L], and white blood cell count 5.4 × 109 /L [normal range (4.0-10.0) × 109/L].
On day 23 (the day after convulsion onset), an 0.8 mL sample of CSF was collected and tested, which showed a clear colorless appearance, negative Pandy’s reaction for detecting protein levels, 1 × 106/L cell count, and no growth of bacteria or fungi.
On day 24, the plasma concentrations of vancomycin were measured with the peak concentration of 59.5 mg/L (normal range 20-40 mg/L) and the trough concentration of 38.6 mg/L (normal range 5-10 mg/L), which were higher than normal.
CT scan of the brain on day 22 revealed widened sulcus fissure and full supratentorial ventricle. There were no abnormal dense shadows in the cerebral parenchyma. Thickening of the nasal sinus mucosa was seen. Magnetic resonance imaging was conducted on day 23 to further evaluate the brain. The results showed that the splenium of the corpus callosum had an abnormal patchy signal shadow on T1 weighted image, T2 total internal reflection fluorescence microscopy and T2 weighted image images. No other abnormal signals in the rest of the brain were observed.
Convulsion with unknown cause, chemotherapy for tumor treatment, retroperitoneal tumor (neuroblastoma post-biopsy; IV stage; MYCN-non-amplified), and hypertension.
Cycle 2 chemotherapy, including 1380 mg CPM for two days, 16.5 mg DOX for three days, and 0.4 mg VCR for three days, was started on day 2 in hospital. On day 5, intravenous cefoperazone-sulbactam 800 mg every 8 h (q8h) for 7 d was administered for anti-infection. On day 12, a stool test showed rotavirus infection, and intravenous meropenem 300 mg q8h was prescribed. On day 15, vancomycin 200 mg q8h with an infusion duration of 40 min and fluconazole 48 mg daily (qd) were prescribed as anti-infective treatment considering her continuous fever. On day 17, the fever recurred with a peak body temperature as high as 39.2℃, and symptomatic treatment lowered the temperature but fever recurred. Intravenous immunoglobulin was added to the treatment plan and anti-infective medication prescriptions continued.
On day 22, during convulsion onset, chloral hydrate and phenobarbital were given for sedation. 250 mL physiological saline solution was infused for fluid expansion.
When the patient was transferred to the PICU, midazolam was infused at a rate of 1 μg/(kg· min), and deslanoside was administered to control her heart rate. Mannitol and glycerol fructose were used alternatively to lower intracranial pressure.
On day 24, the plasma concentrations of vancomycin were reported to be abnormally high; thus, vancomycin administration was stopped. Teicoplanin was given instead and combined with meropenem and fluconazole as the anti-infective treatment strategy. The patient’s blood pressure increased to 125-140/70-90 mmHg and nicardipine was prescribed to lower her blood pressure.
On day 26, the plasma concentration of vancomycin was measured again and was 20.5 mg/L, which was lower than two days previously. On day 30, the patient was transferred back to the Department of Oncology and the anti-infective treatment combination was changed to meropenem only. On day 32, the patient was discharged. Follow-up at one month, two months, and six months later revealed no further convulsion, and due to the previous abnormally high plasma concentrations, the patient did not receive vancomycin.
The common etiology of convulsion can be divided into two major categories[26-29]: Infective and non-infective. Infective causes include purulent meningitis and infective toxic encephalopathy. Non-infective causes include intracranial disorders (epilepsy, space-occupying lesion such as intracranial hemorrhage, and atelencephalia) and extracranial disorders (toxicity caused by a prescription drug overdose, blood glucose and electrolytes disorders, such as hypoglycemia, hyponatremia, hypocalcemia, etc., and genetic metabolic diseases, such as organic acidemia, aminoacidopathy and lipodystrophia).
Differential diagnosis was conducted to determine the underlying cause of the convulsion that led to PICU admission. The patient had myelosuppression after chemotherapy, presenting with low red blood cell, white blood cell and platelet counts. Infection secondary to chemotherapy was possible, but purulent meningitis was ruled out by lumbar puncture, which showed no bacterial growth in the CSF culture. Infective toxic encephalopathy caused by severe infection such as sepsis can also lead to convulsion. However, the patient had no fever and no symptoms of systemic intoxication at that time, and the blood culture results did not show any signs of bacterial growth within five days. Therefore, the unexpected convulsion was most likely caused by non-infective conditions. The patient had no history of epilepsy. Although the patient had a history of asphyxia and anoxia, intracranial hemorrhage was ruled out by CT. Magnetic resonance imaging did not reveal signs of atelecephalia. The patient had no electrolyte or blood glucose abnormalities, and no genetic metabolic disease was detected.
The only abnormal phenomenon noted during the treatment process was the high plasma concentration of vancomycin. The patient started vancomycin on day 15 at a dose of 200 mg q8h and an infusion duration of approximately 40 min, which was within the normal dosing range of 40-60 mg/(kg· d) divided every 6 to 8 h[15,21]. On day 22, the convulsion occurred and the patient was transferred to the PICU. The timeline of medications and laboratory test results are shown in Figure 1. The concentrations of vancomycin were not measured until day 24, and warning values of both peak and trough concentrations were reported, which were 59.5 mg/L and 38.6 mg/L, respectively. Simulation with the Bayesian approach using MwPharm software (Version 1.6.1.128, Mediware, Prague, Czech Republic) showed that the area under the concentration-time curve over 24 h (AUC0-24 h) was 1086.6 mg· h/L. Assuming the minimum inhibitory concentration was 1 mg/L, the AUC/MIC was 1086.6. The simulated curve is displayed in Figure 2.
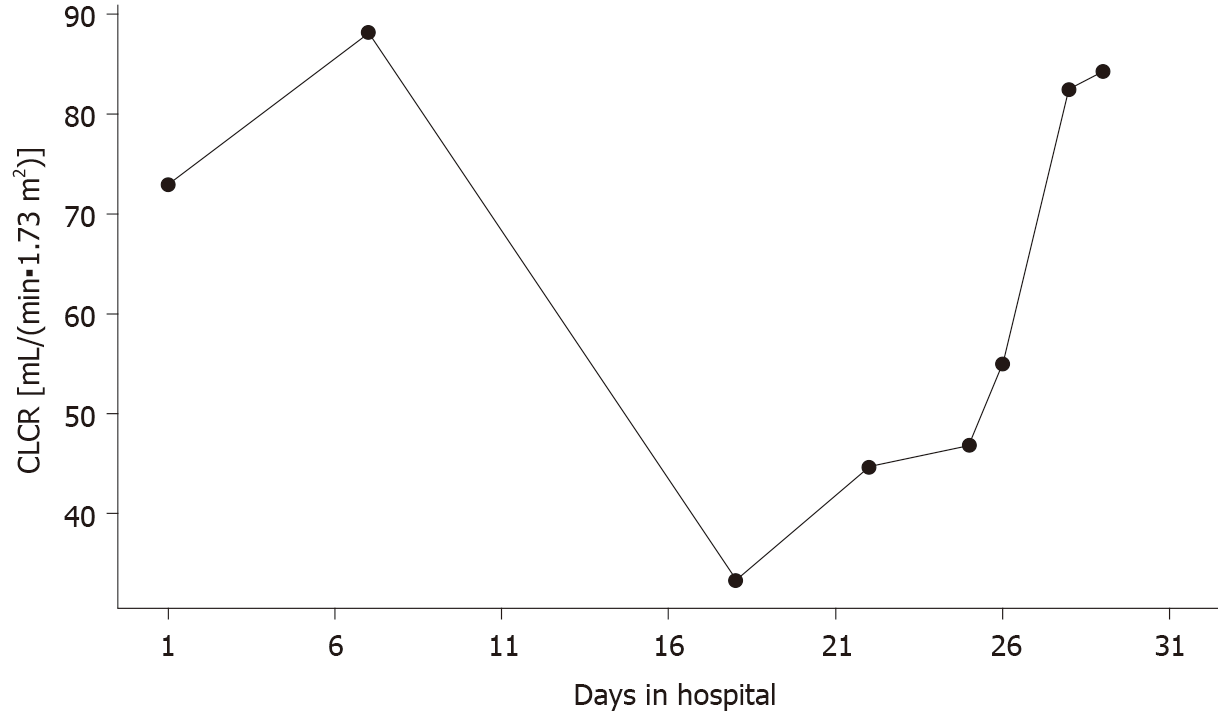
According to previous research, when the AUC is over 600 mg· h /L, the risk of acute kidney injury (AKI) increases[30]. A meta-analysis of 10 studies in pediatric populations by Fiorito et al[31] reported that a vancomycin trough concentration of ≥ 15 mg/L increased the odds ratio of AKI by 2.7-fold. In our patient, the creatinine clearance (CLCR) estimated by the Schwartz formula of CLCR = (0.413 × Height)/Creatinine[32], indicated potential impairment of renal function from the beginning of hospitalization, the changes of which are shown in Figure 3. There was a significant decrease in CLCR on day 18 with the value of 33.3 mL/(min· 1.73 m2). The sudden decrease in renal function was probably due to the increased vancomycin concentration. On the other hand, the decreased renal function facilitated the accumulation of vancomycin in the patient’s body, thus forming the vicious cycle between the vancomycin concentration and renal function. Moreover, it was likely that the concentration of vancomycin was already over the normal therapeutic window before the unexpected convulsion occurred. Researchers have found no correlation between dose and CSF concentration[33], meaning the patient could have a high CSF concentration of vancomycin even when on a normal dose of vancomycin. High plasma vancomycin concentration could indicate a high CSF concentration, thus leading to central nervous symptoms such convulsion as in this patient. According to a previous report of an infant receiving intrathecal vancomycin administration, the elimination half-life of vancomycin in the CSF can be as long as 77.7 h[34]. Another report showed that an anephric child had accumulated concentrations of vancomycin during oral therapy for Clostridium difficile pseudomembranous colitis[23]. The documented serum vancomycin level was 34.0 mg/L and the CSF level was 4.2 mg/L when the patient presented with unexplained fever and encephalopathy, which were resolved by discontinuation of vancomycin and hemodialysis. Therefore, in our case, vancomycin could have accumulated in the CSF and eventually led to the sudden convulsion during vancomycin treatment. Unfortunately, the concentration of vancomycin in CSF was not measured before or after the convulsion, which should be considered in future clinical practice when the plasma concentration of vancomycin is overly high.
Of note, this patient had neuroblastoma and received four cycles of high-risk group chemotherapy. The blood-brain barrier in this patient may have been altered due to the disease state or cytotoxic medications[35]; thus, vancomycin could more easily enter the central nervous system and induce the convulsion. To replace vancomycin as part of the anti-infective treatment plan in patients receiving chemotherapy, teicoplanin can be considered as it has a high plasma protein binding rate (90%-95%) and its penetration into the CSF is lower compared with vancomycin[36,37]. Moreover, teicoplanin has a lower incidence of adverse effects including nephrotoxicity than vancomycin[38]. Teicoplanin is also a better choice for patients with insufficient renal function.
A rare case of vancomycin-related convulsion in a pediatric patient with neuroblastoma receiving chemotherapy is reported. Physiological conditions in children with tumors and receiving anti-tumor chemotherapy may be different to other patients. The dosing strategy of antibiotics, especially those with a narrow therapeutic window and greater toxicity, should be chosen with caution in this special patient group. Thorough evaluation and close monitoring should be conducted throughout the treatment process to decide if dosage adjustment is necessary. When unexpected convulsion occurs but cannot be explained by other causes, plasma and CSF drug concentrations should be measured to determine if the patient is intoxicated by prescribed drugs. Further research into the pharmacokinetics of vancomycin in pediatric patients receiving anti-tumor chemotherapy is warranted to generate more reliable evidence for better clinical practice.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kupeli S S-Editor: Zhang L L-Editor: Webster JR P-Editor: Liu JH
| 1. | Tsubota S, Kadomatsu K. Origin and initiation mechanisms of neuroblastoma. Cell Tissue Res. 2018;372:211-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 111] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 2. | Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1600] [Cited by in RCA: 1623] [Article Influence: 73.8] [Reference Citation Analysis (0)] |
| 3. | Maris JM. Recent advances in neuroblastoma. N Engl J Med. 2010;362:2202-2211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1588] [Cited by in RCA: 1508] [Article Influence: 100.5] [Reference Citation Analysis (0)] |
| 4. | Sharma R, Mer J, Lion A, Vik TA. Clinical Presentation, Evaluation, and Management of Neuroblastoma. Pediatr Rev. 2018;39:194-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Maris JM, Mosse YP, Bradfield JP, Hou C, Monni S, Scott RH, Asgharzadeh S, Attiyeh EF, Diskin SJ, Laudenslager M, Winter C, Cole KA, Glessner JT, Kim C, Frackelton EC, Casalunovo T, Eckert AW, Capasso M, Rappaport EF, McConville C, London WB, Seeger RC, Rahman N, Devoto M, Grant SF, Li H, Hakonarson H. Chromosome 6p22 Locus associated with clinically aggressive neuroblastoma. N Engl J Med. 2008;358:2585-2593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 244] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 6. | Aygun N. Biological and Genetic Features of Neuroblastoma and Their Clinical Importance. Curr Pediatr Rev. 2018;14:73-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 7. | Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64:83-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1416] [Cited by in RCA: 1592] [Article Influence: 144.7] [Reference Citation Analysis (1)] |
| 8. | Twist CJ, Schmidt ML, Naranjo A, London WB, Tenney SC, Marachelian A, Shimada H, Collins MH, Esiashvili N, Adkins ES, Mattei P, Handler M, Katzenstein H, Attiyeh E, Hogarty MD, Gastier-Foster J, Wagner E, Matthay KK, Park JR, Maris JM, Cohn SL. Maintaining Outstanding Outcomes Using Response- and Biology-Based Therapy for Intermediate-Risk Neuroblastoma: A Report From the Children's Oncology Group Study ANBL0531. J Clin Oncol. 2019;37:3243-3255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 9. | Ladenstein R, Pötschger U, Pearson ADJ, Brock P, Luksch R, Castel V, Yaniv I, Papadakis V, Laureys G, Malis J, Balwierz W, Ruud E, Kogner P, Schroeder H, de Lacerda AF, Beck-Popovic M, Bician P, Garami M, Trahair T, Canete A, Ambros PF, Holmes K, Gaze M, Schreier G, Garaventa A, Vassal G, Michon J, Valteau-Couanet D; SIOP Europe Neuroblastoma Group (SIOPEN). Busulfan and melphalan vs carboplatin, etoposide, and melphalan as high-dose chemotherapy for high-risk neuroblastoma (HR-NBL1/SIOPEN): an international, randomised, multi-arm, open-label, phase 3 trial. Lancet Oncol. 2017;18:500-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 238] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 10. | Twist CJ, Naranjo A, Schmidt ML, Tenney SC, Cohn SL, Meany HJ, Mattei P, Adkins ES, Shimada H, London WB, Park JR, Matthay KK, Maris JM. Defining Risk Factors for Chemotherapeutic Intervention in Infants With Stage 4S Neuroblastoma: A Report From Children's Oncology Group Study ANBL0531. J Clin Oncol. 2019;37:115-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Kushner BH, LaQuaglia MP, Bonilla MA, Lindsley K, Rosenfield N, Yeh S, Eddy J, Gerald WL, Heller G, Cheung NK. Highly effective induction therapy for stage 4 neuroblastoma in children over 1 year of age. J Clin Oncol. 1994;12:2607-2613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 120] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | London WB, Frantz CN, Campbell LA, Seeger RC, Brumback BA, Cohn SL, Matthay KK, Castleberry RP, Diller L. Phase II randomized comparison of topotecan plus cyclophosphamide vs topotecan alone in children with recurrent or refractory neuroblastoma: a Children's Oncology Group study. J Clin Oncol. 2010;28:3808-3815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 13. | Rujkijyanont P, Photia A, Traivaree C, Monsereenusorn C, Anurathapan U, Seksarn P, Sosothikul D, Techavichit P, Sanpakit K, Phuakpet K, Wiangnon S, Chotsampancharoen T, Chainansamit SO, Kanjanapongkul S, Meekaewkunchorn A, Hongeng S. Clinical outcomes and prognostic factors to predict treatment response in high risk neuroblastoma patients receiving topotecan and cyclophosphamide containing induction regimen: a prospective multicenter study. BMC Cancer. 2019;19:961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Millan X, Muggia V, Ostrowsky B. Antimicrobial agents, drug adverse reactions and interactions, and cancer. Cancer Treat Res. 2014;161:413-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Dehority W. Use of vancomycin in pediatrics. Pediatr Infect Dis J. 2010;29:462-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Sullins AK, Abdel-Rahman SM. Pharmacokinetics of antibacterial agents in the CSF of children and adolescents. Paediatr Drugs. 2013;15:93-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 17. | Bruniera FR, Ferreira FM, Saviolli LR, Bacci MR, Feder D, da Luz Gonçalves Pedreira M, Sorgini Peterlini MA, Azzalis LA, Campos Junqueira VB, Fonseca FL. The use of vancomycin with its therapeutic and adverse effects: a review. Eur Rev Med Pharmacol Sci. 2015;19:694-700. [PubMed] |
| 18. | Nau R, Sörgel F, Eiffert H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev. 2010;23:858-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 717] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 19. | Rybak MJ. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis. 2006;42 Suppl 1:S35-S39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 562] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 20. | Beach JE, Perrott J, Turgeon RD, Ensom MHH. Penetration of Vancomycin into the Cerebrospinal Fluid: A Systematic Review. Clin Pharmacokinet. 2017;56:1479-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 21. | Rybak MJ, Le J, Lodise TP, Levine DP, Bradley JS, Liu C, Mueller BA, Pai MP, Wong-Beringer A, Rotschafer JC, Rodvold KA, Maples HD, Lomaestro BM. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77:835-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 733] [Article Influence: 183.3] [Reference Citation Analysis (0)] |
| 22. | Leibowitz G, Golan D, Jeshurun D, Brezis M. Mononeuritis multiplex associated with prolonged vancomycin treatment. BMJ. 1990;300:1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 23. | Thompson CM Jr, Long SS, Gilligan PH, Prebis JW. Absorption of oral vancomycin - possible associated toxicity. Int J Pediatr Nephrol. 1983;4:1-4. [PubMed] |
| 24. | Caglayan S, Ozdogru E, Aksit S, Kansoy S, Senturk H. Vancomycin-induced hypertension with transient blindness and generalized seizure. Acta Paediatr Jpn. 1992;34:90-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Brodeur GM, Pritchard J, Berthold F, Carlsen NL, Castel V, Castelberry RP, De Bernardi B, Evans AE, Favrot M, Hedborg F. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11:1466-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1656] [Cited by in RCA: 1587] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 26. | Gavvala JR, Schuele SU. New-Onset Seizure in Adults and Adolescents: A Review. JAMA. 2016;316:2657-2668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 27. | Kotsopoulos I, de Krom M, Kessels F, Lodder J, Troost J, Twellaar M, van Merode T, Knottnerus A. Incidence of epilepsy and predictive factors of epileptic and non-epileptic seizures. Seizure. 2005;14:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Mehta A, Zusman BE, Choxi R, Shutter LA, Yassin A, Antony A, Thirumala PD. Seizures After Intracerebral Hemorrhage: Incidence, Risk Factors, and Impact on Mortality and Morbidity. World Neurosurg. 2018;112:e385-e392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Leung H, Man CB, Hui AC, Kwan P, Wong KS. Prognosticating acute symptomatic seizures using two different seizure outcomes. Epilepsia. 2010;51:1570-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Suzuki Y, Kawasaki K, Sato Y, Tokimatsu I, Itoh H, Hiramatsu K, Takeyama M, Kadota J. Is peak concentration needed in therapeutic drug monitoring of vancomycin? Chemotherapy. 2012;58:308-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 31. | Fiorito TM, Luther MK, Dennehy PH, LaPlante KL, Matson KL. Nephrotoxicity With Vancomycin in the Pediatric Population: A Systematic Review and Meta-Analysis. Pediatr Infect Dis J. 2018;37:654-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 32. | Mian AN, Schwartz GJ. Measurement and Estimation of Glomerular Filtration Rate in Children. Adv Chronic Kidney Dis. 2017;24:348-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 152] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 33. | Jorgenson L, Reiter PD, Freeman JE, Winston KR, Fish D, McBride LA, Handler MH. Vancomycin disposition and penetration into ventricular fluid of the central nervous system following intravenous therapy in patients with cerebrospinal devices. Pediatr Neurosurg. 2007;43:449-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Pau AK, Smego RA Jr, Fisher MA. Intraventricular vancomycin: observations of tolerance and pharmacokinetics in two infants with ventricular shunt infections. Pediatr Infect Dis. 1986;5:93-96. [PubMed] |
| 35. | Banks WA. From blood-brain barrier to blood-brain interface: new opportunities for CNS drug delivery. Nat Rev Drug Discov. 2016;15:275-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 771] [Article Influence: 85.7] [Reference Citation Analysis (0)] |
| 36. | Stahl JP, Croize J, Wolff M, Garaud JJ, Leclercq P, Vachon F, Micoud M. Poor penetration of teicoplanin into cerebrospinal fluid in patients with bacterial meningitis. J Antimicrob Chemother. 1987;20:141-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 37. | Byrne CJ, Roberts JA, McWhinney B, Fennell JP, O'Byrne P, Deasy E, Egan S, Desmond R, Enright H, Ryder SA, D'Arcy DM, McHugh J. Variability in Trough Total and Unbound Teicoplanin Concentrations and Achievement of Therapeutic Drug Monitoring Targets in Adult Patients with Hematological Malignancy. Antimicrob Agents Chemother. 2017;61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 38. | Cavalcanti AB, Goncalves AR, Almeida CS, Bugano DD, Silva E. Teicoplanin vs vancomycin for proven or suspected infection. Cochrane Database Syst Rev. 2010: CD007022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |