Published online May 6, 2021. doi: 10.12998/wjcc.v9.i13.2951
Peer-review started: January 4, 2021
First decision: January 23, 2021
Revised: February 5, 2021
Accepted: March 25, 2021
Article in press: March 25, 2021
Published online: May 6, 2021
Processing time: 107 Days and 20 Hours
The outbreak of coronavirus disease-2019 (COVID-19) has resulted in a global public health emergency. Patients with cirrhosis were deemed more susceptible to viral infection because of their dysregulated immune response. Similar to the general population, cirrhotic patients exhibit various degrees of COVID-19-related liver injury, which could be attributed to direct virus cytotoxicity, systemic immune system activation, drug-related liver injury, reactivation of pre-existing liver disease, and hypoxic hepatitis. The clinical symptoms in patients with cirrhosis and COVID-19 were similar to those in the general population with COVID-19, with a lower proportion of patients with gastrointestinal symptoms. Although respiratory failure is the predominant cause of mortality in cirrhotic patients with COVID-19, a significant proportion of them lack initial respiratory symptoms. Most evidence has shown that cirrhotic patients have relatively higher rates of morbidity and mortality associated with COVID-19. Advanced cirrhosis was also proposed as an independent factor affecting a poor prognosis and the need to consider COVID-19 palliative care. General measures implemented to prevent the transmission of the virus are also essential for cirrhotic patients, and they should also receive standard cirrhosis care with minimal interruptions. The efficacy of the available COVID-19 vaccines in cirrhotic patients still needs investigation.
Core Tip: Several studies have addressed the impact of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in patients with cirrhosis. The presence and stage of cirrhosis is correlated with the development of severe coronavirus disease-2019 (COVID-19), COVID-19-related adverse outcomes, and mortality. It remains unclear whether SARS-CoV-2 infection is a trigger of acute decompensation or acute-on-chronic liver failure in cirrhotic patients based on the current evidence. There is still no clearly proven effective pharmacological treatment for cirrhotic patients with COVID-19. Clinicians should maintain standard cirrhosis care, defer unnecessary liver-specific procedures and have a low threshold for admission if cirrhotic patients are infected with SARS-CoV-2.
- Citation: Su HY, Hsu YC. Patients with cirrhosis during the COVID-19 pandemic: Current evidence and future perspectives . World J Clin Cases 2021; 9(13): 2951-2968
- URL: https://www.wjgnet.com/2307-8960/full/v9/i13/2951.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i13.2951
In December 2019, an outbreak of unexplained pneumonia caused by a novel coronavirus [severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)] occurred in Wuhan, China[1]. The disease was named coronavirus disease 2019 (COVID-19) and has rapidly spread to other countries, causing a global public health crisis. On March 11, 2020, the World Health Organization (WHO) declared that the outbreak of COVID-19 was a pandemic. COVID-19 is generally a self-limiting disease, but it can progress to acute respiratory distress syndrome (ARDS), septic shock, and death, especially in those with advanced age or underlying comorbidities, including hypertension, diabetes mellitus, and cancer[2-5]. The estimated case-fatality rate of COVID-19 ranges between 2% and 6%[2,6,7].
The clinical manifestations in patients with COVID-19 may be nonspecific, but most have fever, cough, followed by dyspnea, fatigue, or sputum production[2,3,8,9]. It is noteworthy that approximately 14% to 53% of patients experience various degrees of liver damage, although most of these injuries are mild and transient, with a satisfactory prognosis in patients without prior liver disease[10-13]. In contrast, COVID-19 in patients with pre-existing liver disease has been reported to result in higher hospitalization and mortality rates[14-22]. Among these preexisting liver diseases, cirrhosis is a chronic liver disease that involves the collapse of the structure of the liver and distortion of the vascular architecture. Cirrhosis is associated with inherent immune dysfunction and an altered gut-liver axis; patients with cirrhosis are particularly at elevated risk of infections and the associated complications[23]. It remains uncertain whether immunocompromised patients with COVID-19 have a higher risk of adverse outcomes. Patients with cancer or solid organ transplant recipients may have an elevated risk of more severe COVID-19[24]; whereas patients taking biologic therapies may not have a greater risk of developing severe COVID-19[24]. No additional risk of death was observed in cancer patients receiving active treatment except in those undergoing chemotherapy[25]. Whether patients with human immunodeficiency virus infection are at higher risk of mortality due to COVID-19 is unclear[24,26,27]. In addition to studies involving the aforementioned immunocomp
As of late December 2020, there were over 79 million confirmed COVID-19 cases in more than 200 countries and 1.7 million cases of mortality, as reported by the WHO[34]. Previous studies reported that 2%-11% of COVID-19 patients had pre-existing liver disease[10,12], and approximately 0.6% of COVID-19 patients had coexisting liver disease in a recent large-scale population-based United Kingdom cohort study[35]. In contrast, the literature on the prevalence of cirrhosis in patients with COVID-19 is limited. Two large-scale studies from the United States demonstrated that 0.3% (19/5700) and 1.8% (50/2780) of patients with COVID-19 had underlying cirrhosis[20,36]. Similarly, two studies from Portugal and China showed that 0.8% (6/756) and 2.4% (3/123) of patients with COVID-19 had pre-existing cirrhosis, respectively[37,38]. Overall, a low proportion of COVID-19 patients have coexisting liver cirrhosis. Nevertheless, there had been speculated that people with liver cirrhosis are more susceptible to SARS-CoV-2 infection due to their altered immune response[39-41]. One study from the United States and another from China reported incidences of SARS-CoV-2 infection in hospitalized patients with liver cirrhosis of 6.6% (37/556) and 16.8% (17/101), respectively[23,42].Taken together, although they account for a small proportion of patients with COVID-19, patients with underlying cirrhosis are more susceptible to viral infection than the general population.
Limited evidence has shown that the clinical manifestations in cirrhotic patients with COVID-19 are similar to those in the general population with COVID-19, with fever and cough remaining the most common symptoms, followed by shortness of breath and sputum production[21,31,33]. Interestingly, whereas similar proportions of cirrhotic and noncirrhotic patients developed respiratory and cardiovascular symptoms, cirrhotic patients were less likely to develop gastrointestinal symptoms (e.g., diarrhea, nausea, vomiting)[19,23,31,33]. Possible explanations include the inherently higher proportion of baseline gastrointestinal symptoms in cirrhotic patients, and their use of medications (e.g., lactulose), which could also result in the underestimation of the proportion of patients with gastrointestinal symptoms attributable to COVID-19[23].
With regard to laboratory test results, the data are scarce; however, the available data showed that patients with cirrhosis and COVID-19 were significantly more likely to have thrombocytopenia than the group of patients with cirrhosis but not COVID-19[30]. Another Asian multinational study revealed significantly elevated aspartate aminotransferase levels in cirrhotic patients after SARS-CoV-2 infection; meanwhile, the degrees to which the aspartate/alanine transferase and bilirubin levels were elevated in these patients was associated with survival[14]. Interestingly, the early (usually occurring within the first week of hospitalization) and rapid worsening of these liver biochemicals (e.g., aminotransferase), rather than bile duct enzymes (e.g., alkaline phosphatase, γ-glutamyl transferase), in these cirrhotic patients may indicate that COVID-19-related liver injury is more often caused by drugs or hypoxia in this population. However, further histological and experimental studies are needed to validate this finding[14]. In summary, although patients with cirrhosis and COVID-19 are presumed to have elevated levels of both liver and bile duct biochemicals (e.g., aminotransferase, bilirubin, alkaline phosphatase, γ-glutamyl transferase) because of the widespread and abundant distribution of angiotensin-converting enzyme 2 (ACE2) receptors in these areas, making them more susceptible to virus-related injury, the mismatch between ACE2 expression in SARS-CoV-2-target organs and ACE2 localization means that ACE2 may not fully explain the liver tropism of SARS-CoV-2, which warrants future larger cohort studies[43].
Acute decompensation (AD) of liver cirrhosis and acute-on-chronic liver failure (ACLF) are two important complications in patients with cirrhosis, resulting in high mortality and poor outcomes[44]. It has been proposed that COVID-19 leads to deterioration of hepatic function, leading to AD and ACLF in cirrhotic patients[17,18,39]. Studies involving patients with cirrhosis and COVID-19 showed that the proportion of patients who developed AD ranged widely from 9.3% to 61.5% (Table 1)[14,18,19,21,30]. The decompensation events included hepatic encephalopathy (4.5% to 27%)[14,18,19,21,23,28,31], variceal bleeding (1% to 30.8%)[14,18,19,21,23,28,30,32,33], worsening ascites (3% to 28%)[14,18,19,21,28,30,31,33], spontaneous bacterial peritonitis (2.9% to 7%)[14,18,19], jaundice (23.3%)[14], and secondary infection (7.1%)[22]. Moreover, the development of ACLF was reported in 4.8% to 34.6% of patients with cirrhosis and COVID-19[14,18,23,30-33]. The diversity of the distribution of decompensation events and ACLF development could be attributed to the high heterogeneity among these studies, due to the inclusion of patients with different ethnicities, cirrhosis etiologies and cirrhosis severity levels, and the use of different ACLF definitions (Table 1). Additionally, the relatively small numbers of patients in several studies may complicate the interpretation of these results. Notably, approximately 20% of patients with cirrhosis and COVID-19 had AD and did not initially present with respiratory symptoms[18,19]. Therefore, regardless of the presence of respiratory symptoms, the European Association for the Study of the Liver (EASL) and American Association for the Study of Liver Diseases (AASLD) recommend that all cirrhotic patients with new or worsening AD or the development of ACLF undergo testing for SARS-CoV-2 during the COVID-19 pandemic[39,45].
| Reference | Patientnumber (n) | Region | Etiology | CirrhosisSeverity1 | ACLF2 | Acute decompensation |
| Qi et al[33] | 21 | China | HCV: 9.5% | CTP-A: 76.2% | 4.8%3 | Variceal bleeding: 19% |
| HBV: 42.9% | CTP-B: 14.3% | |||||
| ALD: 9.5% | CTP-C: 9.5% | Ascites: 23.8% | ||||
| AIH: 4.8% | MELD: 8 (7-11) | |||||
| Liu et al[32] | 17 | China | HCV: 11.8% | CTP-A: 88.2% | APASL:11.8% | Variceal bleeding: 5.9% |
| HBV: 70.6% | CTP-B: 5.9% | |||||
| CTP-C: 5.9% | ||||||
| Iavarone et al[31] | 50 | Italy | HCV: 28% | CTP-A: 40% | EASL: 28% | HE: 22% |
| HBV: 10% | CTP-B: 28% | |||||
| ALD: 24% | CTP-C: 32% | |||||
| NAFLD: 6% | MELD: 9 (6-15) | |||||
| Sarin et al[14] | 43 | Asia | Viral: 60.4% | CTP-A: 53.8% | APASL: 11.6% | AD event: 9.3% |
| MAFLD: 32.5% | CTP-B: 37.2% | Variceal bleeding: 9.3% | ||||
| ALD: 4.6% | CTP-C: 9% | HE: 7% | ||||
| AIH: 2.3% | Ascites: 23.3% | |||||
| Jaundice: 23.3% | ||||||
| SBP: 7% | ||||||
| Moon et al[19] | 103 | International | HCV: 10.5% | CTP-A: 44.7% | N/A | AD event: 25.7% |
| HBV: 11.8% | CTP-B: 29.1% | Variceal bleeding: 1% | ||||
| ALD: 19.7% | CTP-C: 26.2% | HE: 16.5% | ||||
| NAFLD: 22.4% | MELD: 10 | Ascites: 27.2% | ||||
| SBP: 2.9% | ||||||
| Lee et al[22] | 14 | Korea | HCV: 14.3% | CTP-A: 64.3% | N/A | Secondary infection: 7.1% |
| HBV: 35.7% | CTP-B: 35.7% | |||||
| ALD: 35.7% | MELD: 8 (7-12) | |||||
| AIH: 7.1% | ||||||
| Bajaj et al[23] | 37 | United States | HCV: 24.3% | MELD: 17.6 ± 8.6 | NACSELD: 30% | Variceal bleeding: 14% |
| ALD: 24.3% | ||||||
| HE: 14% | ||||||
| NASH: 24.3% | ||||||
| Kimet al[21] | 227 | United States | N/A | Compensated: 59% | N/A | AD event: 29.5% |
| Variceal bleeding: 3.1% | ||||||
| Decompensated: 41% | ||||||
| HE: 10.1% | ||||||
| Ascites: 4.8% | ||||||
| Shalimar et al[30] | 26 | India | HCV: 7.7% | CTP: 8.6 ± 2.3 | EASL: 34.6% | AD event: 61.5% |
| HBV: 11.5% | MELD: 18.1 ± 9.6 | Variceal bleeding: 30.8% | ||||
| ALD: 34.6% | Ascites: 7.7% | |||||
| NAFLD: 7.7% | ||||||
| AIH: 15.4% | ||||||
| Marjot et al[18] | 386 | International | HCV: 11% | CTP-A: 52% | EASL: 23% | AD event: 46% |
| HBV: 21% | CTP-B: 30% | Variceal bleeding: 3% | ||||
| ALD: 38% | CTP-C: 17% | HE: 27% | ||||
| NAFLD: 26% | MELD: 12 (8-19) | Ascites: 28% | ||||
| SBP: 3% | ||||||
| Jeon et al[28] | 67 | Korea | N/A | N/A | N/A | Variceal bleeding: 3% |
| Ascites: 3% | ||||||
| HE: 4.5% |
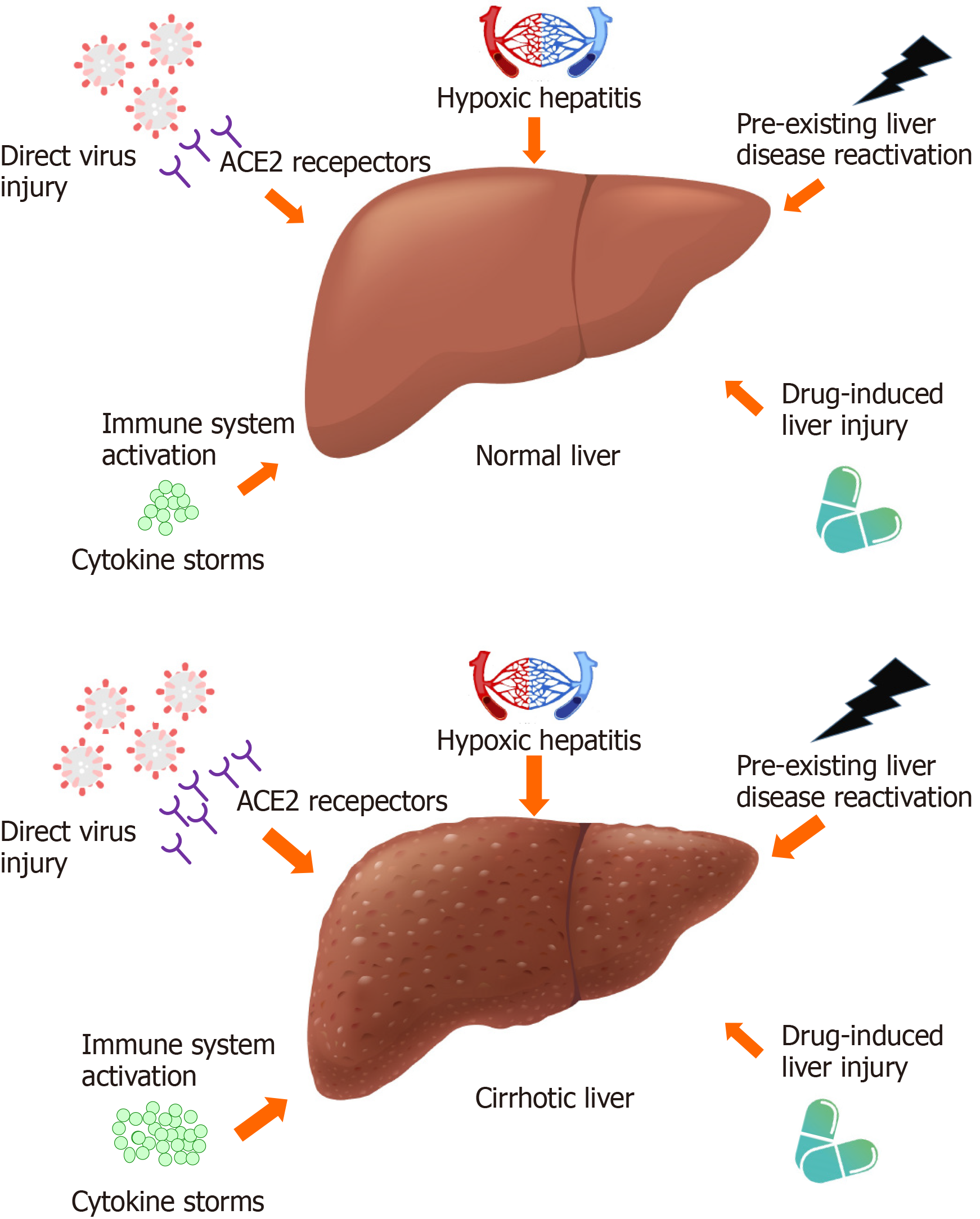
The putative mechanisms underlying liver injury in COVID-19 patients include direct pathogenic virus cytotoxicity, systemic immune system activation and cytokine storms, drug-related liver injury, reactivation of pre-existing liver disease, and hypoxic hepatitis. The major differences between these mechanisms in patients with cirrhotic and noncirrhotic livers are highlighted in Figure 1.
It has been postulated that SARS-CoV-2 uses the same receptor, ACE2, as SARS-CoV to enter host cells, leading to inflammatory responses and cytopathic effects[46,47]. The expression level and distribution status of the ACE2 receptors in the human body may partially indicate the potential infection targets of SARS-CoV-2[48]. ACE2 receptors are widespread across the human body and are highly expressed in type II alveolar cells in the lungs, esophageal epithelial cells, absorptive enterocytes in the ileum and colon, myocardial cells, proximal tubule cells in the kidneys, and bladder urothelial cells[48-50]. In the normal human liver, the expression of ACE2 receptors is particularly high in bile duct epithelial cells (i.e., cholangiocytes) and the vascular endothelium; only a small portion of hepatocytes are positive for ACE2[10,50,51]. The disruption of cholangiocytes due to the cytopathic effect of SARS-CoV-2 results in bile duct injury and further liver injury[12,52,53]. In contrast, ACE2 expression was detected in most hepatocytes within cirrhotic nodules, as well as in cholangiocytes and vascular endothelial cells[51,54]. Patients with cirrhosis also have increased circulating ACE activity and angiotensin II levels, which may facilitate the entry of the virus into host cells, making them more vulnerable to direct virus-related cytotoxicity, leading to a greater severity of hepatic dysfunction and serious clinical consequences[51,55,56].
Similar to other viruses, such as cytomegalovirus and Epstein-Barr virus, SARS-CoV-2 can activate excessive innate immune responses and systemic inflammatory reactions, causing rapid disease progression and adverse outcomes[57,58]. In patients with severe COVID-19, fulminant cytokine storms can occur, involving the release of multiple proinflammatory cytokines and inflammatory markers, including IL-1β, IL-6, IL-8, IL-17, interferon-γ, granulocyte-colony stimulating factor and tumor necrosis factor-α[52,59,60]. Hypercytokinemia can damage the tissue of the heart, kidney, lung, and liver, leading to multiple organ failure and mortality[61]. It is noteworthy that patients with cirrhosis exhibit higher baseline levels of cytokines and are at increased risk of systemic inflammation, which may lead to systemic circulatory and local microcirculatory function impairment, mitochondrial and cell dysfunction, cell death, and organ failure[62,63].
Lymphocytes, an important type of leukocyte, are responsible for a wide range of immune cell regulation, activation, and proliferative functions necessary for maintaining immune homeostasis[64]. The degree of lymphopenia, including the numbers of CD4+ T cells, CD8+ T cells, B cells, and natural killer cells, was associated with disease severity in COVID-19 patients[61,64-67]. Importantly, cirrhosis disrupts the reticulo-endothelial system, which affects immune surveillance activities, impairs immune protein synthesis, damages circulating immune cells, and compromises immune cells functions, resulting in pancytopenia, including a decreased number of lymphocytes, which is a feature of cirrhosis-associated immune dysfunction[68,69]. This could partially explain why cirrhotic patients with COVID-19 were at higher risk for severe disease and mortality than noncirrhotic patients[23,31].
During the early phase of the COVID-19 pandemic, multiple drugs, including antiviral agents (e.g., lopinavir/ritonavir, remdesivir, oseltamivir, ribavirin), antibiotics (e.g., macrolides), and corticosteroids, were prescribed. Many of these drugs are metabolized in the liver, and concerns about drug-related hepatotoxicity have been raised, especially when they are used in combination[70]. Patients who used lopinavir/ritonavir were reported to have a significantly higher proportion with liver function impairment[71,72], and the administration of systemic corticosteroid or antifungal agents was associated with a risk of elevated aminotransferase levels[73]. Chloroquine and hydroxychloroquine have been reported to induce acute liver failure, and they should be used with caution in patients with pre-existing liver disease[70]. Moreover, approximately 15.2% of the patients experienced elevated aminotransferase levels while receiving treatment with remdesivir[74,75], although two studies revealed similar proportion of patients with elevated levels of aminotransferase in the remdesivir and placebo groups[76,77]. The EASL suggested that remdesivir should be used with caution in patients with advanced liver disease or severe liver baseline biochemistry derangement[39]. Overall, the incidence of drug-induced liver injury was estimated to be approximately 25.4% in a meta-analysis[78]. Nonetheless, concern about SARS-CoV-2 drug-induced liver injury may decrease gradually after the development of vaccines, as vaccine-related hepatotoxicity has rarely been reported[79-83].
There is a paucity of studies discussing the impact of medications used to treat cirrhosis on patients with cirrhosis and COVID-19. Most guidelines recommend the maintenance of cirrhosis treatment during the COVID-19 pandemic[39,40,84], although concerns about drug-drug interactions still might exist[84]. In fact, the more liberal use of nonselective beta-blockers, a standard treatment for lowering portal pressure and the risk of variceal bleeding in patients with cirrhosis, was proposed to reduce unnecessary endoscopic procedures during the COVID-19 pandemic[45,84,85]. Venous thromboembolism is another emerging clinical problem in patients with cirrhosis due to their hypercoagulative state and coagulation dysregulation[86]. Evidence from previous studies showed that COVID-19 patients were at increased risk for thromboembolism, which correlated with a worse prognosis[87]. Therefore, the EASL suggests that patients with cirrhosis and COVID-19 should consider continuing treatment with thromboprophylactic agents[39], preferably low-molecular-weight heparin, although the actual dose may need further verification by expert consensus[88,89]. Taken together, after weighing the benefits and risks, resuming treatment for cirrhosis, including with medications intended to prevent the progression or decompensation of liver disease, may be the best strategy in patients with cirrhosis and COVID-19[18].
Patients with pre-existing liver disease are presumed to be more vulnerable to COVID-19-related liver damage due to their immune dysregulation and circulatory disturbances, leading to local liver damage and systemic injury[19,39]. For patients with hepatitis B, the discontinuation of antiviral agents during the COVID-19 pandemic may induce the reactivation of the hepatitis B virus (HBV)[58], and the administration of tocilizumab, a monoclonal antibody against IL-6 used to control cytokine storms in patients with severe COVID-19, is occasionally reported to cause HBV flare-ups and further hepatic injury[90,91]. Additionally, systemic corticosteroid administration might cause HBV reactivation and acute liver failure in patients with chronic HBV infection[84]. Prophylactic antiviral therapy with nucleoside analogues is recommended in patients with known HBV infections undergoing corticosteroid therapy for severe COVID-19[84].
Cirrhosis is a form of advanced liver disease associated with extensive immune dysfunction and abnormal systemic inflammation[68]. SARS-CoV-2 infections in patients with cirrhosis is particularly lethal due to the dysregulated immune responses and disordered coagulation[18]. It is noteworthy that SARS-CoV-2-associated lung injury leading to respiratory failure was the predominant cause of death in patients with cirrhosis, rather than the decompensation of their liver disease[18,19,29]. This phenomenon could be explained by their altered pulmonary dynamics due to the presence of ascites or encephalopathy, and coexisting lung diseases, including hepatopulmonary syndrome, porpopulmonary hypertension, or hepatic hydrothorax, which could exacerbate the risk of respiratory failure in patients with cirrhosis[18,20]. Despite this, these findings should not contradict the recommendations that standard cirrhosis treatment should be continued with minimal interruptions in these patients, as stated in the guidelines[39], as their liver dysfunction may also aggravate SARS-CoV-2 induced respiratory failure[18].
The abundant vascular supply and high metabolic demand in the liver make it vulnerable to circulatory disturbances[58]. Hypoxic hepatitis, also called ischemic hepatitis, is characterized by a rapid and massive serum aminotransferase elevation resulting from reduced liver oxygen delivery and diminished perfusion in a complication of cardiac, circulatory, or respiratory failure[92,93]. The ongoing oxygen reduction and lipid accumulation in hepatocytes during shock and hypoxic conditions could lead to mitochondrial damage and cell death, and subsequent reactive oxygen species activation and high levels of oxygen free radicals further amplify the release of proinflammatory cytokines, resulting in liver injury[40,94]. Given that respiratory failure and the associated hypoxia are important characteristics of COVID-19[40], it is reasonable to assume that hypoxia-reperfusion injury is a crucial cause of secondary liver injury in these patients[40,57,58,94].
Pre-existing liver disease, especially cirrhosis, was also identified as a risk factor for hypoxic hepatitis, leading to a worse prognosis[20,92]. Cirrhotic livers have abnormal portosystemic shunting and impaired functional flow, dysregulated glutathione synthesis, and increased susceptibility to circulatory failure, all of which could exacerbate hypoxic damage to the liver[92,95]. Additionally, a hypovolemic state, such as gastrointestinal bleeding, in cirrhotic patients due to coagulopathy could ultimately contribute to the occurrence of hypoxic hepatitis[96]. The cornerstone of hypoxic hepatitis treatment mostly depends on its early recognition and correction of the underlying disease[92] (i.e., cirrhosis and COVID-19).
It is not surprising that patients with cirrhosis were more likely to have severe COVID-19 than the general population[7], with proportions ranging from 18.6% to 35.3% (Table 2)[14,30,32]. Pre-existing cirrhosis was found to be a risk factor for severe COVID-19[14]. Furthermore, cirrhotic patients are also more likely to develop complications of COVID-19, including ARDS (28.6% to 52%)[22,31-33], respiratory failure requiring mechanical ventilation (MV) (4% to 38%)[14,18,19,22,23,31-33], shock (6% to 30%)[14,22,23,28,31-33], renal failure requiring renal replacement therapy (1.5% to 19%)[18,19,22,23,28,33], the need for extracorporeal membrane oxygenation (9.5%)[33], and the need for intensive care unit (ICU) admission (3% to 43%) (Table 2)[14,18,19,22,23,28,31-33]. It is noteworthy that far greater proportions of COVID-19 patients developed an adverse outcome than receiving the corresponding level of care (i.e., 52% of the patients had ARDS, but only 4% of the patients received MV and were admitted to the ICU) in an Italian study, which reflected the marked scarcity of ICU beds in an area with a high burden of COVID-19[31]. Therefore, the true number of cirrhotic patients who needed intensive care in these studies may have been underestimated because of the limited availability of healthcare resources during the pandemic[19].
| Reference | Patient number (n) | COVID-19 severity | COVID-19 complications | Mortality |
| Qi et al[33] | 21 | N/A | ICU: 23.8% | 23.8% |
| MV: 14.3% | ||||
| Shock: 14.3% | ||||
| ARDS: 28.6% | ||||
| RRT: 9.5% | ||||
| ECMO: 9.5% | ||||
| Liu et al[32] | 17 | Mild1: 64.7% | ICU: 17.6% | 17.6% |
| Severe1: 35.3% | MV: 11.8% | |||
| Shock: 11.8% | ||||
| ARDS: 29.4% | ||||
| Iavarone[31] et al | 50 | N/A | ICU: 4% | 34% |
| MV: 4% | ||||
| Shock: 8% | ||||
| ARDS: 52% | ||||
| Sarin et al[14] | 43 | Severe2: 18.6% | ICU: 25.6% | 16.3% |
| MV: 23.2% | ||||
| Shock: 14% | ||||
| Moon et al[19] | 103 | N/A | ICU: 23.3% | 39.8% |
| MV: 17.5% | ||||
| RRT: 4.9% | ||||
| Lee et al[22] | 14 | N/A | ICU: 35.7%MV: 21.4% | 28.6% |
| RRT: 7.1% | ||||
| Shock: 28.6% | ||||
| ARDS: 35.7% | ||||
| Bajaj et al[23] | 37 | N/A | ICU: 43% | 30% |
| MV: 38% | ||||
| Shock: 30% | ||||
| RRT: 19% | ||||
| Kim et al[21] | 227 | N/A | Death, hospitalization, oxygen support, ICU, vasopressor support, or MV: 70.5% | 25.1% |
| Shalimar et al[30] | 26 | Mild3: 57.7% | N/A | 42.3% |
| Moderate3: 7.7% | ||||
| Severe3: 34.6% | ||||
| Marjot et al[18] | 386 | N/A | ICU: 28% | 32% |
| MV: 18.4% | ||||
| RRT: 5.4% | ||||
| Jeon et al[28] | 67 | N/A | ICU: 3% | 9% |
| Shock: 6% | ||||
| RRT: 1.5% |
Compared with the general population with COVID-19, patients with cirrhosis and COVID-19 had higher mortality rates, ranging from 9% to 42.3% (Table 2)[14,18,19,21-23,28,30-33]. Decompensated liver cirrhosis was identified as an independent risk factor for mortality[21]; patients with cirrhosis are also at higher risk of COVID-19-related hospitalization and mortality[20]. Compared with other studies, cirrhosis was not found to be associated with mortality in COVID-19 patients in a Korean nationwide population-based study[28]. The authors also demonstrated a substantially lower risk of mortality and other complications in patients with cirrhosis and COVID-19[28]. Nevertheless, the enrollment of all cirrhotic patients (including hospitalized and nonhospitalized patients) and the lack of data on cirrhosis etiology and severity in that study may limit the extrapolation of the results[28]. Moreover, a North American multicenter study showed that patients with cirrhosis and COVID-19 had a comparable mortality risk to the group of patients with cirrhosis but not COVID-19 (30% vs 20%, P = 0.16); they also had a similar rate development of ACLF (30% vs 23%, P = 0.11)[23]. Whereas the cirrhosis-alone group had worse baseline liver function [i.e., higher Model for End-Stage Liver Disease (MELD) score] in that study, they had more cirrhosis-specific complications (e.g., variceal bleeding, hepatic encephalopathy) during hospitalization. In contrast, the cirrhosis and COVID-19 group had more COVID-19-related complications (e.g., respiratory failure, need for MV, shock), indicating possible different causes of mortality in the two groups[23]. Taken together, compared with the general population with COVID-19, cirrhotic patients with COVID-19 have a higher risk of in-hospital mortality, but there is still insufficient evidence to support the idea that COVID-19 increases the risk of ACLF or mortality in cirrhotic patients[39].
The baseline cirrhosis severity in patients correlated with their risk of COVID-19-related morbidity and mortality[18,19,31]. The MELD score independently predicted mortality in patients with cirrhosis and COVID-19 in an Italian study[31]. An Asian multinational study concluded that cirrhotic patients with a Child-Turcotte-Pugh (CTP) score of 9 or more at presentation were at higher risk for COVID-19-related mortality[14]. In line with these findings, a large multinational cirrhosis cohort study revealed that the stage of liver disease (i.e., CTP and MELD score) was associated with a stepwise increased risk of COVID-19-related mortality and the need for admission to the ICU, and CTP-B, CTP-C stage, and baseline MELD score were independent factors associated with the risk of mortality[18]. These findings have significant prognostic implications and could help clinicians perform risk stratification and make decisions while treating cirrhotic patients with COVID-19, increasing the level of care earlier in those with advanced liver disease[18]. Furthermore, clinicians could also consider the implementation of palliative care in COVID-19 patients with decompensated cirrhosis who experience rapid clinical deterioration because of their extremely poor prognosis, to ensure appropriate healthcare resource allocation in areas where there is a high burden of COVID-19[18,39,84].
Current recommendations for cirrhotic patient management during the COVID-19 pandemic proposed by the EASL, AASLD, and Asian Pacific Association for the Study of the Liver (APASL) are summarized in Table 3.
| EASL | AASLD | APASL | |
| Outpatient | Common rules of physical distancing | Limited outpatient visitsWearing mask and keeping appropriate distancing | Phone or telemedicine |
| Early admission for patient infected with SARS-CoV-2 | Limited family or friend accompanied | Limited travel | |
| Prevent decompensation (e.g., variceal bleeding, HE, SBP) and avoid admission | Video conference, phone or telemedicine | Continue hepatitis B and C treatment | |
| Telemedicine or remote monitor | Provide prescriptions for 90 d instead of 30 dLimited travel | ||
| Continue hepatitis B and C treatment | Continue hepatitis B and C treatment | ||
| Receive vaccination for Streptococcus pneumoniae and influenza | |||
| Inpatient | Designate non-COVID-19 ward | Designate non-COVID-19 ward | Option of palliative treatment for advanced liver disease with COVID-19 disease |
| Perform SARS-CoV-2 testing in patient with new or worsening decompensation or ACLF | Perform SARS-CoV-2 testing in patient with new or worsening decompensation or ACLF | ||
| Option of palliative treatment for patient with advanced liver disease with COVID-19 | Minimize interaction and transport for patient | ||
| Telemedicine equipmentLimit patient visitors | |||
| Endoscopy | Limit to emergency (e.g., GI bleeding or bacterial cholangitis) in patient with COVID-19 | Limit to emergency (e.g., GI bleeding or bacterial cholangitis) in patient with COVID-19 | Limit to emergency (e.g., GI bleeding or bacterial cholangitis) in patient with COVID-19 |
| SARS-CoV-2 testing prior to endoscopic procedureNo delay in endoscopy in areas with low COVID-19 burden | SARS-CoV-2 testing prior to endoscopic procedure | PPE used in endoscopy for patient and staff | |
| Noninvasive tool for variceal surveying | PPE used in endoscopy for patient and staff | Variceal survey can be arbitrary postponed 3 mo depend on COVID-19 outbreak. | |
| Clean and disinfect the operation room | Noninvasive tool for variceal survey | ||
| Prophylaxis with beta-blocker instead of endoscopic screening | Prophylaxis with beta-blocker instead of endoscopic screening | ||
| HCC surveillance | Deferred in patients with COVID-19 until recovery | Deferred in patients with COVID-19 until recovery | Prioritized for patients at high risk |
| Prioritized for patients at high risk (e.g., elevated alpha-fetoprotein level or advanced cirrhosis | Continued radiological surveillance, but an arbitrary delay of 2 mo is reasonable | Continued radiological surveillance, but an arbitrary delay of 3 mo is reasonable |
Various methods described to reduce SARS-CoV-2 transmission and infection in the WHO protocol for the general public are also vital to cirrhotic patients, including maintaining a social distance at least one meter, wearing a mask, avoiding closed or crowded places, regularly cleaning hands with alcohol-based hand rub, and washing hands thoroughly with soap and water[97]. For cirrhotic patients who need outpatient follow-up, several alternative ways to reduce in-person visits could be considered, including telemedicine, video conference or phone tracking[15,39,40,42,45]. Integrated telehealth provides remote monitoring, focused education, caregiver support, and early intervention to ensure medical compliance[85]. The continuation of antiviral therapy for viral hepatitis was proposed by all three associations[15,39,40,45]. The guidelines regarding prophylaxis for decompensation events (e.g., spontaneous bacterial peritonitis, variceal bleeding, hepatic encephalopathy) should be followed to avoid non-COVID-19 cirrhotic-related admission[15,39]. Moreover, the threshold for hospital admission should be low in patients with cirrhosis and COVID-19 because they are at greater risk of developing adverse outcomes[15,39]. It is noteworthy that approximately 40% of SARS-CoV-2 infections in cirrhotic patients were healthcare-related in one study[31]. Therefore, for cirrhotic patients who need inpatient treatment, the designation of non-COVID wards is essential, and limiting patient visitors and minimizing inpatient interaction and transport are also crucial to reduce the possibility of virus transmission[39,45].
Currently, remdesivir is the only United States Food and Drug Administration-approved therapy for hospitalized COVID-19 patients[98], although several recent large-scale randomized controlled trials revealed conflicting results in the general population with COVID-19[76,99,100]. There have been no studies on the pharmacokinetics and outcomes of remdesivir use in cirrhotic patients[45]; only limited data showed that remdesivir did not confer a significant survival benefit on COVID-19 patients with pre-existing chronic liver disease[21].
Dexamethasone, a potent corticosteroid agent, was shown to reduce the risk of mortality in COVID-19 patients requiring MV[101]. The National Institutes of Health and Infectious Disease Society of America recommended the use of dexamethasone in hospitalized critically ill COVID-19 patients or those who need supplemental oxygen, noninvasive ventilation, or MV[102,103]. Similarly, there have been no studies on the use of dexamethasone or alternative corticosteroids in patients with cirrhosis during the pandemic. No statements have been made in favor of or against the routine use of corticosteroids in patients with cirrhosis and COVID-19 in the current guidelines[39,45].
As previously mentioned, tocilizumab, is a humanized monoclonal antibody targeting IL-6, which is a key driver of cytokine storms in severeCOVID-19. Tocilizumab was postulated to counter dysregulated inflammation and reduce the need for organ support in patients with severe COVID-19[39]; however, it failed to confer any clinical or survival benefit on patients with severe COVID-19 in a recent randomized controlled trial[104]. The National Institutes of Health and Infectious Disease Society of America warned against the routine use of tocilizumab in hospitalized patients, including cirrhotic patients[102,103].
Routine screening for esophageal varices (EVs) and hepatocellular carcinoma (HCC) is essential for patients with cirrhosis[39,105]. Esophagogastroduodeno-scopy is an effective method of assessing bleeding severity and stopping bleeding in cirrhotic patients with EVs. However, as endoscopy is considered an aerosol-generating procedure, its performance is associated with the risk of SARS-CoV-2 transmission[40,45,106]. Current guidelines recommend the postponement of nonurgent or elective endoscopies during the pandemic[107,108], depending on the local prevalence of COVID-19, patient risk factors, and treatment center resources[105]. Urgent or emergent endoscopies should only be performed in cases of active gastrointestinal bleeding or bacterial cholangitis[39,40,84]. If they must be performed, all patients should first be tested for SARS-CoV-2[39,45], and healthcare workers involved in the endoscopy should wear a full set of personal protection equipment, including double gloves and N95 masks[45,84,108]. Regarding deferring HCC screening in patients with COVID-19 until recovery[39,40], a multidisciplinary approach to individual risk evaluation should be implemented based on local resources[39,40,105]. Patients at a high risk of HCC (e.g., active viral hepatitis, advanced cirrhosis, elevated α-fetoprotein level) should be prioritized[39,105]; otherwise, most patients can be monitored at prolonged intervals reaching up to eight months[105]; or delayed follow-up for two to three months[40,45].
In this unprecedented COVID-19 pandemic, although patients with cirrhosis account for a small portion of the total population, they are more vulnerable to viral infection, resulting in both hepatic and extrahepatic complications, which are further associated with a higher risk of mortality and greater healthcare resource utilization. Because of their immune dysfunction, cirrhotic patients have distinct features of COVID-19-related liver injury. Data focusing on their clinical manifestations and laboratory results after SARS-CoV-2 infection are scarce, and more importantly, whether COVID-19 is a trigger of AD or ACLF in patients with cirrhosis is still unknown. As there is no pharmacological therapy proven to be effective in cirrhotic patients with COVID-19, the maintenance of standard care for cirrhosis and the avoidance of virus transmission are the cornerstones of management. As the COVID-19 vaccines become available, their safety and efficacy in cirrhotic patients need further investigation. We hope that both healthcare providers and patients can stand together to be strong enough to weather the storm.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ahboucha S, Feng B S-Editor: Liu M L-Editor: A P-Editor: Li JH
| 1. | Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W; China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18987] [Cited by in RCA: 17630] [Article Influence: 3526.0] [Reference Citation Analysis (0)] |
| 2. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 18866] [Article Influence: 3773.2] [Reference Citation Analysis (7)] |
| 3. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17476] [Cited by in RCA: 18189] [Article Influence: 3637.8] [Reference Citation Analysis (0)] |
| 4. | Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, Greninger AL, Pipavath S, Wurfel MM, Evans L, Kritek PA, West TE, Luks A, Gerbino A, Dale CR, Goldman JD, O'Mahony S, Mikacenic C. Covid-19 in Critically Ill Patients in the Seattle Region - Case Series. N Engl J Med. 2020;382:2012-2022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1819] [Cited by in RCA: 1861] [Article Influence: 372.2] [Reference Citation Analysis (0)] |
| 5. | Poston JT, Patel BK, Davis AM. Management of Critically Ill Adults With COVID-19. JAMA. 2020;323:1839-1841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 207] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 6. | Baud D, Qi X, Nielsen-Saines K, Musso D, Pomar L, Favre G. Real estimates of mortality following COVID-19 infection. Lancet Infect Dis. 2020;20:773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 711] [Cited by in RCA: 689] [Article Influence: 137.8] [Reference Citation Analysis (0)] |
| 7. | Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11409] [Cited by in RCA: 11504] [Article Influence: 2300.8] [Reference Citation Analysis (0)] |
| 8. | Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, Satlin MJ, Campion TR Jr, Nahid M, Ringel JB, Hoffman KL, Alshak MN, Li HA, Wehmeyer GT, Rajan M, Reshetnyak E, Hupert N, Horn EM, Martinez FJ, Gulick RM, Safford MM. Clinical Characteristics of Covid-19 in New York City. N Engl J Med. 2020;382:2372-2374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1644] [Cited by in RCA: 1617] [Article Influence: 323.4] [Reference Citation Analysis (0)] |
| 9. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30097] [Article Influence: 6019.4] [Reference Citation Analysis (3)] |
| 10. | Jothimani D, Venugopal R, Abedin MF, Kaliamoorthy I, Rela M. COVID-19 and the liver. J Hepatol. 2020;73:1231-1240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 364] [Cited by in RCA: 358] [Article Influence: 71.6] [Reference Citation Analysis (1)] |
| 11. | Sun J, Aghemo A, Forner A, Valenti L. COVID-19 and liver disease. Liver Int. 2020;40:1278-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 219] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 12. | Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1348] [Cited by in RCA: 1295] [Article Influence: 259.0] [Reference Citation Analysis (4)] |
| 13. | Phipps MM, Barraza LH, LaSota ED, Sobieszczyk ME, Pereira MR, Zheng EX, Fox AN, Zucker J, Verna EC. Acute Liver Injury in COVID-19: Prevalence and Association with Clinical Outcomes in a Large U.S. Cohort. Hepatology. 2020;72:807-817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 271] [Article Influence: 54.2] [Reference Citation Analysis (2)] |
| 14. | Sarin SK, Choudhury A, Lau GK, Zheng MH, Ji D, Abd-Elsalam S, Hwang J, Qi X, Cua IH, Suh JI, Park JG, Putcharoen O, Kaewdech A, Piratvisuth T, Treeprasertsuk S, Park S, Wejnaruemarn S, Payawal DA, Baatarkhuu O, Ahn SH, Yeo CD, Alonzo UR, Chinbayar T, Loho IM, Yokosuka O, Jafri W, Tan S, Soo LI, Tanwandee T, Gani R, Anand L, Esmail ES, Khalaf M, Alam S, Lin CY, Chuang WL, Soin AS, Garg HK, Kalista K, Batsukh B, Purnomo HD, Dara VP, Rathi P, Al Mahtab M, Shukla A, Sharma MK, Omata M; APASL COVID Task Force; APASL COVID Liver Injury Spectrum Study (APCOLIS Study-NCT 04345640). Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; The APCOLIS Study (APASL COVID-19 Liver Injury Spectrum Study). Hepatol Int. 2020;14:690-700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 216] [Article Influence: 43.2] [Reference Citation Analysis (1)] |
| 15. | Boettler T, Newsome PN, Mondelli MU, Maticic M, Cordero E, Cornberg M, Berg T. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep. 2020;2:100113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 334] [Article Influence: 66.8] [Reference Citation Analysis (1)] |
| 16. | Hashemi N, Viveiros K, Redd WD, Zhou JC, McCarty TR, Bazarbashi AN, Hathorn KE, Wong D, Njie C, Shen L, Chan WW. Impact of chronic liver disease on outcomes of hospitalized patients with COVID-19: A multicentre United States experience. Liver Int. 2020;40:2515-2521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 117] [Article Influence: 23.4] [Reference Citation Analysis (2)] |
| 17. | Ji D, Zhang D, Yang T, Mu J, Zhao P, Xu J, Li C, Cheng G, Wang Y, Chen Z, Qin E, Lau G. Effect of COVID-19 on patients with compensated chronic liver diseases. Hepatol Int. 2020;14:701-710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 18. | Marjot T, Moon AM, Cook JA, Abd-Elsalam S, Aloman C, Armstrong MJ, Pose E, Brenner EJ, Cargill T, Catana MA, Dhanasekaran R, Eshraghian A, García-Juárez I, Gill US, Jones PD, Kennedy J, Marshall A, Matthews C, Mells G, Mercer C, Perumalswami PV, Avitabile E, Qi X, Su F, Ufere NN, Wong YJ, Zheng MH, Barnes E, Barritt AS 4th, Webb GJ. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J Hepatol. 2021;74:567-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 384] [Article Influence: 96.0] [Reference Citation Analysis (0)] |
| 19. | Moon AM, Webb GJ, Aloman C, Armstrong MJ, Cargill T, Dhanasekaran R, Genescà J, Gill US, James TW, Jones PD, Marshall A, Mells G, Perumalswami PV, Qi X, Su F, Ufere NN, Barnes E, Barritt AS, Marjot T. High mortality rates for SARS-CoV-2 infection in patients with pre-existing chronic liver disease and cirrhosis: Preliminary results from an international registry. J Hepatol. 2020;73:705-708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 203] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 20. | Singh S, Khan A. Clinical Characteristics and Outcomes of Coronavirus Disease 2019 Among Patients With Preexisting Liver Disease in the United States: A Multicenter Research Network Study. Gastroenterology 2020; 159: 768-771. e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 270] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 21. | Kim D, Adeniji N, Latt N, Kumar S, Bloom PP, Aby ES, Perumalswami P, Roytman M, Li M, Vogel AS, Catana AM, Wegermann K, Carr RM, Aloman C, Chen V, Rabiee A, Sadowski B, Nguyen V, Dunn W, Chavin K, Zhou K, Lizaola-Mayo B, Moghe A, Debes J, Lee TH, Branch A, Viveiros K, Chan W, Chascsa D, Kwo P, Dhanasekaran R. Predictors of Outcomes of COVID-19 in Patients with Chronic Liver Disease: US Multi-center Study. Clin Gastroenterol Hepatol. 2020 epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 184] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 22. | Lee YR, Kang MK, Song JE, Kim HJ, Kweon YO, Tak WY, Jang SY, Park JG, Lee C, Hwang JS, Jang BK, Suh JI, Chung WJ, Kim BS, Park SY. Clinical outcomes of coronavirus disease 2019 in patients with pre-existing liver diseases: A multicenter study in South Korea. Clin Mol Hepatol. 2020;26:562-576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 23. | Bajaj JS, Garcia-Tsao G, Biggins SW, Kamath PS, Wong F, McGeorge S, Shaw J, Pearson M, Chew M, Fagan A, de la Rosa Rodriguez R, Worthington J, Olofson A, Weir V, Trisolini C, Dwyer S, Reddy KR. Comparison of mortality risk in patients with cirrhosis and COVID-19 compared with patients with cirrhosis alone and COVID-19 alone: multicentre matched cohort. Gut. 2021;70:531-536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 176] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 24. | Fung M, Babik JM. COVID-19 in Immunocompromised Hosts: What We Know So Far. Clin Infect Dis. 2021;72:340-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 381] [Cited by in RCA: 367] [Article Influence: 91.8] [Reference Citation Analysis (0)] |
| 25. | Yekedüz E, Utkan G, Ürün Y. A systematic review and meta-analysis: the effect of active cancer treatment on severity of COVID-19. Eur J Cancer. 2020;141:92-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 26. | Dandachi D, Geiger G, Montgomery MW, Karmen-Tuohy S, Golzy M, Antar AAR, Llibre JM, Camazine M, Díaz-De Santiago A, Carlucci PM, Zacharioudakis IM, Rahimian J, Wanjalla CN, Slim J, Arinze F, Kratz AMP, Jones JL, Patel SM, Kitchell E, Francis A, Ray M, Koren DE, Baddley JW, Hill B, Sax PE, Chow J; HIV-COVID-19 consortium. Characteristics, Comorbidities, and Outcomes in a Multicenter Registry of Patients with HIV and Coronavirus Disease-19. Clin Infect Dis. 2020 epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 160] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 27. | Bhaskaran K, Rentsch CT, MacKenna B, Schultze A, Mehrkar A, Bates CJ, Eggo RM, Morton CE, Bacon SCJ, Inglesby P, Douglas IJ, Walker AJ, McDonald HI, Cockburn J, Williamson EJ, Evans D, Forbes HJ, Curtis HJ, Hulme WJ, Parry J, Hester F, Harper S, Evans SJW, Smeeth L, Goldacre B. HIV infection and COVID-19 death: a population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet HIV. 2021;8:e24-e32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 315] [Cited by in RCA: 326] [Article Influence: 81.5] [Reference Citation Analysis (0)] |
| 28. | Jeon D, Son M, Choi J. Impact of Liver Cirrhosis on the Clinical Outcomes of Patients with COVID-19: A Nationwide Cohort Study of Korea. Korean J Intern Med. 2020;Epub ahead of print. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Frager SZ, Szymanski J, Schwartz JM, Massoumi HS, Kinkhabwala M, Wolkoff AW. Hepatic Predictors of Mortality in Severe Acute Respiratory Syndrome Coronavirus 2: Role of Initial Aspartate Aminotransferase/Alanine Aminotransferase and Preexisting Cirrhosis. Hepatol Commun. 2020;5:424-433. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Shalimar, Elhence A, Vaishnav M, Kumar R, Pathak P, Soni KD, Aggarwal R, Soneja M, Jorwal P, Kumar A, Khanna P, Singh AK, Biswas A, Nischal N, Dar L, Choudhary A, Rangarajan K, Mohan A, Acharya P, Nayak B, Gunjan D, Saraya A, Mahapatra S, Makharia G, Trikha A, Garg P. Poor outcomes in patients with cirrhosis and Corona Virus Disease-19. Indian J Gastroenterol. 2020;39:285-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 31. | Iavarone M, D'Ambrosio R, Soria A, Triolo M, Pugliese N, Del Poggio P, Perricone G, Massironi S, Spinetti A, Buscarini E, Viganò M, Carriero C, Fagiuoli S, Aghemo A, Belli LS, Lucà M, Pedaci M, Rimondi A, Rumi MG, Invernizzi P, Bonfanti P, Lampertico P. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol. 2020;73:1063-1071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 279] [Cited by in RCA: 276] [Article Influence: 55.2] [Reference Citation Analysis (2)] |
| 32. | Liu F, Long X, Ji G, Zhang B, Zhang W, Zhang Z, Chen X. Clinically significant portal hypertension in cirrhosis patients with COVID-19: Clinical characteristics and outcomes. J Infect. 2020;81:e178-e180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Qi X, Liu Y, Wang J, Fallowfield JA, Li X, Shi J, Pan H, Zou S, Zhang H, Chen Z, Li F, Luo Y, Mei M, Liu H, Wang Z, Li J, Yang H, Xiang H, Liu T, Zheng MH, Liu C, Huang Y, Xu D, Kang N, He Q, Gu Y, Zhang G, Shao C, Liu D, Zhang L, Kawada N, Jiang Z, Wang F, Xiong B, Takehara T, Rockey DC; COVID-Cirrhosis-CHESS Group. Clinical course and risk factors for mortality of COVID-19 patients with pre-existing cirrhosis: a multicentre cohort study. Gut. 2021;70:433-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (1)] |
| 34. | World Health Organization. Weekly epidemiological update. 2020 [cited 30 December 2020] Available from: https://www.who.int/publications/m/item/weekly-epidemiological-update---29-december-2020. |
| 35. | Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, Cockburn J, McDonald HI, MacKenna B, Tomlinson L, Douglas IJ, Rentsch CT, Mathur R, Wong AYS, Grieve R, Harrison D, Forbes H, Schultze A, Croker R, Parry J, Hester F, Harper S, Perera R, Evans SJW, Smeeth L, Goldacre B. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4343] [Cited by in RCA: 4205] [Article Influence: 841.0] [Reference Citation Analysis (0)] |
| 36. | Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW; the Northwell COVID-19 Research Consortium; Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6024] [Cited by in RCA: 6515] [Article Influence: 1303.0] [Reference Citation Analysis (0)] |
| 37. | Garrido I, Liberal R, Gaspar R, Macedo G. Cirrhosis management in a major referral center during COVID-19. JHEP Rep. 2020;2:100146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Chen X, Jiang Q, Ma Z, Ling J, Hu W, Cao Q, Mo P, Yao L, Yang R, Gao S, Gui X, Hou W, Xiong Y, Li J, Zhang Y. Clinical Characteristics of Hospitalized Patients with SARS-CoV-2 and Hepatitis B Virus Co-infection. Virol Sin. 2020;35:842-845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 39. | Boettler T, Marjot T, Newsome PN, Mondelli MU, Maticic M, Cordero E, Jalan R, Moreau R, Cornberg M, Berg T. Impact of COVID-19 on the care of patients with liver disease: EASL-ESCMID position paper after 6 months of the pandemic. JHEP Rep. 2020;2:100169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 40. | APASL Covid-19 Task Force. , Lau G, Sharma M. Clinical practice guidance for hepatology and liver transplant providers during the COVID-19 pandemic: APASL expert panel consensus recommendations. Hepatol Int. 2020;14:415-428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 41. | Kushner T, Cafardi J. Chronic Liver Disease and COVID-19: Alcohol Use Disorder/Alcohol-Associated Liver Disease, Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis, Autoimmune Liver Disease, and Compensated Cirrhosis. Clin Liver Dis (Hoboken). 2020;15:195-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 42. | Xiao Y, Pan H, She Q, Wang F, Chen M. Prevention of SARS-CoV-2 infection in patients with decompensated cirrhosis. Lancet Gastroenterol Hepatol. 2020;5:528-529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 43. | Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, Li X, Xu P, Zhang L, Zhao L, Cao Y, Kang J, Yang J, Li L, Liu X, Li Y, Nie R, Mu J, Lu F, Zhao S, Lu J, Zhao J. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 457] [Article Influence: 91.4] [Reference Citation Analysis (0)] |
| 44. | Gustot T, Moreau R. Acute-on-chronic liver failure vs. traditional acute decompensation of cirrhosis. J Hepatol. 2018;69:1384-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 45. | Fix OK, Hameed B, Fontana RJ, Kwok RM, McGuire BM, Mulligan DC, Pratt DS, Russo MW, Schilsky ML, Verna EC, Loomba R, Cohen DE, Bezerra JA, Reddy KR, Chung RT. Clinical Best Practice Advice for Hepatology and Liver Transplant Providers During the COVID-19 Pandemic: AASLD Expert Panel Consensus Statement. Hepatology. 2020;72:287-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 422] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 46. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020; 181: 271-280. e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11946] [Cited by in RCA: 14253] [Article Influence: 2850.6] [Reference Citation Analysis (0)] |
| 47. | Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15248] [Cited by in RCA: 14118] [Article Influence: 2823.6] [Reference Citation Analysis (1)] |
| 48. | Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, Li T, Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1709] [Cited by in RCA: 1731] [Article Influence: 346.2] [Reference Citation Analysis (0)] |
| 49. | Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14:185-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1286] [Cited by in RCA: 1528] [Article Influence: 305.6] [Reference Citation Analysis (0)] |
| 50. | Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, Zhou J, Shi G, Fang N, Fan J. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv. 2020;Preprint. [DOI] [Full Text] |
| 51. | Paizis G, Tikellis C, Cooper ME, Schembri JM, Lew RA, Smith AI, Shaw T, Warner FJ, Zuilli A, Burrell LM, Angus PW. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut. 2005;54:1790-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 281] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 52. | Wu J, Song S, Cao HC, Li LJ. Liver diseases in COVID-19: Etiology, treatment and prognosis. World J Gastroenterol. 2020;26:2286-2293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 105] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (1)] |
| 53. | Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998-1004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 622] [Cited by in RCA: 574] [Article Influence: 114.8] [Reference Citation Analysis (0)] |
| 54. | Casey S, Schierwagen R, Mak KY, Klein S, Uschner F, Jansen C, Praktiknjo M, Meyer C, Thomas D, Herath C, Jones R, Trebicka J, Angus P. Activation of the Alternate Renin-Angiotensin System Correlates with the Clinical Status in Human Cirrhosis and Corrects Post Liver Transplantation. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 55. | Grace JA, Casey S, Burrell LM, Angus PW. Proposed mechanism for increased COVID-19 mortality in patients with decompensated cirrhosis. Hepatol Int. 2020;14:884-885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 56. | Gao F, Zheng KI, Fan YC, Targher G, Byrne CD, Zheng MH. ACE2: A Linkage for the Interplay Between COVID-19 and Decompensated Cirrhosis. Am J Gastroenterol. 2020;115:1544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 57. | Ali N, Hossain K. Liver injury in severe COVID-19 infection: current insights and challenges. Expert Rev Gastroenterol Hepatol. 2020;14:879-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 58. | Yang RX, Zheng RD, Fan JG. Etiology and management of liver injury in patients with COVID-19. World J Gastroenterol. 2020;26:4753-4762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (2)] |
| 59. | Feldmann M, Maini RN, Woody JN, Holgate ST, Winter G, Rowland M, Richards D, Hussell T. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet. 2020;395:1407-1409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 405] [Cited by in RCA: 439] [Article Influence: 87.8] [Reference Citation Analysis (0)] |
| 60. | Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration; UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033-1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6366] [Cited by in RCA: 6746] [Article Influence: 1349.2] [Reference Citation Analysis (0)] |
| 61. | Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20:269-270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 972] [Cited by in RCA: 1118] [Article Influence: 223.6] [Reference Citation Analysis (0)] |
| 62. | Clària J, Stauber RE, Coenraad MJ, Moreau R, Jalan R, Pavesi M, Amorós À, Titos E, Alcaraz-Quiles J, Oettl K, Morales-Ruiz M, Angeli P, Domenicali M, Alessandria C, Gerbes A, Wendon J, Nevens F, Trebicka J, Laleman W, Saliba F, Welzel TM, Albillos A, Gustot T, Benten D, Durand F, Ginès P, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL-CLIF Consortium and the European Foundation for the Study of Chronic Liver Failure (EF-CLIF). Systemic inflammation in decompensated cirrhosis: Characterization and role in acute-on-chronic liver failure. Hepatology. 2016;64:1249-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 555] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 63. | Clària J, Arroyo V, Moreau R. The Acute-on-Chronic Liver Failure Syndrome, or When the Innate Immune System Goes Astray. J Immunol. 2016;197:3755-3761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 64. | Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W, Tian DS. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762-768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2495] [Cited by in RCA: 3336] [Article Influence: 667.2] [Reference Citation Analysis (0)] |
| 65. | Liu J, Li S, Liu J, Liang B, Wang X, Wang H, Li W, Tong Q, Yi J, Zhao L, Xiong L, Guo C, Tian J, Luo J, Yao J, Pang R, Shen H, Peng C, Liu T, Zhang Q, Wu J, Xu L, Lu S, Wang B, Weng Z, Han C, Zhu H, Zhou R, Zhou H, Chen X, Ye P, Zhu B, Wang L, Zhou W, He S, He Y, Jie S, Wei P, Zhang J, Lu Y, Wang W, Zhang L, Li L, Zhou F, Wang J, Dittmer U, Lu M, Hu Y, Yang D, Zheng X. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1171] [Cited by in RCA: 1207] [Article Influence: 241.4] [Reference Citation Analysis (0)] |
| 66. | Tan M, Liu Y, Zhou R, Deng X, Li F, Liang K, Shi Y. Immunopathological characteristics of coronavirus disease 2019 cases in Guangzhou, China. Immunology. 2020;160:261-268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 225] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 67. | Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5228] [Cited by in RCA: 5781] [Article Influence: 1156.2] [Reference Citation Analysis (2)] |
| 68. | Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 847] [Article Influence: 77.0] [Reference Citation Analysis (1)] |
| 69. | Sipeki N, Antal-Szalmas P, Lakatos PL, Papp M. Immune dysfunction in cirrhosis. World J Gastroenterol. 2014;20:2564-2577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 138] [Cited by in RCA: 122] [Article Influence: 11.1] [Reference Citation Analysis (1)] |
| 70. | Metawea MI, Yousif WI, Moheb I. COVID 19 and liver: An A-Z literature review. Dig Liver Dis. 2021;53:146-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (2)] |
| 71. | Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561-1566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 559] [Cited by in RCA: 556] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 72. | Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, Zhou G, Gou J, Qu J, Sun Y, Liu Y, He Q, Chen J, Liu L, Xu L. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73:566-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 623] [Cited by in RCA: 661] [Article Influence: 132.2] [Reference Citation Analysis (0)] |
| 73. | Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, Zhang XJ, Cai J, Lin L, Ouyang S, Wang X, Yang C, Cheng X, Liu W, Li H, Xie J, Wu B, Luo H, Xiao F, Chen J, Tao L, Cheng G, She ZG, Zhou J, Wang H, Lin J, Luo P, Fu S, Ye P, Xiao B, Mao W, Liu L, Yan Y, Chen G, Huang X, Zhang BH, Yuan Y. Longitudinal Association Between Markers of Liver Injury and Mortality in COVID-19 in China. Hepatology. 2020;72:389-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 309] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 74. | Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, Feldt T, Green G, Green ML, Lescure FX, Nicastri E, Oda R, Yo K, Quiros-Roldan E, Studemeister A, Redinski J, Ahmed S, Bernett J, Chelliah D, Chen D, Chihara S, Cohen SH, Cunningham J, D'Arminio Monforte A, Ismail S, Kato H, Lapadula G, L'Her E, Maeno T, Majumder S, Massari M, Mora-Rillo M, Mutoh Y, Nguyen D, Verweij E, Zoufaly A, Osinusi AO, DeZure A, Zhao Y, Zhong L, Chokkalingam A, Elboudwarej E, Telep L, Timbs L, Henne I, Sellers S, Cao H, Tan SK, Winterbourne L, Desai P, Mera R, Gaggar A, Myers RP, Brainard DM, Childs R, Flanigan T. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N Engl J Med. 2020;382:2327-2336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1926] [Cited by in RCA: 1884] [Article Influence: 376.8] [Reference Citation Analysis (0)] |
| 75. | Lescure FX, Bouadma L, Nguyen D, Parisey M, Wicky PH, Behillil S, Gaymard A, Bouscambert-Duchamp M, Donati F, Le Hingrat Q, Enouf V, Houhou-Fidouh N, Valette M, Mailles A, Lucet JC, Mentre F, Duval X, Descamps D, Malvy D, Timsit JF, Lina B, van-der-Werf S, Yazdanpanah Y. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect Dis. 2020;20:697-706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 783] [Cited by in RCA: 777] [Article Influence: 155.4] [Reference Citation Analysis (0)] |
| 76. | Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh MD, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC; ACTT-1 Study Group Members. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020;383:1813-1826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5711] [Cited by in RCA: 5110] [Article Influence: 1022.0] [Reference Citation Analysis (0)] |
| 77. | Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, Fu S, Gao L, Cheng Z, Lu Q, Hu Y, Luo G, Wang K, Lu Y, Li H, Wang S, Ruan S, Yang C, Mei C, Wang Y, Ding D, Wu F, Tang X, Ye X, Ye Y, Liu B, Yang J, Yin W, Wang A, Fan G, Zhou F, Liu Z, Gu X, Xu J, Shang L, Zhang Y, Cao L, Guo T, Wan Y, Qin H, Jiang Y, Jaki T, Hayden FG, Horby PW, Cao B, Wang C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569-1578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2337] [Cited by in RCA: 2485] [Article Influence: 497.0] [Reference Citation Analysis (0)] |
| 78. | Kulkarni AV, Kumar P, Tevethia HV, Premkumar M, Arab JP, Candia R, Talukdar R, Sharma M, Qi X, Rao PN, Reddy DN. Systematic review with meta-analysis: liver manifestations and outcomes in COVID-19. Aliment Pharmacol Ther. 2020;52:584-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 183] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 79. | Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, Voysey M, Aley PK, Angus B, Babbage G, Belij-Rammerstorfer S, Berry L, Bibi S, Bittaye M, Cathie K, Chappell H, Charlton S, Cicconi P, Clutterbuck EA, Colin-Jones R, Dold C, Emary KRW, Fedosyuk S, Fuskova M, Gbesemete D, Green C, Hallis B, Hou MM, Jenkin D, Joe CCD, Kelly EJ, Kerridge S, Lawrie AM, Lelliott A, Lwin MN, Makinson R, Marchevsky NG, Mujadidi Y, Munro APS, Pacurar M, Plested E, Rand J, Rawlinson T, Rhead S, Robinson H, Ritchie AJ, Ross-Russell AL, Saich S, Singh N, Smith CC, Snape MD, Song R, Tarrant R, Themistocleous Y, Thomas KM, Villafana TL, Warren SC, Watson MEE, Douglas AD, Hill AVS, Lambe T, Gilbert SC, Faust SN, Pollard AJ; Oxford COVID Vaccine Trial Group. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396:1979-1993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1071] [Cited by in RCA: 1070] [Article Influence: 267.5] [Reference Citation Analysis (0)] |
| 80. | Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, McCullough MP, Chappell JD, Denison MR, Stevens LJ, Pruijssers AJ, McDermott A, Flach B, Doria-Rose NA, Corbett KS, Morabito KM, O'Dell S, Schmidt SD, Swanson PA 2nd, Padilla M, Mascola JR, Neuzil KM, Bennett H, Sun W, Peters E, Makowski M, Albert J, Cross K, Buchanan W, Pikaart-Tautges R, Ledgerwood JE, Graham BS, Beigel JH; mRNA-1273 Study Group. An mRNA Vaccine against SARS-CoV-2 - Preliminary Report. N Engl J Med. 2020;383:1920-1931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2440] [Cited by in RCA: 2407] [Article Influence: 481.4] [Reference Citation Analysis (0)] |
| 81. | Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Raabe V, Bailey R, Swanson KA, Li P, Koury K, Kalina W, Cooper D, Fontes-Garfias C, Shi PY, Türeci Ö, Tompkins KR, Walsh EE, Frenck R, Falsey AR, Dormitzer PR, Gruber WC, Şahin U, Jansen KU. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1003] [Cited by in RCA: 1054] [Article Influence: 210.8] [Reference Citation Analysis (0)] |
| 82. | Walsh EE, Frenck RW Jr, Falsey AR, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Mulligan MJ, Bailey R, Swanson KA, Li P, Koury K, Kalina W, Cooper D, Fontes-Garfias C, Shi PY, Türeci Ö, Tompkins KR, Lyke KE, Raabe V, Dormitzer PR, Jansen KU, Şahin U, Gruber WC. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N Engl J Med. 2020;383:2439-2450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1747] [Cited by in RCA: 1902] [Article Influence: 380.4] [Reference Citation Analysis (0)] |
| 83. | Kaur SP, Gupta V. COVID-19 Vaccine: A comprehensive status report. Virus Res. 2020;288:198114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 494] [Cited by in RCA: 490] [Article Influence: 98.0] [Reference Citation Analysis (0)] |
| 84. | Wong GL, Wong VW, Thompson A, Jia J, Hou J, Lesmana CRA, Susilo A, Tanaka Y, Chan WK, Gane E, Ong-Go AK, Lim SG, Ahn SH, Yu ML, Piratvisuth T, Chan HL; Asia-Pacific Working Group for Liver Derangement during the COVID-19 Pandemic. Management of patients with liver derangement during the COVID-19 pandemic: an Asia-Pacific position statement. Lancet Gastroenterol Hepatol. 2020;5:776-787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (1)] |
| 85. | Tapper EB, Asrani SK. The COVID-19 pandemic will have a long-lasting impact on the quality of cirrhosis care. J Hepatol. 2020;73:441-445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 153] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 86. | Wu H, Nguyen GC. Liver cirrhosis is associated with venous thromboembolism among hospitalized patients in a nationwide US study. Clin Gastroenterol Hepatol. 2010;8:800-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 87. | Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844-847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3992] [Cited by in RCA: 4038] [Article Influence: 807.6] [Reference Citation Analysis (0)] |
| 88. | Zhai Z, Li C, Chen Y, Gerotziafas G, Zhang Z, Wan J, Liu P, Elalamy I, Wang C; Prevention Treatment of VTE Associated with COVID-19 Infection Consensus Statement Group. Prevention and Treatment of Venous Thromboembolism Associated with Coronavirus Disease 2019 Infection: A Consensus Statement before Guidelines. Thromb Haemost. 2020;120:937-948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 257] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 89. | Tritschler T, Mathieu ME, Skeith L, Rodger M, Middeldorp S, Brighton T, Sandset PM, Kahn SR, Angus DC, Blondon M, Bonten MJ, Cattaneo M, Cushman M, Derde LPG, DeSancho MT, Diehl JL, Goligher E, Jilma B, Jüni P, Lawler PR, Marietta M, Marshall JC, McArthur C, Miranda CH, Mirault T, Morici N, Perepu U, Schörgenhofer C, Sholzberg M, Spyropoulos AC, Webb SA, Zarychanski R, Zuily S, Le Gal G; International Network of VENous Thromboembolism Clinical Research Networks INVENT-VTE. Anticoagulant interventions in hospitalized patients with COVID-19: A scoping review of randomized controlled trials and call for international collaboration. J Thromb Haemost. 2020;18:2958-2967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 90. | Chen LF, Mo YQ, Jing J, Ma JD, Zheng DH, Dai L. Short-course tocilizumab increases risk of hepatitis B virus reactivation in patients with rheumatoid arthritis: a prospective clinical observation. Int J Rheum Dis. 2017;20:859-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 91. | Chen YM, Yang SS, Chen DY. Risk-stratified management strategies for HBV reactivation in RA patients receiving biological and targeted therapy: A narrative review. J Microbiol Immunol Infect. 2019;52:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 92. | Waseem N, Chen PH. Hypoxic Hepatitis: A Review and Clinical Update. J Clin Transl Hepatol. 2016;4:263-268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 93. | Lightsey JM, Rockey DC. Current concepts in ischemic hepatitis. Curr Opin Gastroenterol. 2017;33:158-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 94. | Feng G, Zheng KI, Yan QQ, Rios RS, Targher G, Byrne CD, Poucke SV, Liu WY, Zheng MH. COVID-19 and Liver Dysfunction: Current Insights and Emergent Therapeutic Strategies. J Clin Transl Hepatol. 2020;8:18-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 289] [Cited by in RCA: 278] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 95. | Lu SC. Dysregulation of glutathione synthesis in liver disease. Liver Res. 2020;4:64-73. [RCA] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 96. | Fuhrmann V, Jäger B, Zubkova A, Drolz A. Hypoxic hepatitis - epidemiology, pathophysiology and clinical management. Wien Klin Wochenschr. 2010;122:129-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 97. | World Health Organization. Coronavirus disease (COVID-19) advice for the public. 2020 [cited 6 December 2020]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public. |
| 98. | United States Food and Drug Administration. FDA Approves First Treatment for COVID-19; 2020 [cited 25 December 2020]. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19. |
| 99. | Goldman JD, Lye DCB, Hui DS, Marks KM, Bruno R, Montejano R, Spinner CD, Galli M, Ahn MY, Nahass RG, Chen YS, SenGupta D, Hyland RH, Osinusi AO, Cao H, Blair C, Wei X, Gaggar A, Brainard DM, Towner WJ, Muñoz J, Mullane KM, Marty FM, Tashima KT, Diaz G, Subramanian A; GS-US-540-5773 Investigators. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N Engl J Med. 2020;383:1827-1837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 987] [Cited by in RCA: 987] [Article Influence: 197.4] [Reference Citation Analysis (0)] |
| 100. | WHO Solidarity Trial Consortium. , Pan H, Peto R, Henao-Restrepo AM, Preziosi MP, Sathiyamoorthy V, Abdool Karim Q, Alejandria MM, Hernández García C, Kieny MP, Malekzadeh R, Murthy S, Reddy KS, Roses Periago M, Abi Hanna P, Ader F, Al-Bader AM, Alhasawi A, Allum E, Alotaibi A, Alvarez-Moreno CA, Appadoo S, Asiri A, Aukrust P, Barratt-Due A, Bellani S, Branca M, Cappel-Porter HBC, Cerrato N, Chow TS, Como N, Eustace J, García PJ, Godbole S, Gotuzzo E, Griskevicius L, Hamra R, Hassan M, Hassany M, Hutton D, Irmansyah I, Jancoriene L, Kirwan J, Kumar S, Lennon P, Lopardo G, Lydon P, Magrini N, Maguire T, Manevska S, Manuel O, McGinty S, Medina MT, Mesa Rubio ML, Miranda-Montoya MC, Nel J, Nunes EP, Perola M, Portolés A, Rasmin MR, Raza A, Rees H, Reges PPS, Rogers CA, Salami K, Salvadori MI, Sinani N, Sterne JAC, Stevanovikj M, Tacconelli E, Tikkinen KAO, Trelle S, Zaid H, Røttingen JA, Swaminathan S. Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results. N Engl J Med. 2021;384:497-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1601] [Cited by in RCA: 1748] [Article Influence: 437.0] [Reference Citation Analysis (0)] |
| 101. | RECOVERY Collaborative Group; Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384:693-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6762] [Cited by in RCA: 7376] [Article Influence: 1844.0] [Reference Citation Analysis (1)] |
| 102. | National Institutes of Health. Therapeutic Management of Patients with COVID-19. 2020 [cited 26 December 2020]. Available from: https://files.covid19treatmentguidelines.nih.gov/guidelines/section/section_100.pdf. |
| 103. | Infectious Diseases Society of America. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. 2020 [cited 26 December 2020]. Available from: https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/. |
| 104. | Stone JH, Frigault MJ, Serling-Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, Horick NK, Healy BC, Shah R, Bensaci AM, Woolley AE, Nikiforow S, Lin N, Sagar M, Schrager H, Huckins DS, Axelrod M, Pincus MD, Fleisher J, Sacks CA, Dougan M, North CM, Halvorsen YD, Thurber TK, Dagher Z, Scherer A, Wallwork RS, Kim AY, Schoenfeld S, Sen P, Neilan TG, Perugino CA, Unizony SH, Collier DS, Matza MA, Yinh JM, Bowman KA, Meyerowitz E, Zafar A, Drobni ZD, Bolster MB, Kohler M, D'Silva KM, Dau J, Lockwood MM, Cubbison C, Weber BN, Mansour MK; BACC Bay Tocilizumab Trial Investigators. Efficacy of Tocilizumab in Patients Hospitalized with Covid-19. N Engl J Med. 2020;383:2333-2344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 998] [Cited by in RCA: 1011] [Article Influence: 202.2] [Reference Citation Analysis (0)] |
| 105. | Rosenblatt R, Verna EC. COVID 19: Management of Decompensated Cirrhosis and Liver Transplant Recipients. Clin Liver Dis (Hoboken). 2020;15:200-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 106. | Soetikno R, Teoh AYB, Kaltenbach T, Lau JYW, Asokkumar R, Cabral-Prodigalidad P, Shergill A. Considerations in performing endoscopy during the COVID-19 pandemic. Gastrointest Endosc. 2020;92:176-183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 159] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 107. | Lui RN, Wong SH, Sánchez-Luna SA, Pellino G, Bollipo S, Wong MY, Chiu PWY, Sung JJY. Overview of guidance for endoscopy during the coronavirus disease 2019 pandemic. J Gastroenterol Hepatol. 2020;35:749-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 172] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 108. | Sultan S, Lim JK, Altayar O, Davitkov P, Feuerstein JD, Siddique SM, Falck-Ytter Y, El-Serag HB; AGA Institute. Electronic address: ewilson@gastro.org. AGA Rapid Recommendations for Gastrointestinal Procedures During the COVID-19 Pandemic. Gastroenterology 2020; 159: 739-758. e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 278] [Article Influence: 55.6] [Reference Citation Analysis (0)] |