Published online May 6, 2021. doi: 10.12998/wjcc.v9.i13.3014
Peer-review started: November 6, 2020
First decision: January 23, 2021
Revised: February 1, 2021
Accepted: March 19, 2021
Article in press: March 19, 2021
Published online: May 6, 2021
Processing time: 166 Days and 18.6 Hours
The Updated Sydney system for visual evaluation of gastric mucosal atrophy via endoscopic observation is subject to sampling error and interobserver variability. The Kimura-Takemoto classification system was developed to overcome these limitations.
To compare the morphological classification of atrophic gastritis between the Kimura-Takemoto system and the Updated Sydney system.
A total of 169 patients with atrophic gastritis were selected according to diagnosis by the visual endoscopic Kimura-Takemoto method. Following the Updated Kimura-Takemoto classification system, one antrum biopsy and five gastric corpus biopsies were taken according to the visual stages of the Kimura-Takemoto system. The Updated Kimura-Takemoto classification system was then applied to each and showed 165 to have histological mucosal atrophy; the remaining 4 patients had no histological evidence of atrophy in any biopsy. The Updated Kimura-Takemoto classification was verified as a reference morphological method and applied for the diagnosis of atrophic gastritis. Adding one more biopsy from the antrum to the six biopsies according to the Updated Kimura-Takemoto classification, constitutes the updated combined Kimura-Takemoto classification and Sydney system.
The sensitivity for degree of mucosal atrophy assessed by the Updated Sydney system was 25% for mild, 36% for moderate, and 42% for severe, when compared with the Updated Kimura-Takemoto classification of atrophic gastritis for morphological diagnosis. Four types of multifocal atrophic gastritis were identified: sequential uniform (type 1; in 28%), sequential non-uniform (type 2; in 7%), diffuse uniform (type 3; in 23%), diffuse non-uniform (type 4; in 24%), and "alternating atrophic – non-atrophic" (type 5; in 18%). The pattern of the spread of atrophy, sequentially from the antrum to the cardiac segment of the stomach, which was described by the Updated Kimura-Takemoto system, was histologically confirmed in 82% of cases evaluated.
The Updated Sydney system is significantly inferior to the Updated Kimura-Takemoto classification for morphological verification of atrophic gastritis.
Core Tip: An open question in the clinical management of gastritis is whether the current Updated Sydney system is appropriate for classification. It appears to have become obsolete in view of the increasing knowledge on atrophic gastritis. Thus, we propose a new classification system for atrophic gastritis based on morphological criteria, named as the Updated Kimura-Takemoto classification system. This new system correctly reflects the severity of histological atrophy in the gastric mucosa, exceeding the Updated Sydney system in accuracy of detecting gastric atrophy. Moreover, it has higher sensitivity for detecting mild, moderate and severe atrophic gastritis.
- Citation: Kotelevets SM, Chekh SA, Chukov SZ. Updated Kimura-Takemoto classification of atrophic gastritis. World J Clin Cases 2021; 9(13): 3014-3023
- URL: https://www.wjgnet.com/2307-8960/full/v9/i13/3014.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i13.3014
New advanced high-resolution endoscopic technologies are yielding promising results for detection of precancerous changes and precancerous diseases of the stomach[1]. The Kimura-Takemoto visual endoscopic classification system for atrophic gastritis only allows for detection of atrophic borders present in the gastric mucosa[2]. Using this system, Kishino et al[3] and Song et al[4] proposed the scoring of endoscopic atrophic gastritis to follow: C-0 for no atrophy; C-1 and C-2 for mild atrophy; C-3 and O-1 for moderate atrophy; and O-2 and O-3 for severe atrophy. However, visual endoscopic diagnosis of atrophic gastritis by the Kimura-Takemoto system is known to have variable accuracy[5].
Since gastric cancer risk correlates with severity and extent of atrophic gastritis, it is advisable to verify the categorization of any atrophic gastritis finding by histological analysis. A well-documented example of the prognostic dangers associated with development of gastric cancer involves Helicobacter pylori gastritis, for which risk is dependent upon the extent and severity of mucosal atrophy[6]. There are several classification systems that have been proposed for the histological grading of atrophic gastritis; these include the Operative Link for Gastritis Assessment (commonly known as OLGA) and Operative Link for Gastric Intestinal Metaplasia Assessment (commonly known as OLGIM), in addition to the internationally-accepted Sydney system[7-9].
The relative risk and cumulative risk of gastric cancer have been calculated for the different degrees of atrophic gastritis of the antrum and body determined using histological criteria for mild, moderate and severe atrophy[10]. It is important, now, to determine which method is optimal for assessment of the degree of mucosal atrophy. This will benefit both diagnosis and prognosis, as gastric cancer might start to regress if gastric atrophy and intestinal metaplasia begin to improve, as during treatment. Therefore, it is necessary to monitor and promote the regression of atrophic gastritis, which will require accurate criteria for assessing the degree of mucosal atrophy[11].
To develop an accurate diagnostic method for atrophic gastritis and elaborate the criteria for assessment of extent (i.e., mild, moderate, and severe). Ultimately, the method should allow for appropriate determination of high and low risk of progression to gastric cancer and facilitate the monitoring of regression of mucosal atrophy.
The study was conducted according to the updated Declaration of Helsinki in the Karachay-Cherkess Republic, Russian Federation from 2003 to 2004. The study population was comprised of 169 dyspeptic patients diagnosed with atrophic gastritis by endoscopy using the Kimura-Takemoto visual endoscopic classification system. The patients represented 58 males and 111 females, ranging in age from 18-years-old to 81-years-old (mean age of 66.44 ± 10.22 years). All patients gave informed consent for study inclusion. The study participants were asked to stop any medication with proton pomp inhibitors, H2 antagonists, or non-steroidal anti-inflammatory drugs at least 1 mo prior to performance of study examinations.
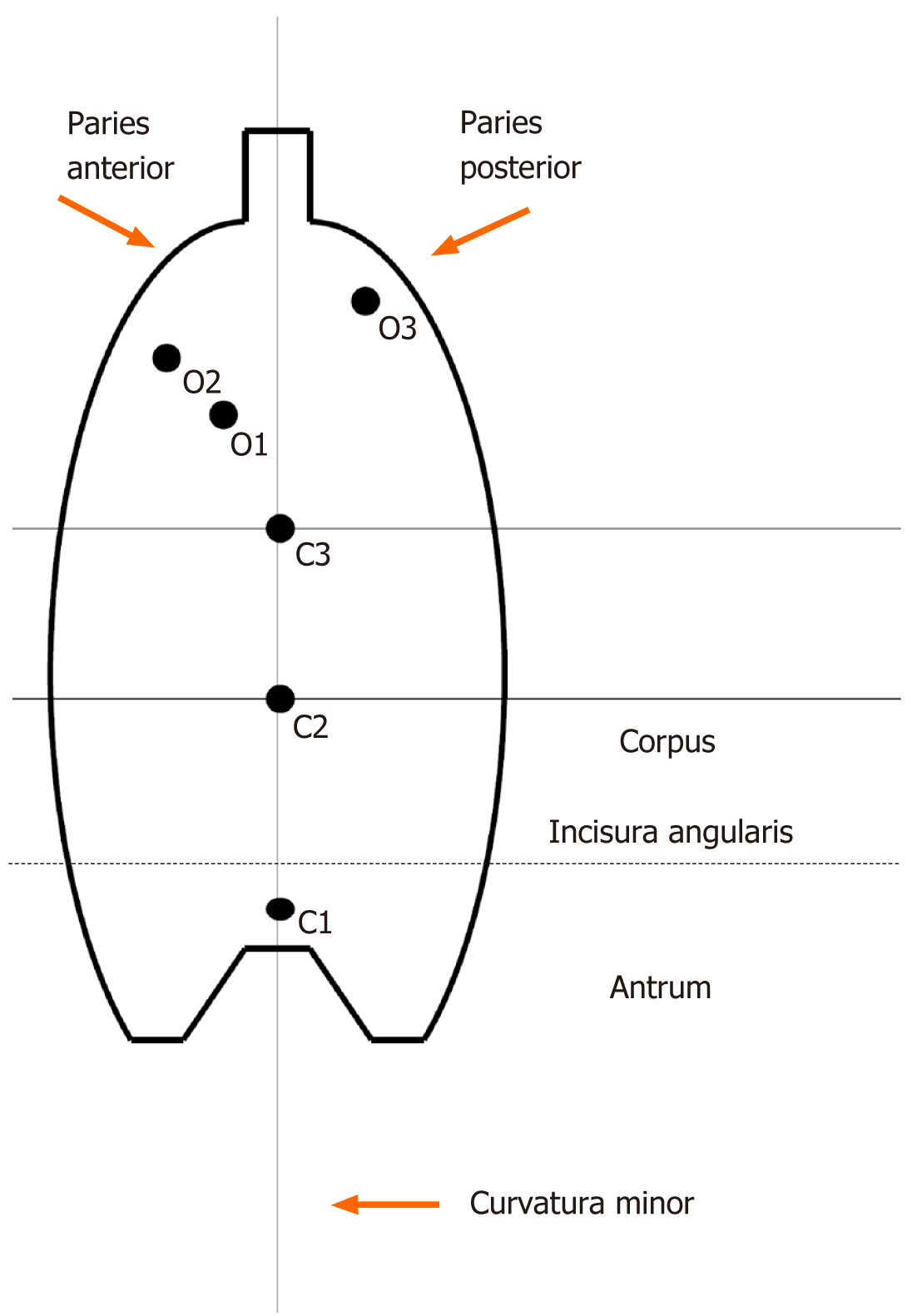
All patients underwent endoscopic visual assessment to determine the type of mucosal atrophy according to Kimura-Takemoto grading, as follows: C-0: No atrophy; C-1: Atrophy exclusively in the antrum; C-2: Border of atrophy lying on the lesser curvature in the lower third of the gastric corpus; C-3: Border of atrophy lying on the lesser curvature in the middle third of the gastric corpus; O-1: Border of atrophy involving the boundary lying between the lesser curvature and the anterior wall of the gastric corpus; O-2: Border of atrophy lying within the anterior wall of the gastric corpus; and O-3: Border of atrophy involving the boundary between the posterior wall of the gastric corpus and large curvature. In addition, six biopsy samples were taken from each patient, for visual histological assessment of atrophic gastritis also using the Kimura-Takemoto grading system (from C-1 to O-3 as above)[2,12]. Of note, the C-1 (antrum) biopsy of the Kimura-Takemoto system corresponded to the three (including incisura angularis) antrum biopsies of the Updated Sydney system. Since a biopsy specimen from the incisura angularis (Updated Sydney system) does not contain parietal nor the main cells of the gastric glands, we considered it as an antral biopsy. Biopsies O-2 and O-3 (corpus) of the Kimura-Takemoto system corresponded to the two corpus biopsies of the Updated Sydney system. This principle of morphological assessment of atrophic gastritis was used to achieve greater accuracy because of the three additional biopsy samples from the gastric corpus (i.e., C-2, C-3, O-1).
Thus, the evaluation of gastric atrophy designed for this study used findings acquired by the Kimura-Takemoto system from endoscopic visualization supplemented with findings from histological examination of six biopsy specimens. This newly proposed system represents an “Updated Kimura-Takemoto classification system for atrophic gastritis” (Figure 1).
The biopsied specimens were fixed in 10% formalin, embedded in paraffin, sliced into sequential 5-μm sections, and stained with hematoxylin and eosin. Periodic acid Schiff/Alcian blue (pH 2.5) staining and Giemsa staining were also performed. The grade of observed stomach mucosal atrophy was estimated from mild to severe according to the Houston visual analogue scale (VAS)[13].
Sensitivity and specificity indicators were used for the comparison of the Updated Sydney system with the Updated Kimura-Takemoto classification system for atrophic gastritis.
Of the 169 patients with atrophic gastritis who were selected by the visual endoscopic Kimura-Takemoto method, 165 showed histological mucosal atrophy using the Updated Kimura-Takemoto method and the remaining 4 patients had no histological evidence of atrophy in any biopsy specimen. Among the 165 patients with verified mucosal atrophy, 121 had at least one of their five biopsy specimens of gastric corpus show severe histological mucosal atrophy.
The method of evaluation of the gastric mucosa using five gastric corpus biopsies assessed by the Updated Kimura-Takemoto endoscopic classification was thus adopted as the reference method for morphological diagnostics. The sensitivity and specificity for the reference method were taken as 100%. The finding of five gastric corpus biopsies showing a histological score in accordance with the VAS was considered to accurately reflect the degree of mucosal atrophy in this part of the stomach. It is very important to note that this approach allows for the differentiation of degree of atrophy (mild, moderate, severe) between each biopsy.
For each patient, two gastric corpus biopsy specimens corresponded topo
The prevalence of severe atrophy in gastric corpus mucosa specimens was assessed morphologically using VASs derived from the Updated Sydney system (in two biopsy specimens) and the Updated Kimura-Takemoto classification system (in five biopsy specimens). As a reminder, two biopsies were taken from the gastric corpus according to the Updated Sydney system and five biopsies were taken according to the Updated Kimura-Takemoto classification system. The principle of determining mild, moderate and severe atrophy was as follows: If only one (from among the two or five, respectively) of the biopsies was classified as mild, moderate or severe atrophy, then such a degree indicated the presence of atrophic gastritis for that patient. Since the conclusion was made in the absence of a more severe degree of atrophy in other biopsy specimens, the degree of atrophy (mild, moderate, or severe) would have to be verified. Non-atrophic gastritis was diagnosed only in the absolute absence of atrophy in all seven biopsies from a patient, by both systems. Four patients were determined to have non-atrophic gastritis.
Comparative analysis of the histological characteristics of biopsy specimens using the two classification systems led to the determination of the sensitivity and specificity indices for the Updated Sydney system in identifying mild, moderate, and severe corpus-predominant atrophic gastritis (Table 1).
| Measure | Atrophy class | ||
| Mild | Moderate | Severe | |
| Sensitivity | 25% | 36% | 42% |
| Specificity | 100% | 100% | 100% |
| Patients with atrophy by the reference method, n | 8 | 36 | 121 |
| Patients with atrophy by the Updated Sydney system, n | 2 | 13 | 51 |
| Pseudo-negative results by the Updated Sydney system, n | 6 | 23 | 70 |
The prevalence of mild, moderate, and severe gastric mucosal atrophy depends on the number of biopsy specimens for the updated histological Sydney system and the updated endoscopic Kimura-Takemoto classification. The data are presented in Table 2 and Table 3. A greater number of atrophic gastritis findings were obtained from a greater number of biopsy specimens when the updated endoscopic Kimura-Takemoto classification system was used. This finding itself served as the basis for the low sensitivity level for the histological detection of atrophy in accordance with the Updated Sydney system (sensitivity of 25% for mild, 36% for moderate, and 42% for severe atrophy, when compared to the Updated Kimura-Takemoto classification system).
| Atrophy class, patients | Biopsy count | |
| 1 | 2 | |
| Mild, n = 2 | 1 (0.6) | 2 (1.2) |
| Moderate, n = 13 | 9 (5.5) | 13 (7.9) |
| Severe, n = 51 | 28 (17.0) | 51 (31.0) |
| Biopsy count | |||||
| Atrophy class, patients | 1 | ≤ 2 | ≤ 3 | ≤ 4 | ≤ 5 |
| Mild, n = 8 | 2 (1.2) | 5 (3.0) | 6 (3.6) | 7 (4.2) | 8 (4.8) |
| Moderate, n = 36 | 21 (12.7) | 27 (16.4) | 30 (18.2) | 34 (20.6) | 36 (21.8) |
| Severe, n = 121 | 40 (24.0) | 79 (48.0) | 96 (58.0) | 109 (66.0) | 121 (73.4) |
In our opinion, the optimal number of biopsies for detection of atrophy in the corpus of the stomach was deemed to be five because further increase in the number of biopsies does not significantly improve the detectability of atrophic gastritis. Kimura and Takemoto determined the direction in which atrophy develops, namely from the pyloric antrum to the greater curvature of the stomach, which they designated as C0-O3. In their opinion, the development of atrophy in the stomach occurs uniformly and sequentially[14,15]. Thus, we structured the assessment of atrophy in the stomach corpus according to histological data from biopsy specimens that were taken by following the endoscopic stages of Kimura-Takemoto C2-O3. The Kyoto Consensus recommended that assessment of the severity of stomach atrophy be based upon the single biopsy specimen of the stomach mucosa that exhibits the most severe degree of atrophy; in other words, if one biopsy specimen from among five taken from a single patient showed severe atrophy, then the patient’s case would be classified wholly as severe atrophy[6].
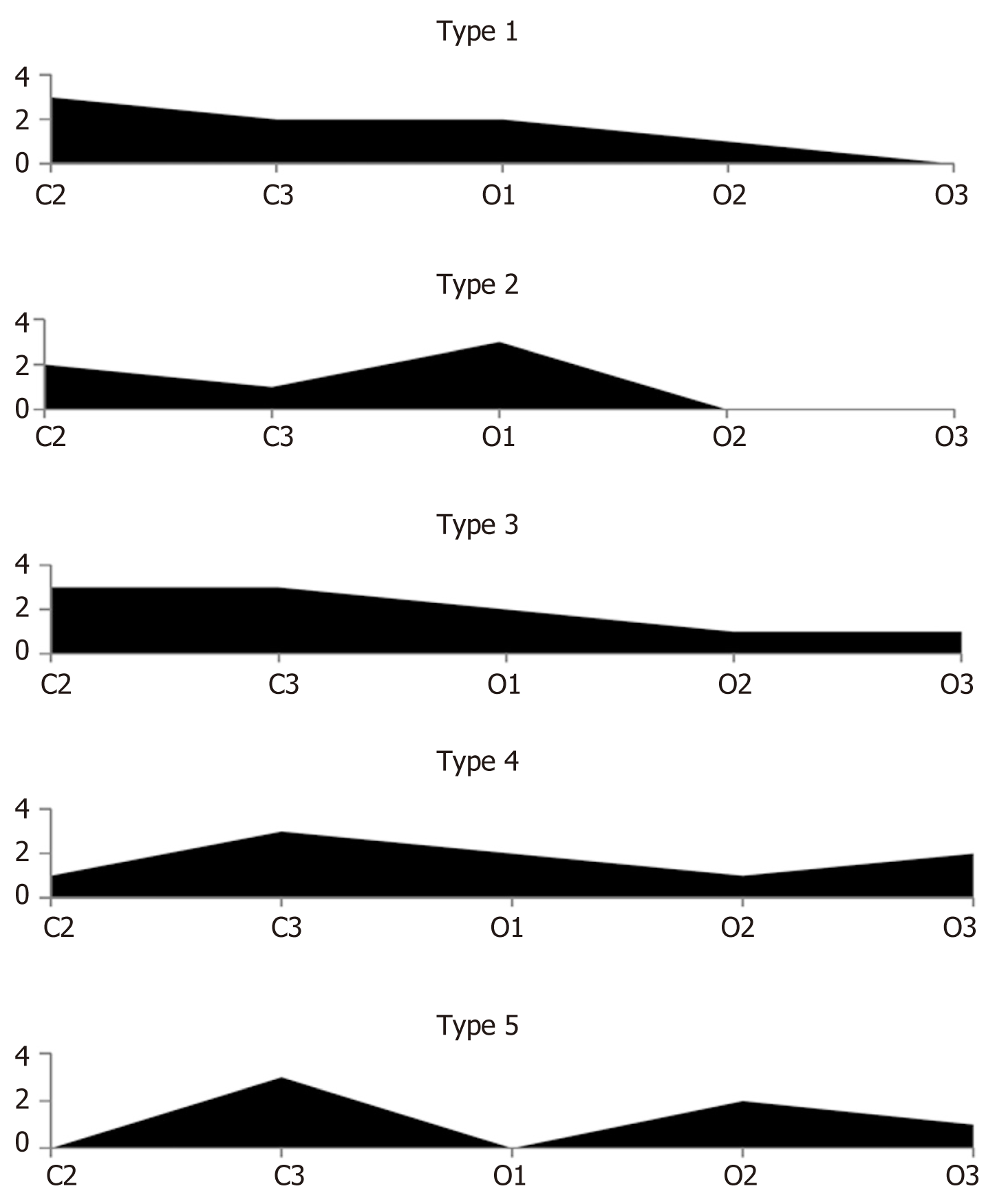
When, in this study, we analyzed the morphological findings from multiple biopsies of the stomach mucosa within the framework of the updated endoscopic Kimura-Takemoto classification alone, some patterns of the development of atrophic changes were identified. Based on these patterns, five types of development of atrophy of the corpus of the stomach were distinguishable (Figure 2 and Table 4).
| Type of gastric atrophy | Patients, n | Patients (%) |
| Sequential uniform type | 46 | 28 |
| Sequential non-uniform type | 11 | 7 |
| Diffuse uniform type | 38 | 23 |
| Diffuse non-uniform type | 40 | 24 |
| "Alternating atrophic – non-atrophic" | 30 | 18 |
| Total | 165 | 100 |
For the first type (sequential uniform), atrophic changes in the stomach mucosa of varying severity are detected in corpus biopsy specimens sequentially, from Kimura-Takemoto stage C2 to O3. The uniformity of the process lies within the fact that, for this type, the atrophy occupies the entire territory of the corpus of the stomach, gradually decreasing in severity from stage to stage, until it goes absent. In the second type (sequential non-uniform), the unevenness of the atrophic process is manifested by the fact that the severity of atrophic changes sequentially occupying the different parts of the corpus of the stomach can be different. The third type (diffuse uniform) is characterized by all five gastric corpus biopsy specimens showing atrophic changes in the mucosa, of varying severity from stage C2 to O3. The uniformity of the process, as for the first type, lies in the fact that atrophy occupies the entire territory of the corpus of the stomach from stage to stage, gradually decreasing in severity from C2 to O3. The fourth type (diffuse non-uniform) is characterized by the uneven development of atrophic changes of varying severity, from stage C2 to O3, present in all five gastric corpus biopsy specimens; this type will have no biopsy specimens that show no atrophic changes, which is the hallmark of the diffuse nature of the atrophic process in the stomach mucosa. In the fifth type (termed “alternating atrophic – non-atrophic”), gastric corpus biopsy specimens with atrophy alternate with biopsy specimens showing no atrophy.
The pattern of atrophy spreading sequentially from the antrum to the large curvature of the stomach, described by Kimura and Takemoto, was histologically confirmed in only 82% of the cases. Thus, visual endoscopic assessment of atrophic gastritis in the stomach corpus cannot be used to stratify the risk of developing stomach mali
Modern endoscopic methods for examining the stomach can effectively identify and verify early gastric cancers. Gastroendomicroscopy with narrow band imaging (commonly referred to as NBI) technology has a high level of sensitivity when compared with routine gastroscopy[1]. The first experience with laser confocal microscopy showed its high efficiency and sensitivity in detecting gastric pathology. Zuo et al[14] rated the value of combined virtual chromoendoscopy (flexible spectral imaging color enhancement) and probe-based confocal laser endomicroscopy (pCLE) for in vivo detection of gastric intestinal metaplasia, gastric intraepithelial neoplasia, and early gastric cancer in 238 patients that were randomized to groups of pCLE with targeted biopsies or with standard biopsies. However, they did not study atrophic gastritis. Atrophic gastritis is a basic precancerous disease and is associated with a high risk for developing gastric cancer[10]. In a review, Sumiyama[15] analyzed 34 articles on current trends in endoscopic diagnosis for early-stage gastric cancer in Japan. Eventually, image enhancement endoscopy, such as that with NBI, was found to optimally highlight the mucosal structures and vascularity revealing fine details of subtle superficial abnormalities of early gastric cancer, which are otherwise difficult to recognize using standard white light endoscopy. Unfortunately, this technology does not help in the diagnosis of atrophic gastritis, a precancer of the stomach.
The only way to verify atrophic gastritis and determine the risk of developing gastric cancer is use of the Updated Sydney system[14]. Li et al[16] proposed a new pCLE classification system for gastric pit patterns and vessel architecture. Although this new pCLE classification system correlates well with specific histological conditions and has been reproducible by multiple investigators, it is inferior to the Updated Sydney system for the diagnosis of atrophic gastritis. Additional studies have cultivated its use for tumor differential detection in the stomach and esophagus by combination with endoscopic submucosal dissection, and it has demonstrated efficacy for the treatment of neoplasms[17,18]. In our study, we compared the Updated Sydney system and the Kimura-Takemoto visual endoscopic classification system for atrophic gastritis. This is very important because atrophic gastritis is the cancerization process by which gastric cancer mainly develops; by combining primary and secondary prevention strategies, gastric cancer can be a preventable disease[19].
The visual endoscopic diagnostic systems of Kimura-Takemoto, blue-light imaging and linked-color imaging do not assess the severity of mucosal atrophy and other precancerous changes. All three require the addition of a targeted biopsy, to verify neoplasms[20-22]. For the diagnosis of atrophic gastritis, these techniques are not used. The Kyoto Global Consensus Twelfth Statement recommends that, in most hands, visual endoscopy is an inadequate tool for diagnosing atrophy and intestinal metaplasia and, therefore, it remains mandatory that a biopsy is carried out, allowing histomorphological assessment of the gastric mucosa according to the Sydney system. In addition, the Kyoto Global Consensus Fourteenth Statement cited that the risk of gastric cancer correlates with the severity of atrophic gastritis[6]. We have adapted the histological VAS of the Updated Sydney system (five biopsies) to the Kimura-Takemoto classification system (six biopsies). For this, six biopsies were used. They corresponded to the boundaries of atrophy identified in the Kimura-Takemoto system (C0, C1, C2, C3, O1, O2, O3) and each were subjected to histological examination. For C0, atrophy is absent in the mucosal specimen.
With this approach, we were able to determine the cumulative risk, not by the area of atrophy with the visual endoscopic classification of Kimura-Takemoto but by the severity of the histological atrophy of the biopsy (five biopsies from the gastric corpus). Using this diagnostic method, the number of patients with confirmed pronounced corpus-atrophic gastritis (who are at high risk of developing stomach cancer) was detected at a rate of two and a half times greater than that achieved when using the Updated Sydney system. Such a diagnostic method will allow effective morphological control of the regression or development of atrophic gastritis. A group of authors from Japan and the United Kingdom reported on a study using a combined diagnosis and multivariate analysis of atrophic gastritis. The visual assessment of atrophic gastritis was carried out using the Kimura-Takemoto classification system. Morphological assessment was carried out using the Updated Sydney system with five biopsies. In addition, serological detection of atrophy was carried out using the markers of pepsinogen-1, pepsinogen-2, and the PG-1:PG-2 ratio[23]. Unfortunately, however, that method is not amenable to mass practical use, despite its inexplicit advantages over the traditional Updated Sydney system.
We recommend the Updated Kimura-Takemoto classification system described in our study for the diagnosis and monitoring of atrophic gastritis, including its development and control of atrophy regression. The incidence of gastric cancer can be effectively reduced as a result of a set of preventive measures, implemented based on mass serological screening of atrophic gastritis[24]. The effectiveness of such preventive screening depends on many factors, namely the sensitivity and specificity of serological markers of atrophic gastritis[25]. The Updated Kimura-Takemoto classification system for atrophic gastritis has a good prospect of use in about the second diagnostic stage, after preliminary serological screening of atrophic gastritis. This will further increase the effectiveness of prevention measures targeting gastric cancer.
Using the Updated Kimura-Takemoto classification system allowed us to identify five types of mucosal atrophy developing in the gastric corpus. This greatly improved our overall understanding of the mechanisms and morphological processes that underlie atrophic corpus-gastritis. It is important that the area of the gastric corpus is several times larger than the area of the antrum. All these details must be taken into account for the practical implementation of measures for the prevention of gastric cancer. The recognition of new types of corpus atrophic gastritis also serves to clarify the concept of multifocal gastritis.
The Updated Sydney system is able to define antrum-predominant atrophic gastritis, corpus-predominant atrophic gastritis, and multifocal atrophic gastritis (antrum + corpus). Now, however, we also need a method that will allow for the determination of antrum-predominant atrophic gastritis and multifocal corpus-predominant atrophic gastritis. Multifocal corpus atrophic gastritis contains monofocal, bifocal, trifocal, quadrifocal and pentafocal corpus atrophic gastritis; corpus atrophic gastritis of mild, moderate or severe degree will be present in any one (or more) of the five biopsy specimens, taken according to the C-2, C-3, O-1, O-2 and O-3 scale. The mild, moderate, severe mucosal atrophy are possible in two, three, four, and five biopsies from the list of C-2, C-3, O-1, O-2, O-3 scale in any combination. Of critical importance is the detection of severe mucosal atrophy in one or more of the biopsy specimens, as the main diagnosis of severe atrophy is stomach precancer[6].
The Updated Kimura-Takemoto classification system adds new possibilities for the differential diagnosis of severe atrophic gastritis and the best risk stratification for gastric cancer.
The Updated Sydney system is significantly inferior to the Updated Kimura-Takemoto classification for morphological verification of atrophic gastritis.
The Updated Sydney system does not meet modern requirements for the study of atrophic gastritis. Thus, for effective prevention of gastric cancer, a new classification of gastritis is needed.
Development of a further updated version of the Updated Kimura-Takemoto morphological classification will allow for more efficient implementation of the visual endoscopic classification of atrophic gastritis.
The proposed Updated Kimura-Takemoto morphological classification of gastritis will further efforts to achieve the overall goal of preventing gastric cancer through more accurate identification and morphological monitoring of severe atrophic gastritis (gastric precancer).
Kimura and Takemoto originally developed a visual endoscopic assessment system for the gastric mucosa, naming the stages of development of atrophic gastritis. In accordance with these stages, we obtained six biopsies from each patient and performed subsequent histological examination. These biopsies included specimens from the antrum (n = 1) and the gastric corpus (n = 5). Of note, for each patient, two of the gastric corpus biopsy specimens corresponded topographically to the Updated Sydney system and the Updated Kimura-Takemoto system.
The results of the study demonstrated a significantly better sensitivity of the updated morphological Kimura-Takemoto classification for the diagnosis of atrophic gastritis (including severe atrophic gastritis) compared to the Updated Sydney system.
A new morphological method for the detection, morphological verification and morphological monitoring of atrophic gastritis is proposed: The Updated Kimura-Takemoto morphological classification system. The Kimura-Takemoto hypothesis that atrophy of the gastric mucosa extends from the antrum to the greater curvature of the gastric corpus was confirmed morphologically via the newly developed system in 82% of patients with indicated atrophic gastritis.
Study of the morpho-functional relationships in atrophic gastritis using the Updated Kimura-Takemoto classification system is promising for finding the best serological markers of atrophic gastritis for future serological screening of precancerous changes in the gastric mucosa.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: Gastroenterological Scientific Society of Russia.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Russia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Romano L, Shibata T S-Editor: Liu M L-Editor: A P-Editor: Zhang YL
| 1. | Pasechnikov V, Chukov S, Fedorov E, Kikuste I, Leja M. Gastric cancer: prevention, screening and early diagnosis. World J Gastroenterol. 2014;20:13842-13862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 247] [Cited by in RCA: 303] [Article Influence: 27.5] [Reference Citation Analysis (2)] |
| 2. | Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;1:87-97. [RCA] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 742] [Article Influence: 43.6] [Reference Citation Analysis (3)] |
| 3. | Kishino M, Nakamura S, Shiratori K. Clinical and Endoscopic Features of Undifferentiated Gastric Cancer in Patients with Severe Atrophic Gastritis. Intern Med. 2016;55:857-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Song JH, Kim SG, Jin EH, Lim JH, Yang SY. Risk Factors for Gastric Tumorigenesis in Underlying Gastric Mucosal Atrophy. Gut Liver. 2017;11:612-619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Jin EH, Chung SJ, Lim JH, Chung GE, Lee C, Yang JI, Kim JS. Training Effect on the Inter-observer Agreement in Endoscopic Diagnosis and Grading of Atrophic Gastritis according to Level of Endoscopic Experience. J Korean Med Sci. 2018;33:e117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, Haruma K, Asaka M, Uemura N, Malfertheiner P; faculty members of Kyoto Global Consensus Conference. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353-1367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1322] [Cited by in RCA: 1180] [Article Influence: 118.0] [Reference Citation Analysis (0)] |
| 7. | Rugge M, Capelle LG, Fassan M. Individual risk stratification of gastric cancer: evolving concepts and their impact on clinical practice. Best Pract Res Clin Gastroenterol. 2014;28:1043-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Rugge M, Genta RM. Staging and grading of chronic gastritis. Hum Pathol. 2005;36:228-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 188] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 9. | Rugge M, Correa P, Di Mario F, El-Omar E, Fiocca R, Geboes K, Genta RM, Graham DY, Hattori T, Malfertheiner P, Nakajima S, Sipponen P, Sung J, Weinstein W, Vieth M. OLGA staging for gastritis: a tutorial. Dig Liver Dis. 2008;40:650-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 226] [Article Influence: 13.3] [Reference Citation Analysis (1)] |
| 10. | Sipponen P, Kekki M, Haapakoski J, Ihamäki T, Siurala M. Gastric cancer risk in chronic atrophic gastritis: statistical calculations of cross-sectional data. Int J Cancer. 1985;35:173-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 234] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Toyoshima O, Yamaji Y, Yoshida S, Matsumoto S, Yamashita H, Kanazawa T, Hata K. Endoscopic gastric atrophy is strongly associated with gastric cancer development after Helicobacter pylori eradication. Surg Endosc. 2017;31:2140-2148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Kodama M, Okimoto T, Ogawa R, Mizukami K, Murakami K. Endoscopic atrophic classification before and after H. pylori eradication is closely associated with histological atrophy and intestinal metaplasia. Endosc Int Open. 2015;3:E311-E317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3221] [Cited by in RCA: 3550] [Article Influence: 122.4] [Reference Citation Analysis (3)] |
| 14. | Zuo XL, Li Z, Li CQ, Zheng YY, Xu LD, Chen J, Lin R, Song J, Yu CH, Yue M, Zhou Q, Liu ZY, Li YQ. Probe-based endomicroscopy for in vivo detection of gastric intestinal metaplasia and neoplasia: a multicenter randomized controlled trial. Endoscopy. 2017;49:1033-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Sumiyama K. Past and current trends in endoscopic diagnosis for early stage gastric cancer in Japan. Gastric Cancer. 2017;20:20-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 16. | Li Z, Zuo XL, Li CQ, Liu ZY, Ji R, Liu J, Guo J, Li YQ. New Classification of Gastric Pit Patterns and Vessel Architecture Using Probe-based Confocal Laser Endomicroscopy. J Clin Gastroenterol. 2016;50:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Kollar M, Spicak J, Honsova E, Krajciova J, Vackova Z, Martinek J. Role of confocal laser endomicroscopy in patients with early esophageal neoplasia. Minerva Chir. 2018;73:417-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Kim JW. Usefulness of Narrow-Band Imaging in Endoscopic Submucosal Dissection of the Stomach. Clin Endosc. 2018;51:527-533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 19. | Rugge M, Sugano K, Scarpignato C, Sacchi D, Oblitas WJ, Naccarato AG. Gastric cancer prevention targeted on risk assessment: Gastritis OLGA staging. Helicobacter. 2019;24:e12571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Weigt J, Malfertheiner P, Canbay A, Haybaeck J, Bird-Lieberman E, Link A. Blue Light Imaging and Linked Color Imaging for the Characterization of Mucosal Changes in Chronic Gastritis: A Clinicians View and Brief Technical Report. Dig Dis. 2020;38:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Shah PA, Shah BB, Rai VK, Khan E, Goenka MK. A study on confocal endomicroscopy in comparison with histopathology for polypoidal lesions of the gastrointestinal tract: A prospective single-centre experience. Indian J Gastroenterol. 2019;38:332-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Ruff S, Curtin B, Quezado M, Heller T, Koh C, Steinberg SM, Connolly M, Hernandez JM, Davis JL. Evaluation of confocal endoscopic microscopy for detection of early-stage gastric cancer in hereditary diffuse gastric cancer (HDGC) syndrome. J Gastrointest Oncol. 2019;10:407-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Kono S, Gotoda T, Yoshida S, Oda I, Kondo H, Gatta L, Naylor G, Dixon M, Moriyasu F, Axon A. Can endoscopic atrophy predict histological atrophy? World J Gastroenterol. 2015;21:13113-13123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (2)] |
| 24. | Kotelevets SM, Chekh SA. Screening, Monitoring, and Treatment of Precancerous Atrophic Gastritis in the Prospective Study for Seven Years. Asian Pac J Cancer Prev. 2020;21:331-336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Kotelevets SM, Chekh SA. Serological Criteria for Mild, Moderate and Severe Atrophy in Atrophic Gastritis. Biol Med J. 2015;7:235. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |