Published online Apr 16, 2021. doi: 10.12998/wjcc.v9.i11.2634
Peer-review started: December 7, 2020
First decision: December 28, 2020
Revised: January 13, 2021
Accepted: February 9, 2021
Article in press: February 9, 2021
Published online: April 16, 2021
Processing time: 115 Days and 21 Hours
Double outlet right ventricle (DORV) is a rare and complex congenital heart defect, and the surgical repairs vary with type and pathophysiology consequences. Due to prolonged progressive hypoxemia, severe polycythemia is common in patients with DORV, which ultimately leads to coagulation dysfunction and increases the risk of thrombosis and infarction. Consequently, the anesthetic management is challenging and how to manage severe polycythemia and avoid hypoxia-related complications in such patients is of great significance.
Herein, we report the anesthetic management of a 10-year-old female patient with a DORV. She lived in the low-oxygen Qinghai-Tibet Plateau, and presented with severe polycythemia (hemoglobin, 24.8 g/dL; hematocrit, 75%). She underwent a modified Fontan surgery, which was satisfactory and without any perioperative complications. Our anesthetic management highlights the importance of perioperative hemodilution in decreasing the risk of thromboembolism and the importance of correcting coagulopathy in preventing hemorrhage.
Anesthetic management is challenging in rare cyanotic congenital heart disease patients with severe polycythemia. It is important to adopt perioperative hemodilution and correction of coagulopathy in preventing thrombosis and hemorrhage.
Core Tip: We present the successful anesthetic management of a double outlet right ventricle (DORV) patient with severe polycythemia. Anesthetic management is challenging in rare cyanotic congenital heart disease patients with severe polycythemia. A thorough understanding of the physiopathology of DORV and polycythemia is essential for successful anesthesia.
- Citation: Tan LC, Zhang WY, Zuo YD, Chen HY, Jiang CL. Anesthetic management of a child with double outlet right ventricle and severe polycythemia: A case report. World J Clin Cases 2021; 9(11): 2634-2640
- URL: https://www.wjgnet.com/2307-8960/full/v9/i11/2634.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i11.2634
Double outlet right ventricle (DORV) is a rare and complex conotruncal malformation, which occurs in approximately less than 1% of all congenital heart defects[1]. Children with DORV are often diagnosed because of progressive cyanosis and pronounced murmurs. They will suffer from tachypnea and poor growth, and ultimately develop pulmonary hypertension and Eisenmenger’s syndrome if not treated. DORV has different classification schemes, and surgical plan depends on specific type of DORV and other associated anomalies, such as Nikaidoh procedure, Rastelli procedure, switch procedure, and modified Fontan operation[2]. Although erythrocytosis frequently occurs in patients with cyanotic congenital heart disease (CCHD), DORV patients with a hemoglobin (Hb) level ≥ 24 g/dL are still rare. It is worth noting that severe decompensated erythrocytosis can dramatically increase the risks of thrombosis and infarction, and cause coagulation disorders. Herein, we present the case of 10-year-old girl from the Qinghai-Tibet Plateau with a DORV and an elevated Hb level of 24.8 g/L, who underwent a modified Fontan operation. Written authorization for the case report was obtained from the patient’s family.
A 10-year-old girl from the Liangshan Prefecture in Sichuan Province, Qinghai-Tibet Plateau, China, presented to Department of Cardiovascular Surgery of our hospital complaining of cardiac murmurs for 6 years.
The patient was diagnosed with DORV and advised to undergo surgical treatment 6 years ago. However, she did not have the surgery because of economic reasons. Her situation progressively worsened, and she was eventually admitted to our center.
The patient had a disease-free personal and family history.
The physical examination revealed delayed growth (height, 119 cm; weight, 19 kg), a blood pressure of 94/65 mmHg, a pulse rate of 98 beats per min, and a respiratory rate of 24 breaths/min. Her baseline oxygen saturation was maintained at about 80%.
Blood tests showed an Hb level of 24.8 g/dL, hematocrit (Hct) of 75%, mean corpuscular volume of 93.3 fL, mean corpuscular Hb of 31 pg, mean corpuscular Hb concentration of 33.2 g/dL, and a platelet count of 108 × 109 cells/L. The blood coagulation test was also abnormal, with a prothrombin time of 16.7 s, active partial thrombin time of 61.8 s, and an international normalized ratio of 1.51. Other blood tests showed no significant abnormalities.
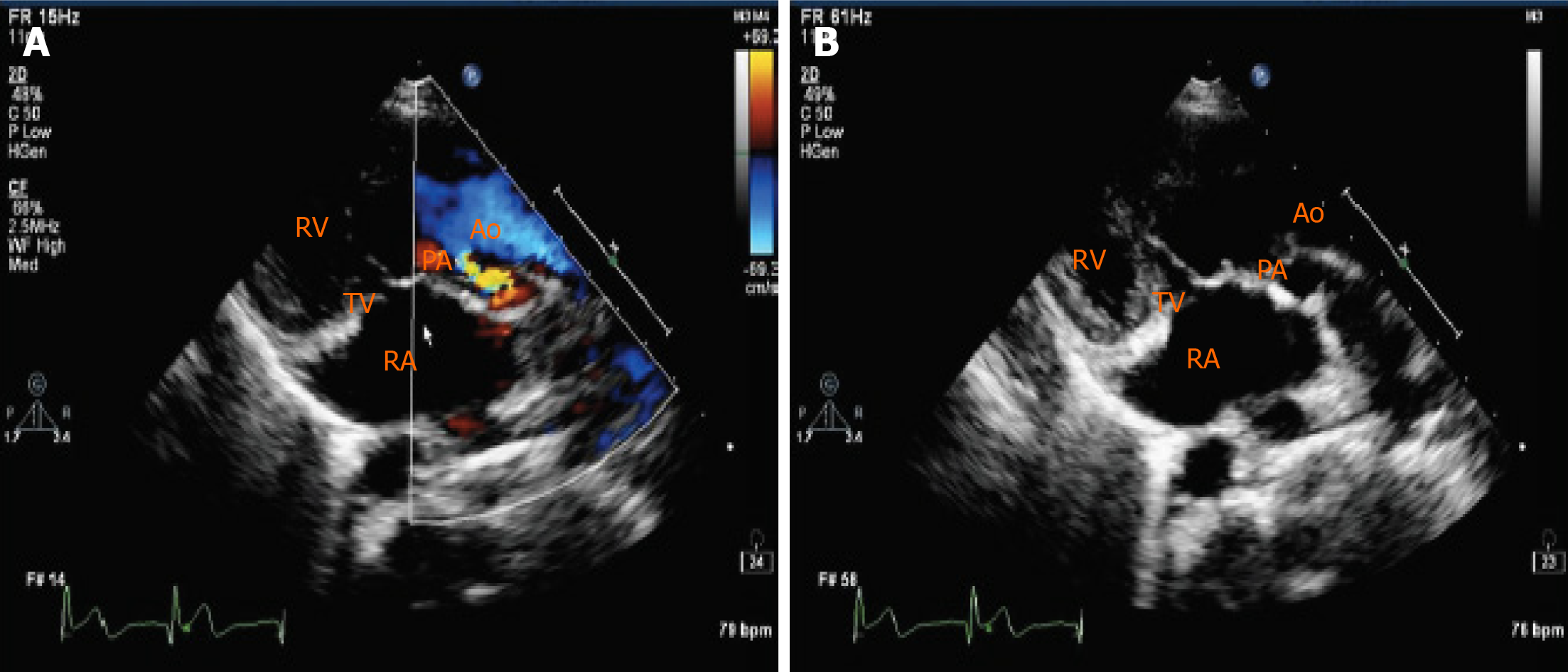
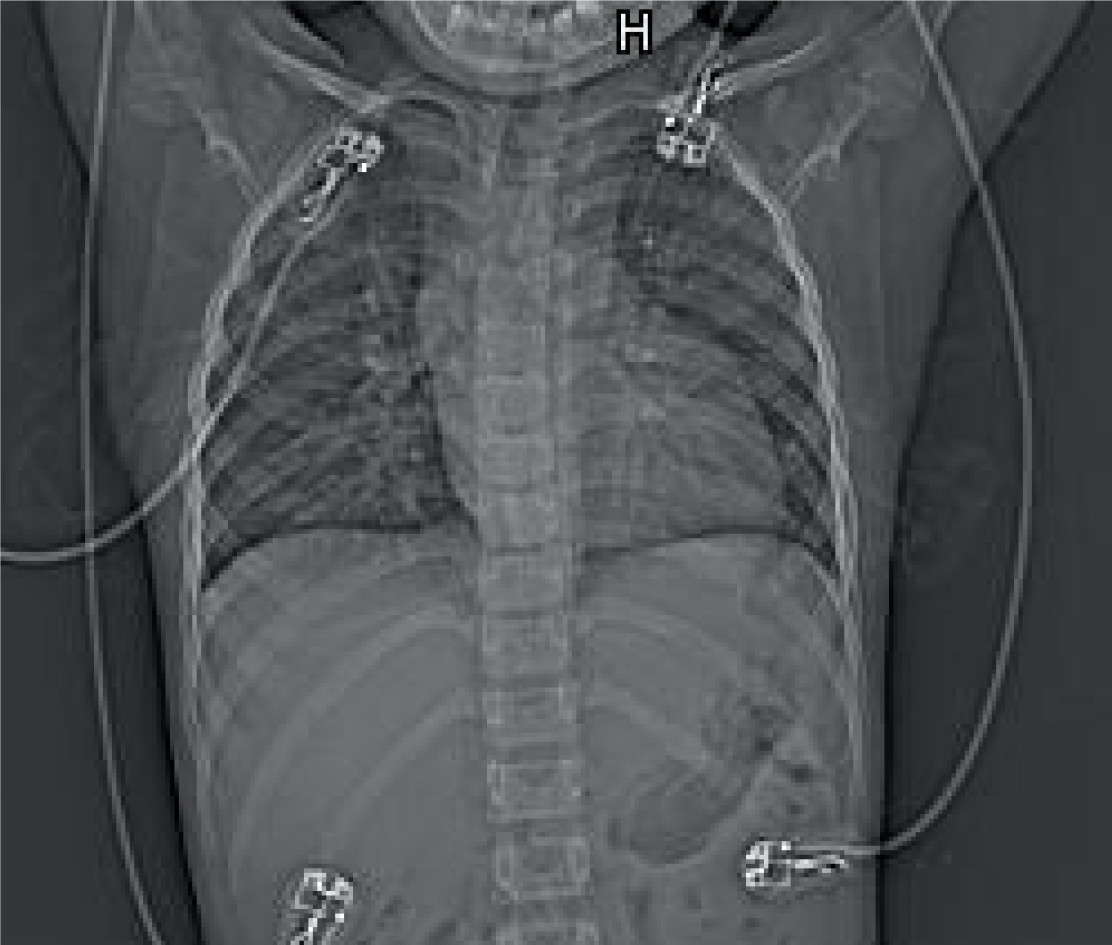
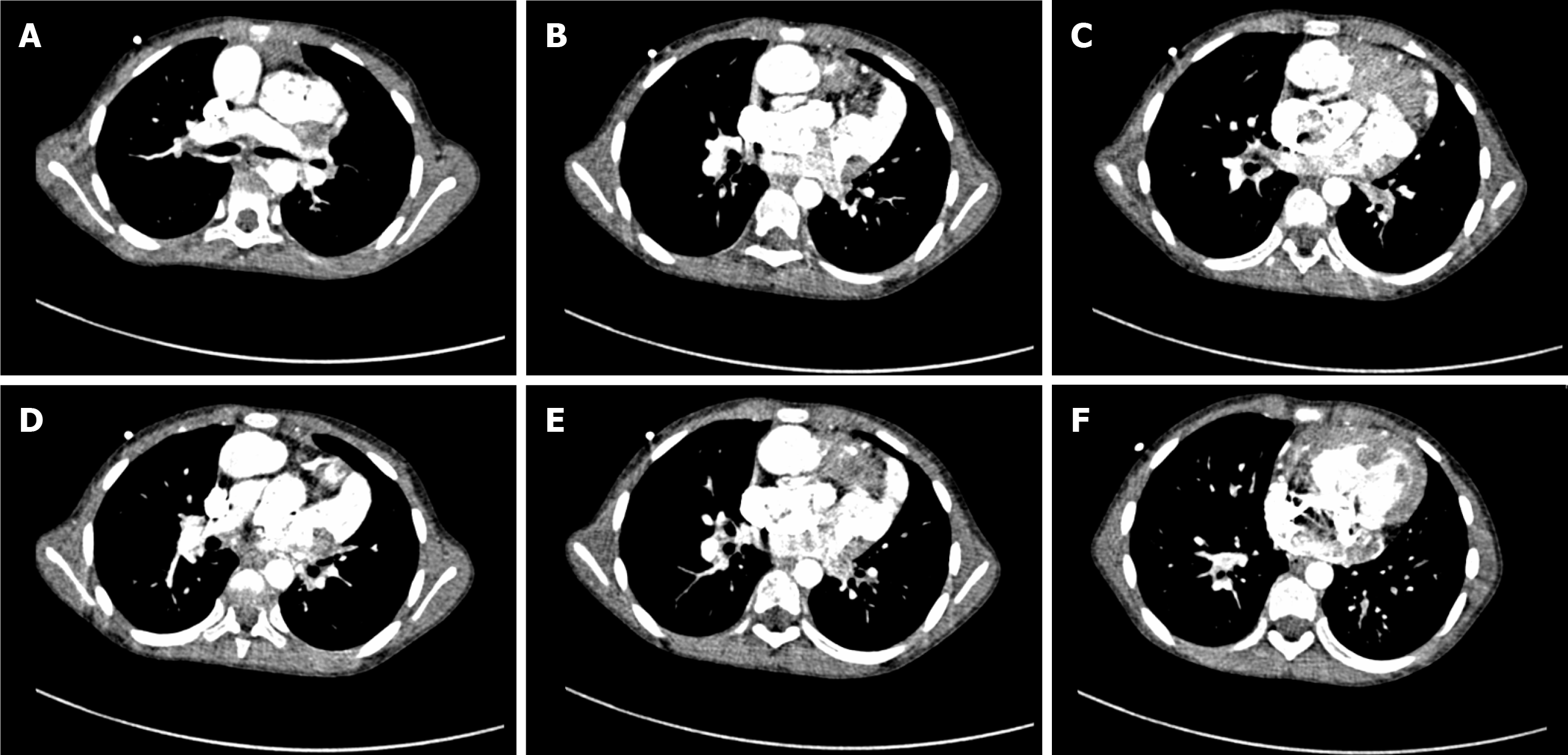
Transthoracic echocardiography (TTE) revealed a DORV, a ventricular septal defect (VSD) with a bidirectional shunt at the ventricular level, an atrial septal defect (ASD) with a right-to-left shunt at the atrial level, severe pulmonary valve stenosis, right ventricular hypertrophy, and transposition of the great arteries, but with normal left ventricular functions (ejection fraction, 66%) (Figure 1). Chest radiography demonstrated an abnormal cardiac morphology (Figure 2), while chest computed tomography showed a DORV with a VSD, an ASD, subvalvular pulmonary artery stenosis, and transposition of the great arteries (Figure 3). Right cardiac catheterization was performed after admission. Angiography showed normal distal pulmonary artery development, and multiple aorta pulmonary collateral arteries, two of which were successfully occluded during the procedure. The mean pulmonary artery pressure was measured as 13 mmHg.
The final diagnosis of the present case was DORV, VSD, ASD, transposition of the great arteries, and severe pulmonary valve stenosis.
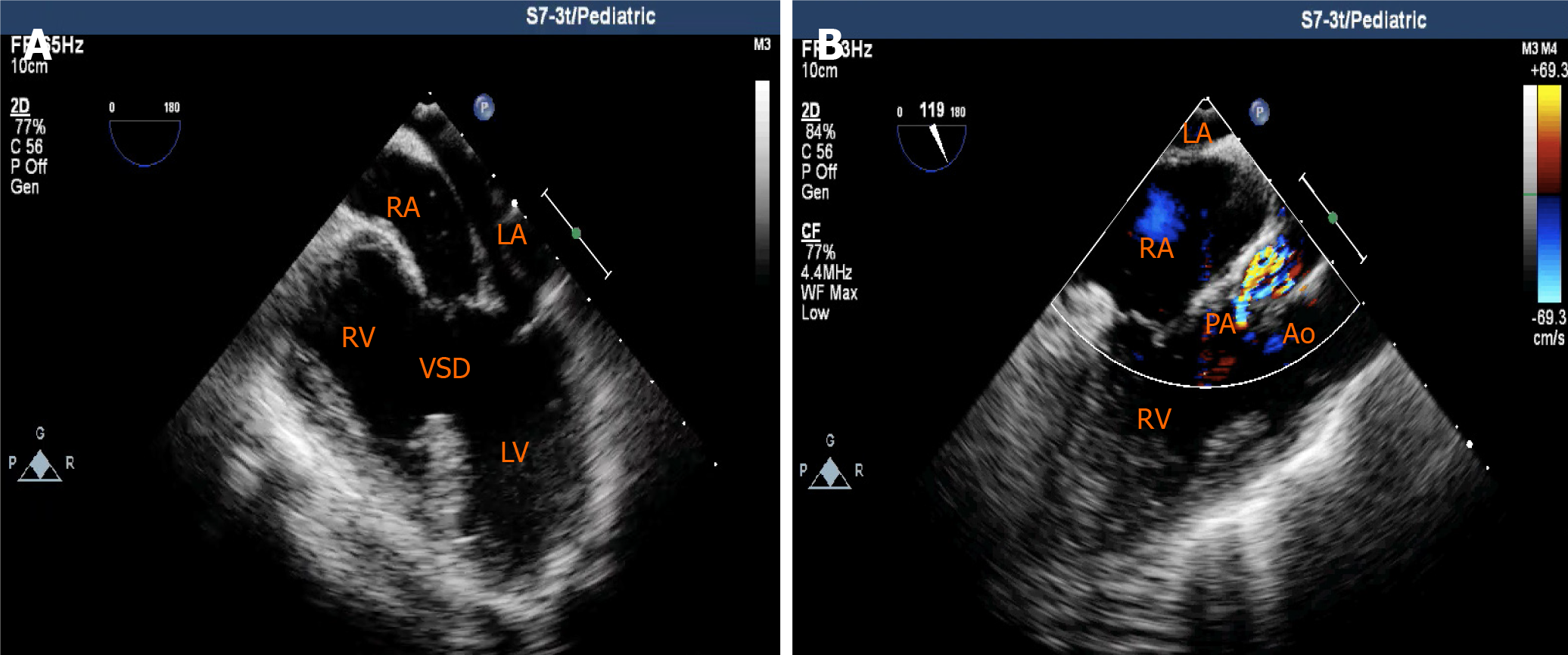
Considering normal distal pulmonary artery development, normal left ventricular function, and acceptable pulmonary vascular resistance (PVR), a modified Fontan operation was then planned. General anesthesia was selected because of the patient’s severe and complicated condition. Routine monitoring was performed. After anesthesia, induction was performed with sufentanil (12.5 μg), midazolam (1.5 mg), and cisatracurium (6 mg), and the patient was smoothly intubated without a marked decrease in oxygen saturation. The radial artery and internal jugular vein were then catheterized to monitor blood pressure and central venous pressure (CVP), respectively. Continuous transesophageal echocardiography (TEE) examination was performed to assess cardiac function and volume status throughout the procedure (Figure 4) (Videos 1-4). Anesthesia was maintained with sevoflurane (1%-3%), propofol (2-6 mg/kg/h), and remifentanil (0.1-0.2 μg/kg/min). Intraoperative mechanical ventilation strategies included a tidal volume of 6-8 mg/kg and maintaining an end-tidal CO2 between 25-30 mmHg. After induction, preoperative hemodilution was performed by infusing crystalloid solution (350 mL) over 1 h to decrease blood viscosity and prevent thrombotic complications. Tranexamic acid was administered before incision to inhibit the fibrinolytic process and prevent bleeding. Crystalloid solution and human albumin were also added to priming solution used for cardiopulmonary bypass (CPB) to further dilute circulating red blood cells. Hct was maintained at 45% to 55% in the CPB. A fenestration modified Fontan operation was performed. After performing a total cavapulmonary connection, the surgeon implemented a right atrium fenestration about 3 millimeter. The surgical procedure was uneventful. The left atrium was then catheterized to continuously assess left atrial pressure prior to discontinuing CPB. After discontinuing CPB, sufficient blood volume (based on dynamic TEE monitoring and blood gas analysis) and a higher CVP were maintained to provide adequate preload for the right ventricle, to facilitate blood flow towards the pulmonary circulation. The patient received 670 mL of crystalloid solution, 200 mL of plasma, 1 unit of platelets, and a 300 mL transfusion of autologous recovered blood. The patient was then shifted to the intensive care unit (ICU). Fortunately, postoperative bleeding was mild and she was continuously administered plasma and platelets to correct coagulation disorder. Changes in Hb levels and coagulative function are shown in Table 1. In the ICU, the patient was placed in a head-high position to facilitate vena cava reflux. The tracheal tube was extubated on postoperative day 1, and she underwent thoracentesis and drainage on postoperative day 2.
| Variable | Preoperative | Postoperative day 1 | Postoperative day 4 | Postoperative day 8 |
| Hb (g/dL) | 24.8 | 16.7 | 15.7 | 15.3 |
| PT (s) | 16.7 | 18.6 | 29.7 | 15.5 |
| INR | 1.51 | 1.70 | 2.77 | 1.4 |
| APTT (s) | 61.8 | 56.9 | 51.6 | 43.8 |
The patient was transferred to general ward on postoperative day 4 and was discharged from the hospital 1 wk after surgery without complications.
Perioperative management of polycythemic CCHD patients undergoing complex cardiac surgery requires adequate planning and careful implementation. The present study demonstrated the successful anesthetic management of a rare DORV patient with severe polycythemia who underwent a modified Fontan surgery. The patient showed a favorable postoperative course without any complications. In this case, hemodilution and management of coagulation functions were key goals. Because of chronic slow progressive hypoxemia and central cyanosis, patients with DORV may experience serious erythrocytosis and coagulation disorders. Additionally, our patient had lived in the Qinghai-Tibet Plateau in China for 10 years. The low-oxygen environment of the plateau may have also contributed to the severe erythrocytosis (Hb levels up to 24.8 g/dL). Under such conditions, thrombosis and infarction can be clinically-devastating complications. However, phlebotomy is not recommended for the prevention of cerebrovascular events, as it can cause iron deficiency, reduce exercise tolerance, and impair oxygen transport capacity[3]. In the present case, we performed hemodilution rather than phlebotomy to ameliorate hyperviscosity. Crystalloid solution was administrated to maintain blood volume and decrease blood viscosity from the beginning of anesthesia. During extracorporeal circulation, hemodilution was also adopted throughout. Intraoperative volume management was constantly regulated according to dynamic TEE monitoring and blood gas analysis to maintain the balance between adequate blood viscosity and appropriate volume load. Sahoo et al[4] showed that hemodilution in CCHD patients has beneficial effects including improved shunt patency and less postoperative blood loss, and it is safe to reduce Hct to 45%[4]. Coagulation dysfunction is also common in cyanotic patients, and involves a multi-systemic mechanism including thrombocytopenia, shortened platelet survival, and deficiencies in coagulation factors[5-11]. Therefore, an individualized intraoperative anticoagulation and antifibrinolytics strategy should be adopted for children with cyanosis. As an alternative treatment, measuring preoperative antithrombin activity and supplementing its activity prior to CPB can preserve the efficacy of heparin during CPB. In addition, epsilon aminocaproic acid and tranexamic acid are two widely available lysine analogs used to inhibit the fibrinolytic process to reduce clinical bleeding[12]. In the present case, postoperative management of coagulation functions was refractory and tenacious. Her coagulopathy suggested an increased risk of postoperative hemorrhage, and for another, anticoagulation should be implemented as soon as possible after surgery to prevent thromboembolic complications. The coagulation disorders were corrected until well after surgery following repeated transfusions. Thromboelastography (TEG) can also be used to monitor perioperative coagulation function for TEG can substantiate changes in coagulation status produced by hemodilution. TEG measurements have become an accepted measure to assess changes in coagulation after volume replacement[13,14]. Maintenance of perioperative hemodynamics is also vital to the success of surgery. Adequate preoxygenation, proper sedation, and prevention of hypoxic crisis before induction should be implemented. Perioperative fluid management is critical for adequate volume control, and is required to maintain sufficient CVP to facilitate blood flow towards the pulmonary circulation while maintaining sufficient colloid osmotic pressure. After CPB, maintaining low pulmonary vascular resistance while maintaining a high right atrial pressure and a low left atrial pressure is key for hemodynamic stability[15,16]. Hypercapnia must be prevented, and any drugs that may increase PVR should be avoided. Adequate hyperventilation and use of positive vasoactive agents can enhance myocardial systole, decrease PVR, and maintain cardiac output. Postoperatively, we ensure that patients smoothly adapt to their unique Fontan circulation hemodynamics. Since positive pressure mechanical ventilation increases intra-alveolar pressure, which can increase PVR and decrease cardiac output, positive end-expiratory pressure can be avoided and the lowest possible inspiratory pressure should be maintained.
We present the anesthetic management of a high-risk CCHD patient with severe polycythemia who underwent a modified Fontan surgery. Our anesthetic management highlights the importance of hemodilution for ameliorating hyperviscosity to decrease the risk of thromboembolism. Furthermore, it is equally important to correct coagulopathy to prevent hemorrhage. Maintenance of stable intraoperative hemodynamics to help the patient adapt smoothly to their unique Fontan circulation is also vital to the success of the surgery.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Naem A S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Li JH
| 1. | Gottschalk I, Abel JS, Menzel T, Herberg U, Breuer J, Gembruch U, Geipel A, Brockmeier K, Berg C, Strizek B. Prenatal diagnosis, associated findings and postnatal outcome of fetuses with double outlet right ventricle (DORV) in a single center. J Perinat Med. 2019;47:354-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Spaeth JP. Perioperative Management of DORV. Semin Cardiothorac Vasc Anesth. 2014;18:281-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Diller GP, Dimopoulos K, Broberg CS, Kaya MG, Naghotra US, Uebing A, Harries C, Goktekin O, Gibbs JS, Gatzoulis MA. Presentation, survival prospects, and predictors of death in Eisenmenger syndrome: a combined retrospective and case-control study. Eur Heart J. 2006;27:1737-1742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 199] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 4. | Sahoo TK, Chauhan S, Sahu M, Bisoi A, Kiran U. Effects of hemodilution on outcome after modified Blalock-Taussig shunt operation in children with cyanotic congenital heart disease. J Cardiothorac Vasc Anesth. 2007;21:179-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Cohen S, Gurvitz MZ, Beauséjour-Ladouceur V, Lawler PR, Therrien J, Marelli AJ. Cancer risk in congenital heart disease-what is the evidence? Can J Cardiol. 2019;35:1750-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Maurer HM, McCue CM, Robertson LW, Haggins JC. Correction of platelet dysfunction and bleeding in cyanotic congenital heart disease by simple red cell volume reduction. Am J Cardiol. 1975;35:831-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 32] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Niwa K, Perloff JK, Kaplan S, Child JS, Miner PD. Eisenmenger syndrome in adults: ventricular septal defect, truncus arteriosus, univentricular heart. J Am Coll Cardiol. 1999;34:223-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 102] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Shebl SS, El-Shehaby WAN, Said YS, Darwish AH, Elfadaly NH, Amer E. Thrombo-hemorrhagic liability in children with congenital heart diseases. Hematol Oncol Stem Cell Ther. 2018;11:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Peters AM, Rozkovec A, Bell RN, Hallidie-Smith KA, Goodwin JF, Lavender JP. Platelet kinetics in congenital heart disease. Cardiovasc Res. 1982;16:391-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Karsenty C, Zhao A, Marijon E, Ladouceur M. Risk of thromboembolic complications in adult congenital heart disease: A literature review. Arch Cardiovasc Dis. 2018;111:613-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Willems A, Patte P, De Groote F, Van der Linden P. Cyanotic heart disease is an independent predicting factor for fresh frozen plasma and platelet transfusion after cardiac surgery. Transfus Apher Sci. 2019;58:304-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Zabala LM, Guzzetta NA. Cyanotic congenital heart disease (CCHD): focus on hypoxemia, secondary erythrocytosis, and coagulation alterations. Paediatr Anaesth. 2015;25:981-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Rupa-Matysek J, Trojnarska O, Gil L, Szczepaniak-Chicheł L, Wojtasińska E, Tykarski A, Grajek S, Komarnicki M. Assessment of coagulation profile by thromboelastometry in adult patients with cyanotic congenital heart disease. Int J Cardiol. 2016;202:556-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Phillips RC, Shahi N, Leopold D, Levek C, Shirek G, Hilton S, Hyslop R, Gien J, Kinsella JP, Buckvold S, Liechty KW, Kim JS, Marwan AI. Thromboelastography-guided management of coagulopathy in neonates with congenital diaphragmatic hernia supported by extracorporeal membrane oxygenation. Pediatr Surg Int. 2020;36:1027-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Ohuchi H. Where is the "Optimal" Fontan Hemodynamics? Korean Circ J. 2017;47:842-857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 16. | Coutsos M, Sala-Mercado JA, Ichinose M, Li Z, Dawe EJ, O'Leary DS. Muscle metaboreflex-induced coronary vasoconstriction limits ventricular contractility during dynamic exercise in heart failure. Am J Physiol Heart Circ Physiol. 2013;304:H1029-H1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |