Published online May 6, 2021. doi: 10.12998/wjcc.v9.i13.2983
Peer-review started: September 10, 2020
First decision: December 28, 2020
Revised: January 10, 2021
Accepted: February 11, 2021
Article in press: February 11, 2021
Published online: May 6, 2021
Processing time: 224 Days and 10.4 Hours
Complex hypospadias is a surgical challenge.
To present the long-term outcomes of two-stage repair of complex hypospadias using a scrotal septal flap.
This was a retrospective study of patients with complex hypospadias who were operated on between January 1st, 2001, and January 1st, 2019, at a single hospital using a scrotal septal flap (two-stage surgery) or prepuce flap (one-stage surgery; control group). In the scrotal group, the urethra was first repaired using oral mucosa; in the second stage, a scrotal septal flap was used as a second imper-meable layer. Maximal/average urinary flow rates after surgery were compared. All patients were followed for ≥ 6 mo (range: 6-96 mo).
Ninety-seven patients were included (46 in the scrotal group and 51 in the prepuce group). The maximal urinary flow rate was 15.4 ± 2.1 mL/s in the scrotal group and 14.3 ± 3.0 mL/s in the control group (P = 0.035). The average urinary flow rate was 8.4 ± 2.3 mL/s in the scrotal group and 7.5 ± 1.5 mL/s in the control group (P = 0.019). The proportion of patients achieving good therapeutic effects was higher in the scrotal group than in the control group [24 (52.2%) vs 16 (31.4%), P = 0.042; 34 (73.9%) vs 25 (49.0%), P = 0.014]. The scrotal flap two-stage surgery was independently associated with a higher maximal urinary flow rate (OR = 2.416, 95%CI: 1.026-5.689, P = 0.044) and with a higher average flow rate (OR = 2.484, 95%CI: 1.054-5.854, P = 0.038).
In complex hypospadias, a scrotal septal flap could be a versatile and reliable option for resurfacing the penis.
Core Tip: The proportion of patients achieving good therapeutic effects for maximal and average urinary flow rates was higher in the scrotal group than in controls. The scrotal flap two-stage surgery was independently associated with higher maximal and average urinary flow rates.
- Citation: Chen S, Yang Z, Ma N, Wang WX, Xu LS, Liu QY, Li YQ. Scrotal septal flap and two-stage operation for complex hypospadias: A retrospective study. World J Clin Cases 2021; 9(13): 2983-2993
- URL: https://www.wjgnet.com/2307-8960/full/v9/i13/2983.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i13.2983
Hypospadias is a common abnormality during fetal development of the penis, in which the urethra does not open at its usual location in the head of the penis. It is the second-most common birth abnormality of the male reproductive system, with an incidence of 0.3%-0.7% in live male births[1-4]. The location of the urethral meatus can be within the glans, at the corona, the shaft of the penis, the scrotum, or the perineum[4-7]. Hypospadias is accompanied by urethral deficiency, chordee, a ventrally deficient hooded foreskin, dorsal hood prepuce, and incomplete foreskin around the glans. Several patients with hypospadias also present scrotal splitting, penoscrotal transposition, small penis, cryptorchidism, and inguinal hernia. Hypospadias has impacts on both urinary and sexual function[4,6].
Since the 19th century, many surgical methods have been proposed[4,8-12], but none of these methods have been reported to achieve an ideal result in complex hypo-spadias[13,14]. Theoretically, surgical treatment aims to repair both cosmetic and functional abnormalities. Cosmetically, the following goals should be achieved: (1) achieving a normal outer genital form; (2) straightening the chordee; and (3) revising the location of the urinary meatus[12]. Functionally, repair should allow standing urination, have no surgery-related complications (urethral fistula, urethral stenosis, and urethral diverticulum), decrease the occurrence of lower urinary tract symptoms, and cure ejaculatory dysfunction[12].
Complex hypospadias is a surgical challenge characterized by (1) a urethral meatus in the proximal penis, penoscrotal, scrotum, or perineum; or (2) redo operation[15-17]. For patients with complex hypospadias, the surgical outcomes mainly depend on tissue availability for repair and a well-scheduled treatment. Even though all kinds of graft tissues (skin, bladder, and oral mucosa) have several disadvantages, the two-stage repair technique for complex hypospadias is critical. Oral mucosa is an appropriate urethral substitute; it is easy to harvest, hairless, resilient, and easy to handle. For patients with complex hypospadias, finding an appropriate flap to repair the foreskin deficiency and cover the wound is challenging. Liu et al[18] suggested using a scrotal septal skin flap to cover the wound at the ventral side of the reconstructed urethra.
In the present study, we report a series of patients with complex hypospadias treated by two-stage hypospadias repair using a scrotal septal flap. The present study aimed to present the long-term outcomes of two-stage hypospadias repair with a scrotal septal flap for complex hypospadias.
This retrospective study included patients with complex hypospadias operated between January 1st, 2001, and January 1st, 2019, at a single hospital. This study was approved by the Medical Ethics Review Board of the Plastic Surgery Hospital (No. ZX201968). Written Informed consent was obtained from the study participants.
The inclusion criteria were: (1) complex hypospadias; (2) > 18 years of age; (3) completed urethra reconstruction surgery; and (4) available data on ultrasound, magnetic resonance imaging (MRI), and urinary flow rate. Those with other congenital malformations were excluded.
The patients were grouped according to the flaps used during surgery: Scrotal septal flap or prepuce flap (control group). Surgery with a scrotal septal flap was a two-stage operation, while surgery with a prepuce flap was a one-stage operation. The prepuce flap was not the recommended surgical procedure for proximal penile and penoscrotal hypospadias. However, the reasons why this surgery was performed during the study period included (1) in low-income areas, a large number of patients cannot afford two-stage surgery, and they need a simple and inexpensive operation; and (2) some patients admitted to our center refused a two-stage operation, and they requested a one-stage repair. Patients with sufficient residual prepuce tissue for repair could undergo a one-stage surgery. Those with insufficient residual prepuce tissue had to undergo scrotal septal flap and two-stage surgery for urethral reconstruction.
Stage one: All patients were operated on under general anesthesia. The surgeons were divided into two teams: The oral team and the penis team. Both teams were led by a surgeon with > 30 years of experience.
The surgeons in the oral team identified the parotid duct opposite the upper second molar. The size of the harvested buccal mucosal strips was estimated. Two mucosal strips were obtained (length of 3.5-5.5 cm and width of 0.8-1.5 cm). The width of the strip determined the diameter of the repaired urethra, while the distance between the normal meatus and the abnormal opening indicated the length of the strip. The donor sites were sutured. Chloramphenicol powder was wrapped in a gauze roll. The gauze roll wall had two layers: The outer layer was ordinary gauze, while the inner layer was a piece of oiled silk. The roll diameter was approximately 0.5-1.2 cm, depending on the width of the mucosal strips. The mucosal strips were sutured longitudinally with each other and covered the fabricated gauze roll to form a mucosal tube.
The penis team surgeons made an incision around the displaced meatus and on the ventral side of the penis, coronary sulcus, and glans. The Y-shaped incision in the glans would become the future meatus. The fibrous tissues were dissected, straightening the curved penis. For re-operative patients who presented urethral stenosis or urethral fistula, an attempt was made to use a urethral probe. The failing urethra was then incised vertically until normal urethra was observed. The urethral stricture was removed. A ventral tunnel was created using the surrounding penile tissue. The tunnel was located between the subcutaneous and tunica albuginea and extended from the normal urethral meatus to the incised glans. The fabricated mucosal tube was then inserted into the tunnel. Both ends of the tube were sutured to the surrounding tissues. A Foley catheter was placed in the bladder. There were gauze ties in the distal and proximal parts of the mucosal tube. An outer dressing was used to provide stress to adhere to the mucosal tube in the tunnel wall. The gauze roll was removed after one month (Supplementary Figure 1).
Stage two: Urethra reconstruction surgery with scrotal septal flap.
After 6-12 mo, the second surgery was carried out. The distance between the repaired urethral mucosa's proximal meatus and the normal urethral meatus was measured (length of approximately 1.0-2.5 cm). Next, they were anastomosed by an absorbable suture around the catheter. The adventitial tissue was harvested to cover the wound as a waterproof layer. A scrotal septal skin flap was harvested as another waterproof layer. The size of the wound was measured before the flap was harvested. The island flap was transferred to cover the wound. A small latex drain was left in the donor site (Supplementary Figure 2).
All patients in this group were operated on using the onlay island prepuce flap described by Elder et al[19] and Rodriguez et al[20].
Demographic and clinical data were recorded, including age, the subtype of hypospadias, and treatment history. During the follow-up visits after surgery, the maximal and average urinary flow rates were measured according to the 2019 standards of the International Continence Society (ICS) to determine the urination status and therapeutic effect[21]. According to the 2019 ICS standards, a maximal urinary flow rate > 15 mL/s was considered normal and thus was considered a good therapeutic effect in this study. An average urinary flow rate > 7.5 mL/s was considered a good effect.
Postoperative complications such as mucosa fibrosis, complete neourethral stricture, fistula, meatal stenosis, and diverticulitis were evaluated during the follow-up visits by ultrasound and MRI. For ultrasound, saline was injected into the urethra to examine complications and to identify minor lesions. Before saline injection, the urethra could not be seen (Video 1). When ultrasound was performed, a physician injected saline through the urethral meatus (Video 2 and Video 3). Water transferred pressure to the urethra, showing stenosis, fistula, and diverticulitis (Video 4 and Video 5). An MRI urogram was used to observe the form of the urethra. After urinating, the residual urine in the urethra was an excellent medium to show the form of the urethra.
All patients were followed for at least 6 mo (range: 6 to 96 mo) after surgery.
Stata/SE 15.1 (Stata Corp., College Station, TX, United States) was used for statistical analysis. Continuous variables are presented as means ± SDs, or as medians and interquartile ranges according to their distribution, as determined by the Kolmogorov-Smirnov test. Categorical variables were reported as frequency with percentage. Continuous data were compared with the Student’s t-test. The rates of complications were compared using the Chi-square test. A multivariable analysis was performed by logistic regression (enter method) with good surgical effect as the dependent variable. Two-sided P values < 0.05 were considered statistically significant.
Ninety-seven patients were included in this study, with 46 in the scrotal septal flap group and 51 in the prepuce flap group. The characteristics of the patients are shown in Supplementary Table 1.
The maximal urinary flow rate was 15.4 ± 2.1 mL/s in the scrotal group and 14.3 ± 3.0 mL/s in the control group (P = 0.0352). A good therapeutic effect was achieved in 24 (52.2%) patients in the scrotal group and 16 (31.4%) in the control group (P = 0.042).
The average urinary flow rate was 8.4 ± 2.3 mL/s in the scrotal group and 7.5 ± 1.5 mL/s in the control group (P = 0.019). A good therapeutic effect was achieved in 34 (73.9%) patients in the scrotal group and 25 (49.0%) in the control group (P = 0.014).
The multivariable analyses showed that compared with the prepuce flap one-stage surgery, the scrotal flap two-stage surgery was independently associated with a higher maximal urinary flow rate (OR = 2.416, 95%CI: 1.026-5.689, P = 0.044, Supplementary Table 2) and with a higher average urinary flow rate (OR = 2.484, 95%CI: 1.054-5.854, P = 0.038, Supplementary Table 3).
Three patients had a complete neourethral stricture in the scrotal group compared with one in the control group (P = 0.259). Three patients in the scrotal group had a fistula, compared with 12 in the control group (P = 0.021). The number of patients with meatal stenosis was two in the scrotal group and nine in the control group (P = 0.039). There was no significant difference in the occurrence of diverticulitis between the two groups (3 vs 2, P = 0.563).
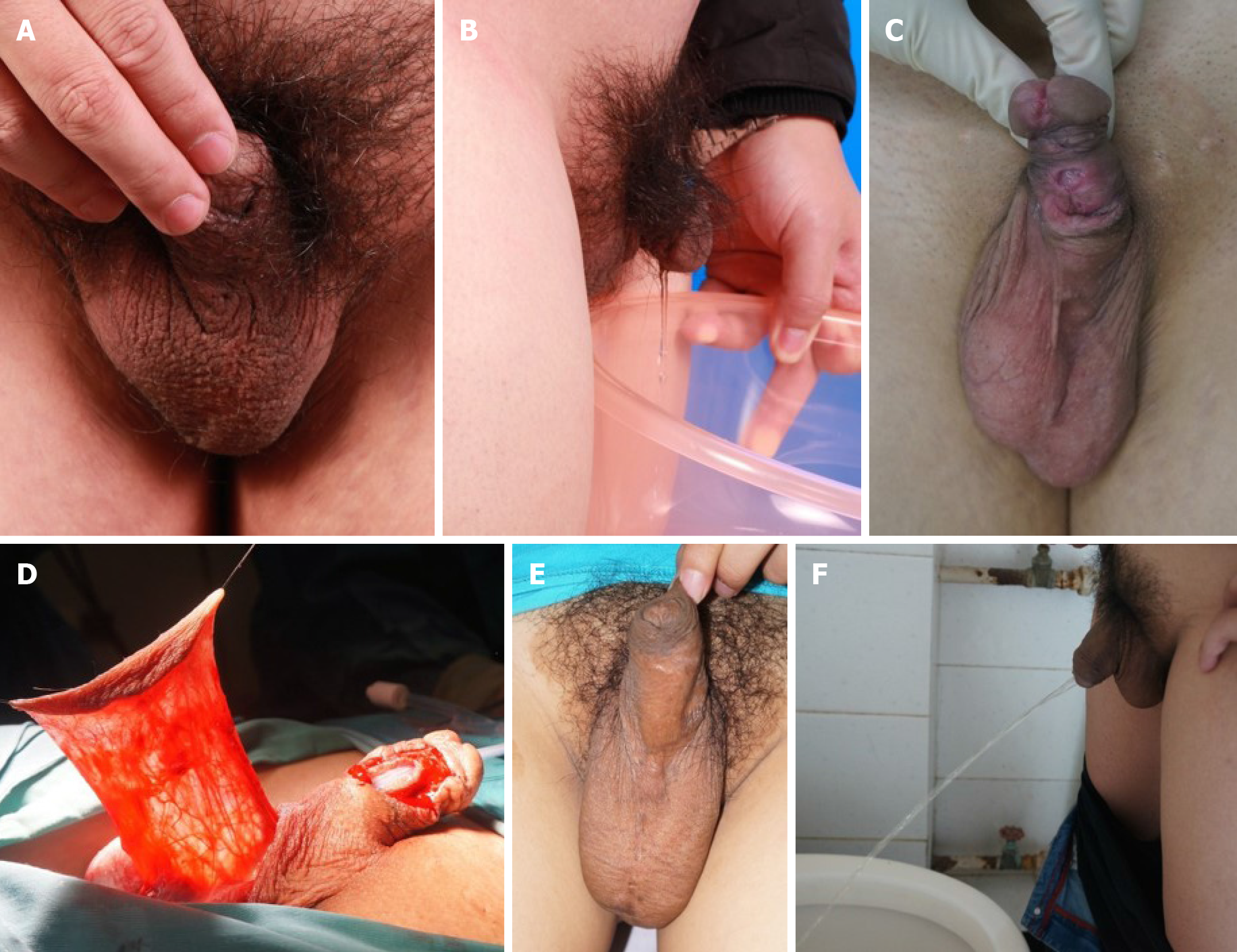
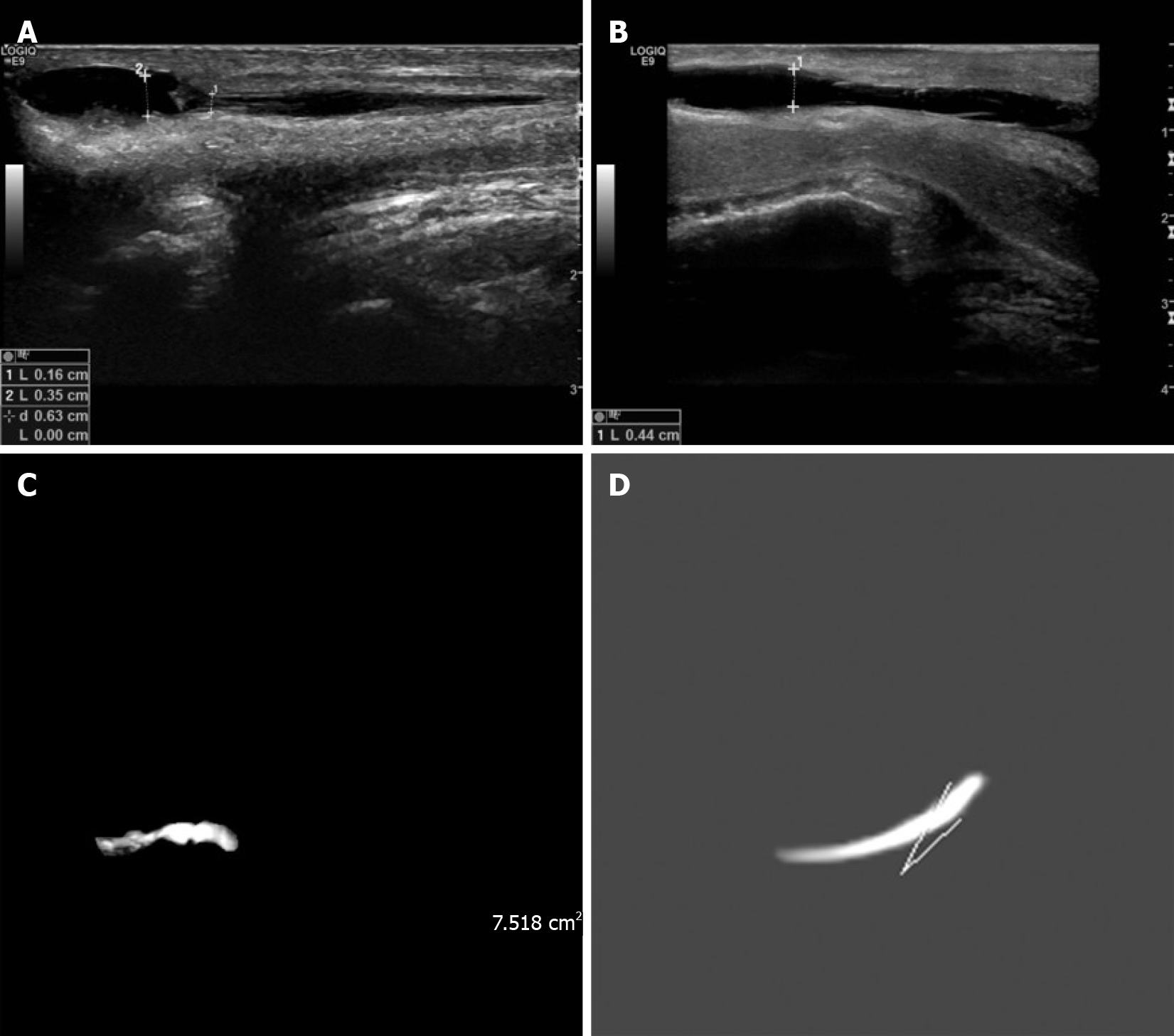
A 24-year-old male patient was diagnosed with proximal penile hypospadias. He underwent surgery about ten years before admission, but he was not satisfied with his appearance (Figure 1A), and he could not urinate well (Figure 1B). We conducted a two-stage operation in this patient. In the first surgery, the fabricated urethra from ten years ago was removed, and two mucosal strips (1.2 cm × 4.5 cm) were harvested. After one year, he underwent the second surgery (Figure 1C and D). He was followed for 30 mo (Figure 1E and F). The ultrasound images are shown in Figure 2A and B. Before the operation, there was stenosis of the urethra (Figure 2A), which was relieved at 30 mo after the second operation (Figure 2B). The MRI findings are shown in Figure 2C and D.
Complex hypospadias is a surgical challenge[15-17]. This study aimed to present the long-term outcomes of two-stage repair of complex hypospadias using a scrotal septal flap. The results suggest that the scrotal septal flap could be a versatile and reliable option for resurfacing the penis in adult patients with complex hypospadias.
In 1985, Li et al[22] used for the first time a scrotal septal flap to repair hypospadias. Since that time, the scrotal septal flap has been extensively used in Asia. Anterior scrotal artery branches from the superficial external pudendal arteries connect with the posterior scrotal artery to form an interconnecting circulation in the scrotal skin. Both anterior and posterior scrotal arteries could act as a scrotal septal vascular pedicle flap (Figure 3A). In 2009, Carrera et al[23] studied scrotal microvascularization and applied the findings to the design of reliable skin flaps for complex urethral reconstruction. They found that the special anatomical distribution of the scrotal branches stemming from the perineal arteries enables the construction of adequate, reliable longitudinal median island scrotal flaps for hypospadias reconstructive surgery.
Initially, Li et al[22] used the scrotal septal flap to create a new urethra. However, this method had many issues, such as hair growing inside the urethra, urethral stenosis, and calculi. Most surgeons currently utilize the scrotal septal flap to cover the wound of the penis or to reconstruct the penis. In the present study, we used the scrotal septal flap to cover the wound in a two-stage hypospadias repair. A scrotal septal pedicle flap was used instead of a random flap to achieve a reliable blood supply. The mean size of the flap was 2.8 cm × 3.4 cm, which was enough to cover the wound. For many patients, hair in the midline of the scrotum was observed, which, when the flap was transferred to the penis, might influence cosmetic satisfaction, but without affecting the function. Laser hair removal could help solve such a problem.
The dartos is a thin sheet of smooth muscle closely associated with the skin of the scrotum. It plays a substantial role in the thermoregulation of the scrotum. When the temperature is extremely low, the tunica dartos muscle contracts, causing the scrotal skin to wrinkle and leading to a smaller heat loss[24,25]. In 1996, Maloney et al[25] reported that the dartos is innervated by a sympathetic branch of the autonomic nervous system. Theoretically, the sympathetic branch cannot be controlled by the patient’s will, but some stimuli can trigger the contraction. In the present study, we observed that micturition could trigger the contraction. Hence, this contraction could provide pressure on the urethra to reduce the residual urine, but further research is required to examine this issue.
The main complications observed in the present study were urethral fistulae. Three patients in the scrotal group and 12 in the control group had to undergo re-operations to repair the fistula. When mucosal cells have covered the fistula's wall, conservative treatment is not feasible (Figure 3B). Thus, during surgery to repair the fistula, the surgeon needs to remove the fistula's mucosal wall and suture the surrounding mucosal layer to close the fistula. The subcutaneous tissue and skin of the transferred scrotal flap are flexible, so it is easy to free them to form new waterproof layers. In the one-stage operation, there is only one waterproof layer formed by the prepuce (Figure 3C). In the present study, the scrotal septal flap transfer will form a second waterproof layer (Figure 3D). The two layers can reduce the risk of fistula and facilitate the repair of a fistula since more tissue is available. The limitation of the scrotal septal flap is that for scrotal and perineum hypospadias, the flap does not exist. For these patients, a random scrotal flap is another option. For children who underwent the first surgery in preadolescence, further research is essential to investigate penile development, psychosexual function, and reproductive ability, as well as eventual secondary repair in adulthood. Cruz-Diaz et al[26] reviewed the evolution of the use of buccal mucosa grafts in hypospadias repair with urethral stricture. Three patients in the scrotal group had a complete neourethral stricture. The main reason was that the grafted oral mucosa did not survive well. These three patients had undergone previous surgical treatment. The scar tissues in the penis probably affected the blood supply, which probably affected survival of the oral mucosa.
Therefore, the two-stage hypospadias repair using oral mucosa to repair the urethra and a scrotal septal flap to repair the penis had a good therapeutic effect, resulting in restored urinary function and cosmetic appearance. In addition, the complication rate was lower than in patients who underwent a one-stage procedure with a prepuce flap. Liu et al[18] reported good outcomes using a penile flap to reconstruct the urethra and a scrotal septal flap to reconstruct the penis. A one-stage repair using oral mucosa to repair the urethra and a scrotal septal flap to repair the penis has been suggested by Chen et al[27] and achieved good therapeutic effects. However, cases of early urinary leakage were observed. The two-stage hypospadias repair using oral mucosa to repair the urethra and a scrotal septal flap to repair the penis was also analyzed by Pandey et al[28] in patients with complex hypospadias, who reported good therapeutic effects and few complications. However, a control group was not included in the study. Using the same approach, Zhang et al[29] reported its success in patients with several previous failed surgeries. Nevertheless, additional studies are necessary to verify whether this method could be optimized and improved.
This study has limitations. There is no objective and precise method to determine the cosmetic result of hypospadias treatment. Patient satisfaction is crucial but could not be assessed because satisfaction data were not reliably written in the charts. The specimen weight could not be analyzed. The sample size was small. In our hospital, we focused on the two-stage repair of hypospadias, and our skill with one-stage surgery is more limited, which could have affected the results of the present study. Multicenter prospective trials are necessary to address these issues.
In conclusion, the scrotal septal flap could be a versatile and reliable option for resurfacing the penis in patients undergoing complex hypospadias repair. It could be especially suitable for re-operative hypospadias in which prepuce tissue is limited. This flap fully respects Gillies’ principle of “replacing like with like” and could achieve an ideal result both functionally and cosmetically.
Complex hypospadias is a surgical challenge and all kinds of graft tissues have several disadvantages.
A scrotal septal pedicle flap was used instead of a random flap to achieve a reliable blood supply.
To present the long-term outcomes of two-stage repair of complex hypospadias using a scrotal septal flap.
This retrospective study included patients with complex hypospadias using a scrotal septal flap or prepuce flap, and compared the maximal/average urinary flow rates after surgery.
The maximal urinary flow rate, average urinary flow rate and the proportion of patients achieving good therapeutic effects were higher in the scrotal group. The scrotal flap two-stage surgery was independently associated with a higher maximal urinary flow rate and a higher average flow rate.
The scrotal septal flap could be a versatile and reliable option for resurfacing the penis in patients undergoing complex hypospadias repair.
This flap could be especially suitable for re-operative hypospadias in which prepuce tissue is limited.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yang G S-Editor: Fan JR L-Editor: Webster JR P-Editor: Liu JH
| 1. | Schneuer FJ, Holland AJ, Pereira G, Bower C, Nassar N. Prevalence, repairs and complications of hypospadias: an Australian population-based study. Arch Dis Child. 2015;100:1038-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | Paulozzi LJ. International trends in rates of hypospadias and cryptorchidism. Environ Health Perspect. 1999;107:297-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 319] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 3. | Baskin LS, Ebbers MB. Hypospadias: anatomy, etiology, and technique. J Pediatr Surg. 2006;41:463-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 237] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 4. | Leung AK, Robson WL. Hypospadias: an update. Asian J Androl. 2007;9:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Radmayr C, Bogaert G, Dogan HS; European Society for Paediatric Urology and European Association of Urology (ESPU/EAU). Guidelines on Paediatric Urology. 2019. |
| 6. | Marrocco G, Grammatico P, Vallasciani S, Gulia C, Zangari A, Marrocco F, Bateni ZH, Porrello A, Piergentili R. Environmental, parental and gestational factors that influence the occurrence of hypospadias in male patients. J Pediatr Urol. 2015;11:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | van der Zanden LF, van Rooij IA, Feitz WF, Franke B, Knoers NV, Roeleveld N. Aetiology of hypospadias: a systematic review of genes and environment. Hum Reprod Update. 2012;18:260-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 193] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 8. | Springer A. Assessment of outcome in hypospadias surgery - a review. Front Pediatr. 2014;2:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Marrocco G, Vallasciani S, Fiocca G, Calisti A. Hypospadias surgery: a 10-year review. Pediatr Surg Int. 2004;20:200-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Shukla AK, Singh AP, Sharma P, Shukla J. Two Stages Repair of Proximal Hypospadias: Review of 700 Cases. J Indian Assoc Pediatr Surg. 2017;22:158-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Kulkarni SB, Joglekar O, Alkandari MH, Joshi PM. Redo hypospadias surgery: current and novel techniques. Res Rep Urol. 2018;10:117-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Calonge WM, Sapino G. An Overview of Hypospadias Surgery. In Amr S (ed) Issues in Flap Surgery. London: IntechOpen 2018. [DOI] [Full Text] |
| 13. | van der Horst HJ, de Wall LL. Hypospadias, all there is to know. Eur J Pediatr. 2017;176:435-441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 155] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 14. | Aldamanhori R, Chapple CR. Management of the patient with failed hypospadias surgery presenting in adulthood. F1000Res. 2017;6:1890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Fam MM, Hanna MK. Resurfacing the Penis of Complex Hypospadias Repair ("Hypospadias Cripples"). J Urol. 2017;197:859-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Elia R, Pafitanis G, Ciudad P, Chen HC. Accessory penis: A rare method of peno-urethral separation of sexual function and voiding following successful complex hypospadias reconstruction with a free ileum flap. Arch Plast Surg. 2019;46:381-385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Weiser AC, Franco I, Herz DB, Silver RI, Reda EF. Single layered small intestinal submucosa in the repair of severe chordee and complicated hypospadias. J Urol. 2003;170:1593-5; disussion 1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Liu X, Li Y, Li S, Tang Y, Li Q. A new use of scrotal septal skin flap in repairing hypospadias. Ann Plast Surg. 2011;67:164-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Elder JS, Duckett JW, Snyder HM. Onlay island flap in the repair of mid and distal penile hypospadias without chordee. J Urol. 1987;138:376-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 134] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Chapter 43: Hypospadias. In Rodriguez ED, Losee JE, Neligan PC, Liu DZ (eds): Plastic Surgery. Craniofacial, Head and Neck Surgery. Pediatric Plastic Surgery. Dublin: Elsevier, 2012. |
| 21. | International Continence Society. ICS 2019 Standards. Bristol: International Continence Society, 2019. |
| 22. | Li SY, Li SK, Zhuang HX. The use of scrotal septal neurovascular pedicle island skin flap in one-stage repair of hypospadias. Ann Plast Surg. 1985;15:529-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Carrera A, Gil-Vernet A, Forcada P, Morro R, Llusa M, Arango O. Arteries of the scrotum: a microvascular study and its application to urethral reconstruction with scrotal flaps. BJU Int. 2009;103:820-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Waites GM. Thermoregulation of the scrotum and testis: studies in animals and significance for man. Adv Exp Med Biol. 1991;286:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Maloney SK, Mitchell D. Regulation of ram scrotal temperature during heat exposure, cold exposure, fever and exercise. J Physiol. 1996;496 (Pt 2):421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Cruz-Diaz O, Castellan M, Gosalbez R. Use of buccal mucosa in hypospadias repair. Curr Urol Rep. 2013;14:366-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Chen W, Li S, Li Y, Li Q. [Combined buccal mucosal graft and scrotal flap for reconstruction of urethra in primary hypospadias repair]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009;23:663-665. [PubMed] |
| 28. | Pandey A, Barta-Kelemen AM, Borisenkov M, Keller H. The Staged Urethroplasty with Vascularised Scrotal Flap and Buccal Mucosa Graft after Failed Hypospadias Surgery: A Reliable Technique with a Novel Tool. Urol Int. 2017;99:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Zhang S, Zhou C, Li F, Li S, Zhou Y, Li Q. Scrotal-Septal Fasciocutaneous Flap Used as a Multifunctional Coverage for Prior Failed Hypospadias Repair. Urol Int. 2016;96:255-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |