Published online Apr 16, 2021. doi: 10.12998/wjcc.v9.i11.2662
Peer-review started: December 14, 2020
First decision: December 28, 2020
Revised: January 3, 2021
Accepted: February 9, 2021
Article in press: February 9, 2021
Published online: April 16, 2021
Processing time: 108 Days and 4 Hours
Pseudogout is a benign joint lesion caused by the deposition of calcium pyro-phosphate dihydrate crystals, but it is invasive. Pseudogout of the temporo-mandibular joint (TMJ) is uncommon, and it rarely invades the skull base or penetrates into the middle cranial fossa. The disease has no characteristic clinical manifestations and is easily misdiagnosed.
We present two cases of tophaceous pseudogout of the TMJ invading the middle cranial fossa. A 46-year-old woman with a history of diabetes for more than 10 years was admitted to the hospital due to swelling and pain in the right temporal region. Another patient, a 52-year-old man with a mass in the left TMJ for 6 years, was admitted to the hospital. Maxillofacial imaging showed a calcified mass and severe bone destruction of the skull base in the TMJ area. Both patients underwent excision of the lesion. The lesion was pathologically diagnosed as tophaceous pseudogout. The symptoms in these patients were relieved after surgery.
Tophaceous pseudogout should be considered when there is a calcified mass in the TMJ with or without bone destruction. A pathological examination is the gold standard for diagnosing this disease. Surgical treatment is currently the recommended treatment, and the prognosis is good after surgery.
Core Tip: Tophaceous pseudogout is a benign lesion caused by calcium pyrophosphate dihydrate crystal deposition and uncommonly involves the temporomandibular joint. We report two cases of pseudogout in the temporomandibular joint invading the middle cranial fossa, which is rare. Surgical treatment is recommended for this disease, and the prognosis is good. A pathological examination is the gold standard for the diagnosis of pseudogout. Large rod or rhombus crystal deposits within the mass are pathological features of tophaceous pseudogout.
- Citation: Tang T, Han FG. Calcium pyrophosphate deposition disease of the temporomandibular joint invading the middle cranial fossa: Two case reports. World J Clin Cases 2021; 9(11): 2662-2670
- URL: https://www.wjgnet.com/2307-8960/full/v9/i11/2662.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i11.2662
Tophaceous pseudogout, also known as calcium pyrophosphate deposition disease (CPPD), is a recurrent acute attack of arthritis caused by calcium pyrophosphate dihydrate crystals deposited in articular cartilage and surrounding tissues such as labra, synovia, articular capsules, and tendons[1,2]. Its clinical manifestations can be similar to those of gout. CPPD most commonly occurs in large joints, such as the knee, hip, shoulder, and elbow, and rarely involves metacarpophalangeal joints, temporomandibular joints (TMJs), the skull base, or the spine[3,4]. Pritzker et al[5] first described pseudogout in the TMJ in 1976. The incidence of CPPD is positively correlated with age and often occurs in middle-aged and elderly people. The prevalence of CPPD is higher in women than in men, with a male-to-female ratio of 1:(2-7)[6].
CPPD is a benign crystalline joint disease with a variety of clinical manifestations[7]. When the TMJ is involved, it can be manifested as facial swelling and pain, tinnitus, unilateral conductive hearing loss, limited mouth opening, etc.[7]. Here, we introduce two cases of pseudogout of the TMJ destroying the skull base and infiltrating the middle cranial fossa. To our knowledge, such cases are rare, with a total of 9 cases having been reported in the literature[8,9]. The purpose of our report was to focus on the invasiveness of CPPD to adjacent structures of the TMJ and discuss the relevant imaging features and differential diagnosis.
Case 1: A 46-year-old woman was admitted to the hospital due to right temporal pain and discomfort for more than 3 mo.
Case 2: A 52-year-old man was admitted due to a mass in the left TMJ for 6 years.
Case 1: The patient had right temporal swelling and pain, along with mouth opening and chewing discomfort. She had a slightly deviated angle of the mouth, slight malocclusion of teeth and dizziness.
Case 2: Six years ago, the patient found a mass in the left TMJ area, and the mass gradually grew. Now, he has pain and tinnitus. No abnormalities in terms of dental occlusion, eyes closed and cheek blowing were observed.
Case 1: She had diabetes for more than 10 years.
Case 2: He did not have a history of medical issues.
The two patients did not have a personal or family history of related issues.
Case 1: Her right maxillofacial region was swollen, and a mass approximately 2 cm × 2 cm in size in the right preauricular region was hard upon palpation. She had slightly restricted mouth opening and a slight left deviation of the angle of the mouth; there was no tenderness in the parotid region on either side.
Case 2: He had maxillofacial asymmetry. The left TMJ area showed a large mass approximately 4 cm × 4 cm in size, with obvious tenderness. The mass was hard, with poor mobility, and slightly adhered to surrounding tissues with unclear boundaries. The range of motion of the TMJ was acceptable bilaterally.
Case 1: Her blood glucose concentration was 16.09 mmol/L, and the other blood test results were within normal limits.
Case 2: The laboratory examination results (including electrolyte level, liver and kidney function, blood cell count, ferritin level, and parathyroid and thyroid function) were normal.
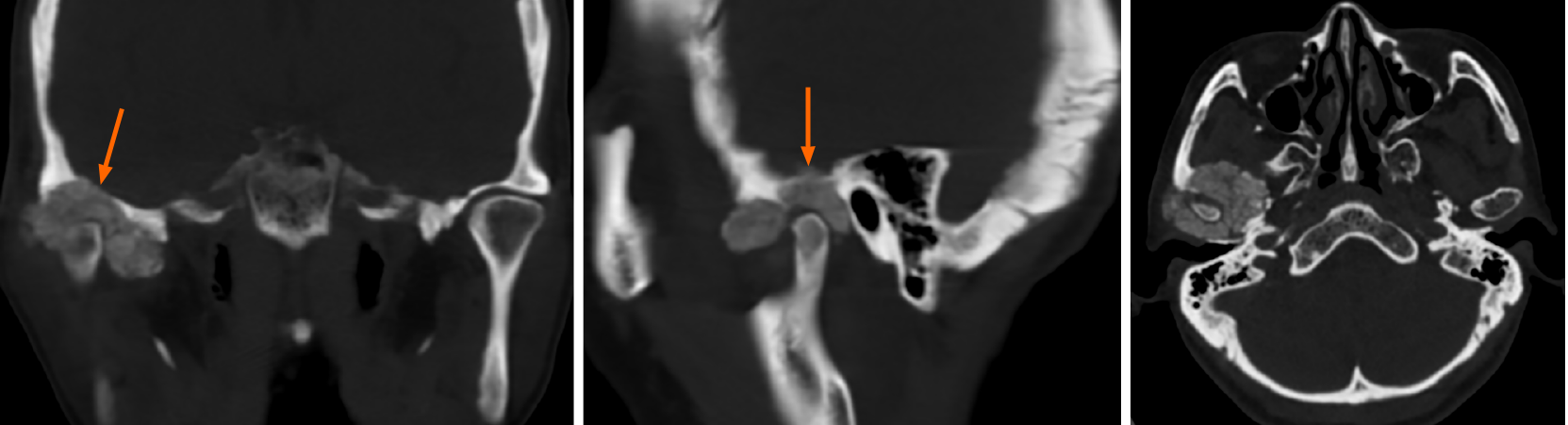
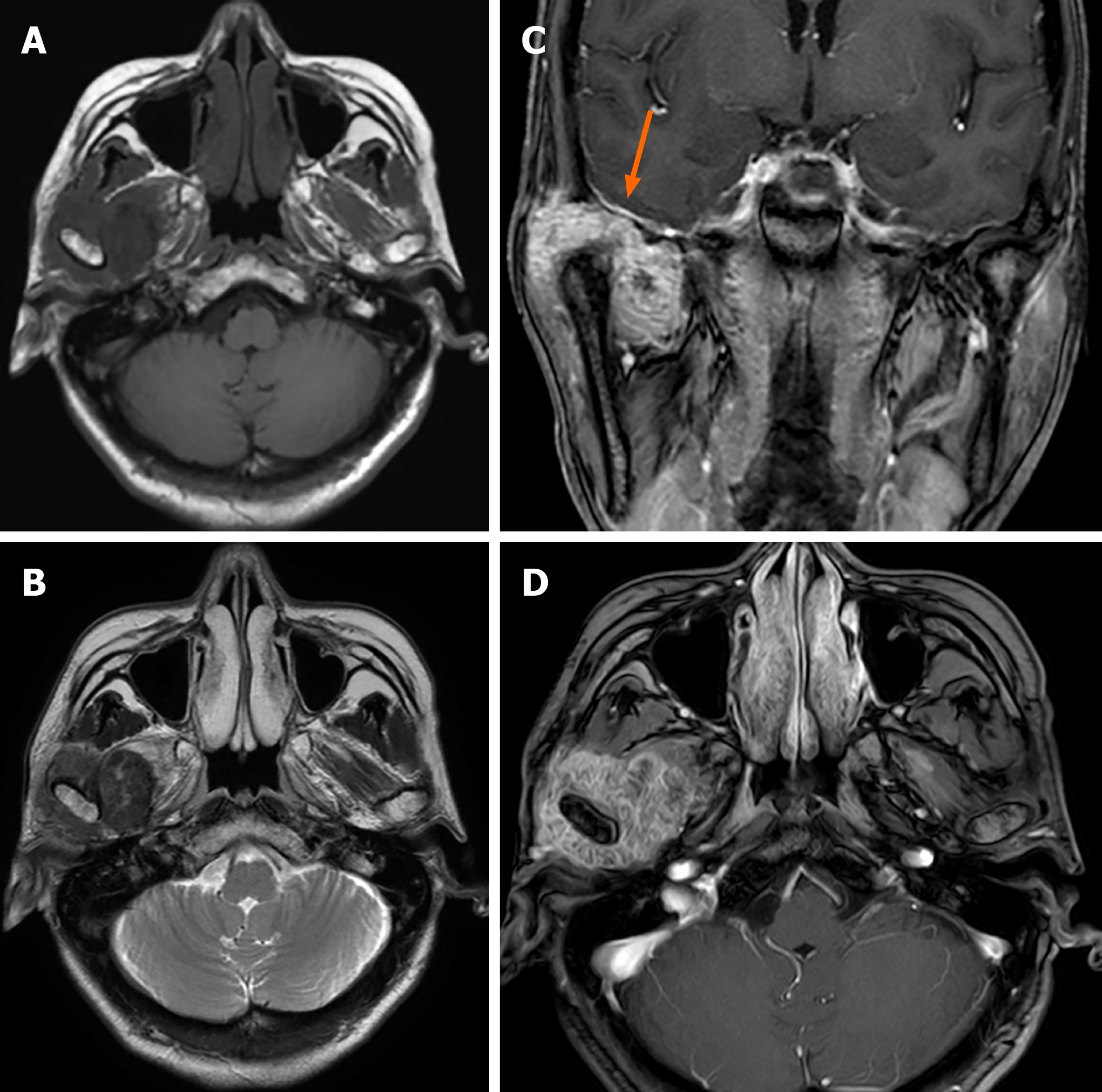
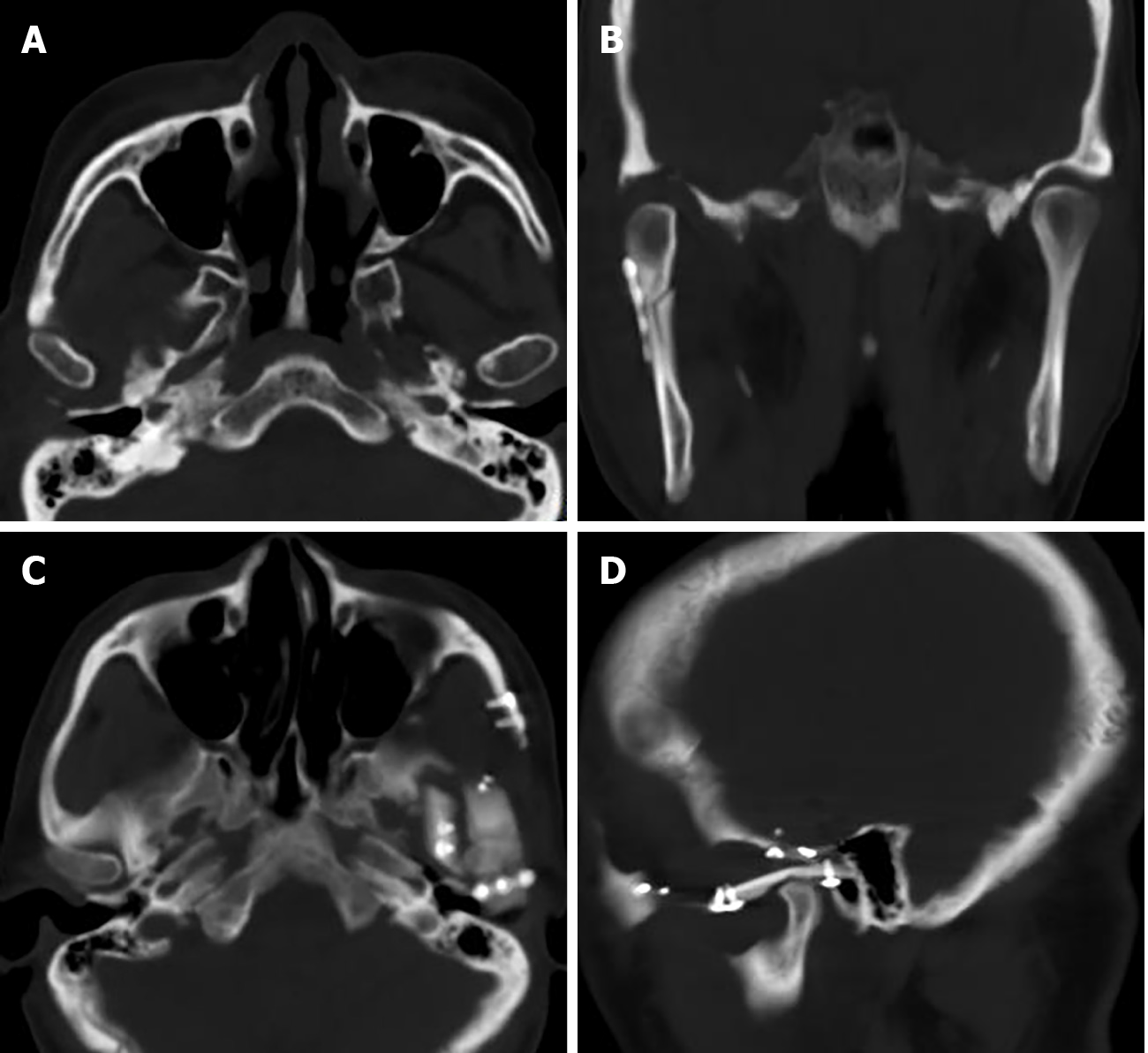
Case 1: Computed tomography (CT) showed an irregular calcified mass in the right TMJ with a CT value of 450-1120 HU and a clear boundary. The mass grew around the right condyle, and the bone of the mandibular head was destroyed. The mass destroyed the glenoid fossa and extended to the skull base (Figure 1). Magnetic resonance imaging (MRI) showed that the mass was T1WI hypointense and T2WI hypointense with inhomogeneous significant enhancement. It was further shown that the lesion was in contact with the dura mater on the inferior surface of the right temporal lobe (Figure 2).
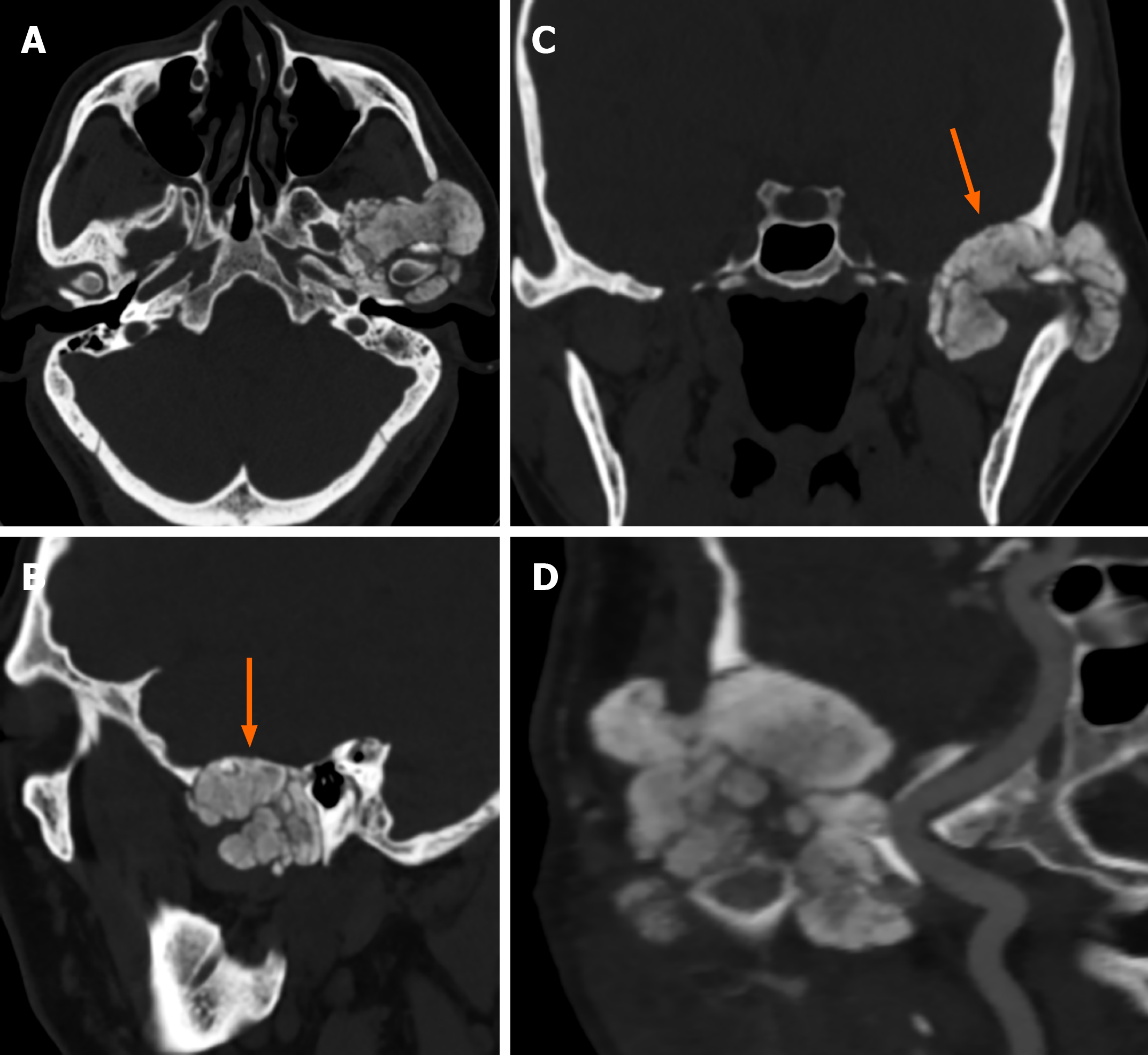
Case 2: CT showed irregular patchy, clustered calcified high-density foci in the left TMJ with clear borders. The lesion enclosed the left mandibular head, and no definite abnormalities were found in the left mandibular bone. The left temporal bone, zygomatic arch, left greater wing of the sphenoid bone and mastoid process of the middle ear were destroyed. The mastoid mucosa of the left middle ear was thickened (Figure 3).
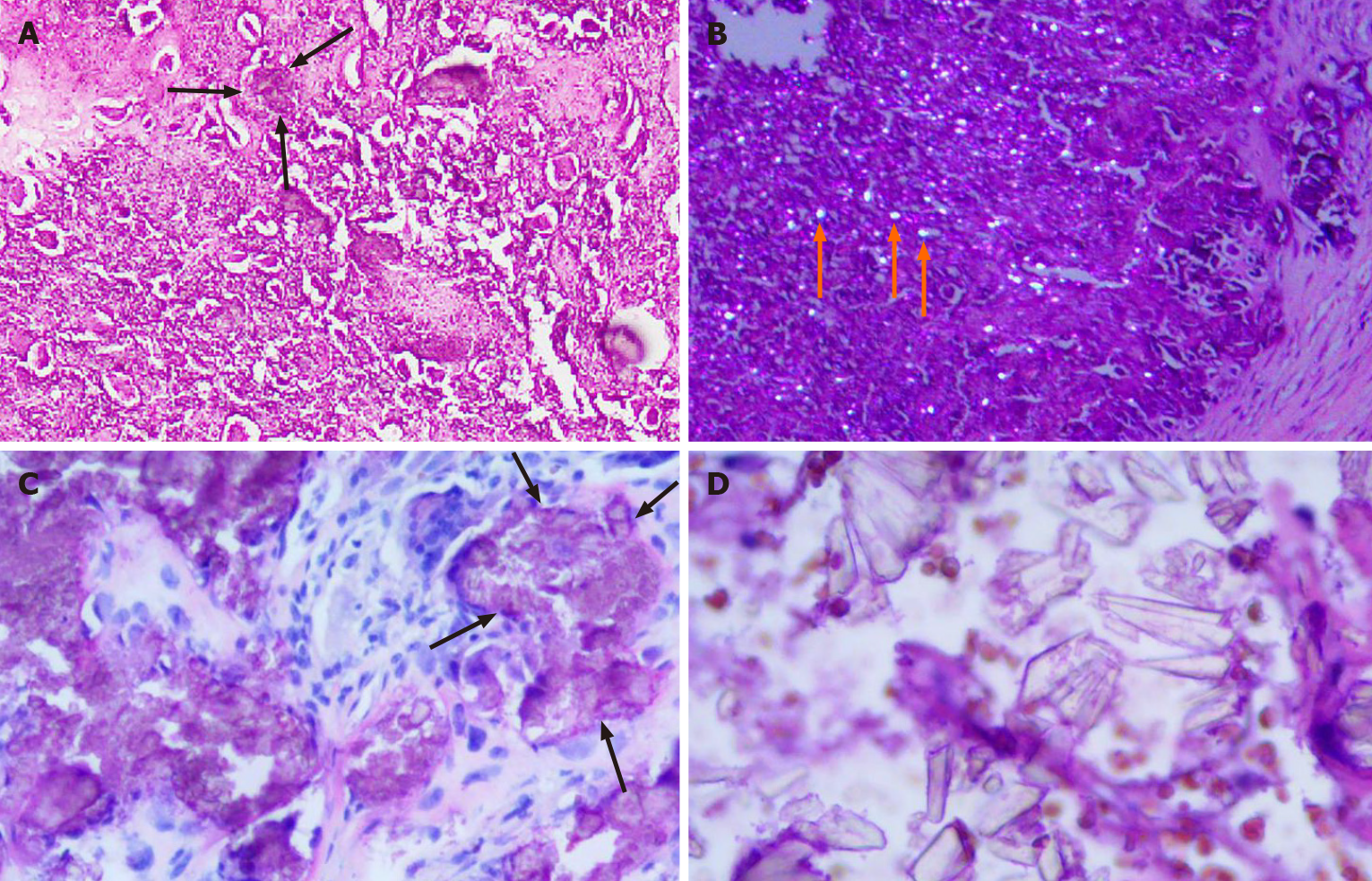
The final diagnosis in both cases was tophaceous pseudogout of the TMJ (Figure 4).
The lesion was excised from the preauricular incision. The mass in the right TMJ eroded the right zygomatic arch and skull base bone. During the operation, the whole mass and part of the parotid gland were removed, and a small amount of cerebrospinal fluid leakage was found. Bone wax was used to fill the bone defect, a temporalis muscle flap was used to repair the articular disc, and temporomandibular arthroplasty was performed.
The lesion was resected using the preauricular approach. In the left TMJ, there was an irregular mass with an intact capsule and a clear boundary. The left zygomatic arch and skull base bone were destroyed. There was obvious adhesion between the mass and dura. The lesion, partial parotid gland and left zygomatic arch were resected, and the left TMJ was reconstructed.
The patient's temporal pain was completely relieved, and the discomfort during mouth opening and chewing significantly improved. The problems of mouth angle deviation and tooth closure were greatly improved. One week after surgery, a CT scan showed that the mass in the right TMJ was absent (Figure 5A and B).
CPPD can occur in one or more joints[10]. Tophaceous pseudogout may be related to metabolic disorders, heredity, aging and joint injury, especially metabolic disorders and genetic factors. The related diseases mainly include diabetes mellitus, hypertension, hypothyroidism, hyperparathyroidism, hyperuricemia/gout, hemochromatosis, nonspecific joint pain, and hypomagnesemia[7,11]. These diseases lead to abnormal pyrophosphate metabolism[11]. The proteoglycan of the articular cartilage is destroyed, and calcium and pyrophosphate are released into synovial fluid. Calcium combines with pyrophosphate to form calcium pyrophosphate molecular crystals, which are deposited in cartilage, ligaments, tendons and joints. It can cause joint surface abrasion and stimulate the joint to produce inflammatory reactions at the same time. Family genetic factors are mostly related to functional mutations in the ANKH gene[12]. The ANKH gene encodes a membrane transporter that transports pyrophosphate produced by cartilage stromal cell metabolism from intracellular to extracellular regions via the cell membrane. When ANKH is mutated, transporter activity increases, which leads to increased extracellular pyrophosphate levels, further causing calcium pyrophosphate molecular crystals to deposit in the joint. In this report, one patient had a history of diabetes for more than 10 years, and the other patient did not have any special abnormalities.
The imaging features were periarticular calcified masses involving articular cartilage. X-ray can show bone destruction and articular cartilage calcification of the TMJ. However, the sensitivity of this method is not high, which may lead to misdiagnosis and missed diagnosis[4]. CT can clearly show the size of the lesion, calcification and extent of bone destruction. The mandibular condyle is most often involved, as well as the mastoid process and the greater wing of the sphenoid bone[13]. Both cases in our study involved the temporal bone and the greater wing of the sphenoid bone, and one involved the middle ear mastoid process. MRI can better identify the extent of lesion involvement and the relationships between the lesion and articular disc, parotid gland, facial nerve and inferior temporal fossa. Articular cartilage calcification in CPPD often develops later than arthropathy on imaging, and MRI can detect lesions earlier than X-ray or CT[4,14]. MRI showed a mass with abnormal signals in the articular cavity, which was mostly T1WI hypointense and T2WI hypointense with significant inhomogeneous enhancement. Masses can grow along articular cartilage, with cartilage and synovial sac involvement[8,9,13-15]. CT is superior to MRI in demonstrating calcification and has suggestive value for disease diagnosis. This disease should be diagnosed on the basis of the common CT and MRI manifestations. Calcification of the articular disc has strong suggestive significance.
Tophaceous pseudogout occurring in the TMJ has similar clinical or imaging findings to other diseases[16-18]. It requires differential diagnosis from synovial chondromatosis (SC), neoplastic calcification, ossifying myositis, gout, chondroblastoma, and chondrosarcoma[7,19-21]. Both cases were misdiagnosed as SC before the operation. SC is a nonneoplastic disease characterized by connective tissue metaplasia, resulting in synovial cartilage formation. SC of the TMJ is rare, but clinically, it resembles pseudogout[22]. The key point of identification is that SC is characterized by the formation of small, multiple cartilaginous nodules in the joint space[19]. In a previous study[7], one patient was misdiagnosed with a malignant tumor and underwent radiotherapy. Tumorous calcifications are mostly lobulated calcified masses in the soft tissues around the joints and generally do not involve the joint space. Calcification of chondrogenic tumors is usually heterogeneous, and the contour of the lesion is not clear on CT, which helps in the diagnosis[9]. The serum uric acid level is high in gout patients, bone destruction is obvious, and the degree of calcification is mild. Gout usually occurs in the metatarsophalangeal joints of men[23,24]. Ossifying myositis is associated with a history of trauma and is mostly located within muscles. The disease does not involve the joint space and rarely causes bone changes[25]. These manifestations differ from the characteristics of the cases described in this paper. Although the imaging features of tophaceous pseudogout in the TMJ have certain specificity, other diseases cannot be completely excluded. The final diagnosis depends on a histopathological diagnosis. Lambrecht et al[26] and related literature reports agree that the presence of diamond crystal morphology under a birefringence microscope is the gold standard for the diagnosis of CPPD.
The treatment of pseudogout of the TMJ is selected according to the patient's symptoms and the severity of bone destruction[7]. Conservative treatment, such as physical therapy, drug therapy (nonsteroidal anti-inflammatory drugs, colchicine, etc.) and joint lavage can be used for patients with mild symptoms and noninvasive lesions[7,20,21]. Follow-up examinations are necessary. Surgical treatment is advocated when intolerable symptoms and/or erosive disease occur[7,27-29]. Surgical resection has been shown to be effective in relieving symptoms and TMJ mobility. Condylectomy, discectomy, and total resection are often combined[7-9]. Of the 9 reported cases of pseudogout involving the middle cranial fossa, 6 underwent surgical resection[8,9]. The symptoms were relieved in all cases. In this study, both patients underwent surgical treatment, with good prognoses. Pseudogout is benign but invasive. Long-term follow-up after surgical resection indicate that the prognosis is good. Pseudogout occurring in the TMJ rarely invades the skull base or middle cranial fossa. Pseudogout should be considered when a calcified mass with or without bone destruction occurs in the TMJ with corresponding clinical symptoms.
In conclusion, cases of TMJ pseudogout that invade the middle cranial fossa are rare, and the clinical manifestations of this disease are atypical. These features make it difficult to diagnose. Preoperative imaging is helpful in the diagnosis and treatment of the disease.
We greatly appreciate the patients and their families and the medical staff involved in this study.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jeong SH S-Editor: Gao CC L-Editor: Webster JR P-Editor: Yuan YY
| 1. | Mccarty DJ, Hollander JL. Identification of urate crystals in gouty synovial fluid. Ann Intern Med. 1961;54:452-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 274] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Canhão H, Fonseca JE, Leandro MJ, Romeu JC, Pimentão JB, Costa JT, Queiroz MV. Cross-sectional study of 50 patients with calcium pyrophosphate dihydrate crystal arthropathy. Clin Rheumatol. 2001;20:119-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Abreu M, Johnson K, Chung CB, De Lima JE Jr, Trudell D, Terkeltaub R, Pe S, Resnick D. Calcification in calcium pyrophosphate dihydrate (CPPD) crystalline deposits in the knee: anatomic, radiographic, MR imaging, and histologic study in cadavers. Skeletal Radiol. 2004;33:392-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Steinbach LS, Resnick D. Calcium pyrophosphate dihydrate crystal deposition disease: imaging perspectives. Curr Probl Diagn Radiol. 2000;29:209-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Pritzker KP, Phillips H, Luk SC, Koven IH, Kiss A, Houpt JB. Pseudotumor of temporomandibular joint: destructive calcium pyrophosphate dihydrate arthropathy. J Rheumatol. 1976;3:70-81. [PubMed] |
| 6. | Frediani B, Filippou G, Falsetti P, Lorenzini S, Baldi F, Acciai C, Siagkri C, Marotto D, Galeazzi M, Marcolongo R. Diagnosis of calcium pyrophosphate dihydrate crystal deposition disease: ultrasonographic criteria proposed. Ann Rheum Dis. 2005;64:638-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Zweifel D, Ettlin D, Schuknecht B, Obwegeser J. Tophaceuos calcium pyrophosphate dihydrate deposition disease of the temporomandibular joint: the preferential site? J Oral Maxillofac Surg. 2012;70:60-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Houghton D, Munir N, Triantafyllou A, Begley A. Tophaceous pseudogout of the temporomandibular joint with erosion into the middle cranial fossa. Int J Oral Maxillofac Surg. 2020;49:1286-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Hotokezaka Y, Hotokezaka H, Katayama I, Fujita S, Sasaki M, Eida S, Uetani M. A case of tophaceous pseudogout of the temporomandibular joint extending into the cranium. Oral Radiol. 2020;36:203-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Abdelsayed RA, Said-Al-Naief N, Salguerio M, Holmes J, El-Mofty SK. Tophaceous pseudogout of the temporomandibular joint: a series of 3 cases. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117:369-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Filippou G, Frediani B, Gallo A, Menza L, Falsetti P, Baldi F, Acciai C, Lorenzini S, Galeazzi M, Marcolongo R. A "new" technique for the diagnosis of chondrocalcinosis of the knee: sensitivity and specificity of high-frequency ultrasonography. Ann Rheum Dis. 2007;66:1126-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Barskova VG, Kudaeva FM, Bozhieva LA, Smirnov AV, Volkov AV, Nasonov EL. Comparison of three imaging techniques in diagnosis of chondrocalcinosis of the knees in calcium pyrophosphate deposition disease. Rheumatology (Oxford). 2013;52:1090-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Srinivasan V, Wensel A, Dutcher P, Newlands S, Johnson M, Vates GE. Calcium pyrophosphate deposition disease of the temporomandibular joint. J Neurol Surg Rep. 2012;73:6-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Matsumura Y, Nomura J, Nakanishi K, Yanase S, Kato H, Tagawa T. Synovial chondromatosis of the temporomandibular joint with calcium pyrophosphate dihydrate crystal deposition disease (pseudogout). Dentomaxillofac Radiol. 2012;41:703-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Reynolds JL, Matthew IR, Chalmers A. Tophaceous calcium pyrophosphate dihydrate deposition disease of the temporomandibular joint. J Rheumatol. 2008;35:717-721. [PubMed] |
| 16. | Marsot-Dupuch K, Smoker WR, Gentry LR, Cooper KA. Massive calcium pyrophosphate dihydrate crystal deposition disease: a cause of pain of the temporomandibular joint. AJNR Am J Neuroradiol. 2004;25:876-879. [PubMed] |
| 17. | Nakagawa Y, Ishibashi K, Kobayashi K, Westesson PL. Calcium pyrophosphate deposition disease in the temporomandibular joint: report of two cases. J Oral Maxillofac Surg. 1999;57:1357-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Kurihara K, Mizuseki K, Saiki T, Wakisaka H, Maruyama S, Sonobe J. Tophaceous pseudogout of the temporomandibular joint: report of a case. Pathol Int. 1997;47:578-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Kudoh K, Kudoh T, Tsuru K, Miyamoto Y. A case of tophaceous pseudogout of the temporomandibular joint extending to the base of the skull. Int J Oral Maxillofac Surg. 2017;46:355-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Aoyama S, Kino K, Amagasa T, Kayano T, Ichinose S, Kimijima Y. Differential diagnosis of calcium pyrophosphate dihydrate deposition of the temporomandibular joint. Br J Oral Maxillofac Surg. 2000;38:550-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Merrill RG, Yih WY, Shamloo J. Synovial chondrosarcoma of the temporomandibular joint: a case report. J Oral Maxillofac Surg. 1997;55:1312-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Guarda-Nardini L, Piccotti F, Ferronato G, Manfredini D. Synovial chondromatosis of the temporomandibular joint: a case description with systematic literature review. Int J Oral Maxillofac Surg. 2010;39:745-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Schlee S, Bollheimer LC, Bertsch T, Sieber CC, Härle P. Crystal arthritides - gout and calcium pyrophosphate arthritis : Part 2: clinical features, diagnosis and differential diagnostics. Z Gerontol Geriatr. 2018;51:579-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Desai MA, Peterson JJ, Garner HW, Kransdorf MJ. Clinical utility of dual-energy CT for evaluation of tophaceous gout. Radiographics. 2011;31:1365-75; discussion 1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 25. | Lacout A, Jarraya M, Marcy PY, Thariat J, Carlier RY. Myositis ossificans imaging: keys to successful diagnosis. Indian J Radiol Imaging. 2012;22:35-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 26. | Lambrecht N, Nelson SD, Seeger L, Bose S. Tophaceous pseudogout: a pitfall in the diagnosis of chondrosarcoma. Diagn Cytopathol. 2001;25:258-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Nicholas BD, Smith JL 2nd, Kellman RM. Calcium pyrophosphate deposition of the temporomandibular joint with massive bony erosion. J Oral Maxillofac Surg. 2007;65:2086-2089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Smolka W, Eggensperger N, Stauffer-Brauch EJ, Brekenfeld C, Iizuka T. Calcium pyrophosphate dihydrate crystal deposition disease of the temporomandibular joint. Oral Dis. 2005;11:104-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Kathju S, Cohen R, Lasko LA, Aynechi M, Dattilo DJ. Pseudogout of the temporomandibular joint: immediate reconstruction with total joint arthroplasty. Head Neck. 2010;32:406-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |