Published online Apr 6, 2021. doi: 10.12998/wjcc.v9.i10.2409
Peer-review started: December 21, 2020
First decision: December 30, 2020
Revised: January 4, 2021
Accepted: January 26, 2021
Article in press: January 26, 2021
Published online: April 6, 2021
Processing time: 98 Days and 11.6 Hours
Acquired haemophilia is a rare coagulation disorder characterized by autoantibodies against coagulation factor VIII leading to severe and potentially life-threatening haemorrhages. The underlying disorder causing the development of an autoimmune phenomenon is not always known, but 10%-15% could be linked to malignancies. Patients with cancer who require surgical resection represent a treatment challenge not solely due to increased risk of bleeding but also due to adverse events of immunosuppressive therapy.
We present the case of a 67-year-old man with non-metastatic adenocarcinoma of the distal bile duct who developed concomitant acquired haemophilia a month after having been diagnosed with malignant disease. Haemostasis was established with recombinant activated factor VII, and immunosuppressive therapy was started immediately. An extensive surgical procedure was performed in order to remove the cancer and, therefore, eliminate the inhibitory autoantibodies. Due to a complicated postoperative course, relatively short period of treatment and likelihood of micrometastases, no improvement in the patient’s status was observed. Diagnosis and treatment of acquired haemophilia as well as other coagulation disorders in patients with cancer are discussed.
Prompt diagnosis of acquired haemophilia is required in order to start appropriate treatment and reduce mortality. Among patients with cancer, other causes of abnormal bleeding related to malignancy should be considered.
Core Tip: Acquired haemophilia is a rare coagulation disorder characterized by autoantibodies against coagulation factor VIII. Immediate consultation with a reference haemophilia centre should be made for obtaining early diagnosis and treatment. In patients with cancer, a wide range of haemostatic disorders should be taken into consideration and distinguished from acquired haemophilia. Although invasive procedures should be avoided in patients with acquired haemophilia, surgical resection of the underlying malignancy is sometimes required. This subset of patients represents a treatment challenge not solely due to increased risk of bleeding but also due to adverse events of immunosuppressive therapy.
- Citation: Krašek V, Kotnik A, Zavrtanik H, Klen J, Zver S. Acquired haemophilia in patients with malignant disease: A case report. World J Clin Cases 2021; 9(10): 2409-2418
- URL: https://www.wjgnet.com/2307-8960/full/v9/i10/2409.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i10.2409
Acquired haemophilia (AH) is a coagulation disorder caused by neutralizing autoantibodies against coagulation factor (F) VIII leading to severe and potentially life-threatening haemorrhages. It occurs at a rate of approximately 1.5/million/year[1] and is predominantly seen in elderly patients with reported median age at diagnosis being 68-78 years[2-6]. In contrast to congenital haemophilia, it affects both sexes equally and has a distinct pattern of bleeding that is unusual and unlike anything that has been seen before. The mortality rate is high, reaching above 20%, making early diagnosis and treatment of vital importance[3,4]. The underlying cause is unknown in about 50% of cases, while the remaining cases may be associated with malignancies, autoimmune diseases, pregnancy, certain medications or infections[3]. Solid cancers most commonly linked to AH are prostate, lung and colon neoplasms, but it can also be seen in lymphoproliferative malignancies[2]. Herein, AH should be distinguished from other causes of abnormal bleeding related to cancer in order to start appropriate treatment and reduce mortality[7]. Without effective treatment of the underlying malignancy, complete elimination of inhibitory autoantibodies is unlikely. Perioperative management of patients with AH and cancer who require surgical resection is challenging.
We present the case of a 67-year-old man with AH due to non-metastatic adenocarcinoma of the distal bile duct and discuss a diagnostic and therapeutic approach to a cancer patient with bleeding diathesis.
A 67-year-old Caucasian male patient presented to the Emergency Department with fever, pain, swelling and bruising of his left thigh. He denied trauma to the leg.
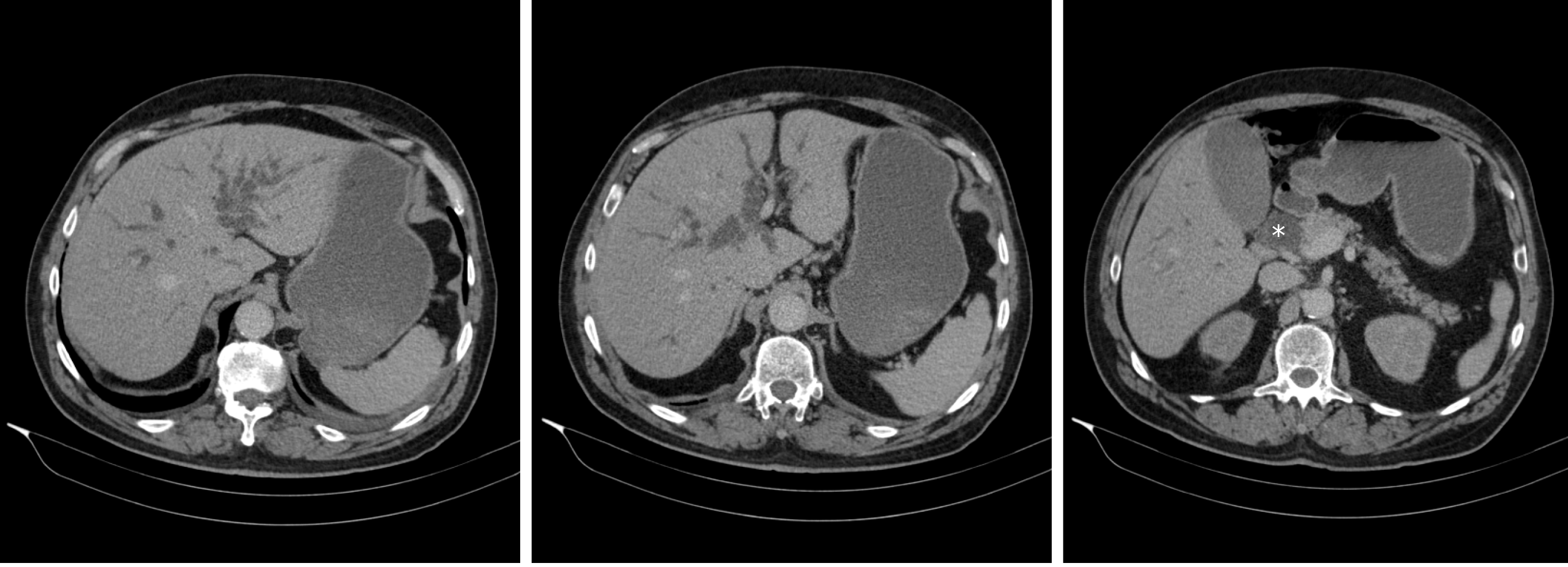
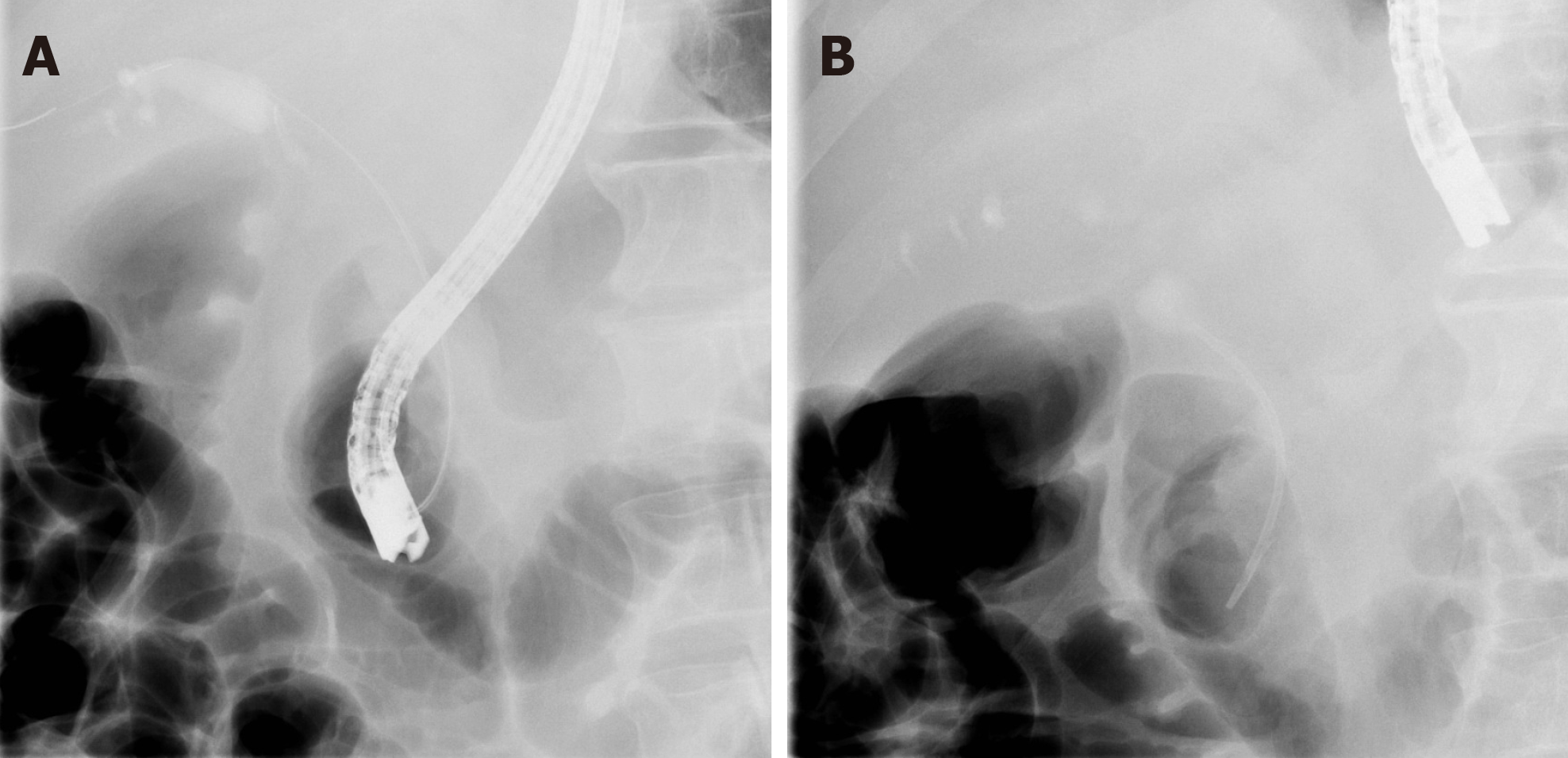
A month before presentation, he was admitted for evaluation of painless jaundice. At the time of admission, his laboratory results showed acute kidney failure (serum creatinine 446 µmol/L, urea 10.7 mmol/L), normocytic anaemia (haemoglobin 90 g/L, mean corpuscular volume 86 fL), elevated liver enzymes [aspartate aminotransferase (AST) 2.6 µkat/L, alanine aminotransferase (ALT) 3.6 µkat/L, gamma-glutamyl transferase (GGT) 12.1 µkat/L, alkaline phosphatase (AP) 12.1 µkat/L] and elevated bilirubin levels (186/145 µmol/L), indicating cholestasis. Malignant stenosis of the distal common bile duct was discovered upon abdominal computed tomography (CT) scan with no evidence of distant metastases (Figure 1). He underwent endoscopic retrograde cholangiopancreatography with stent placement in order to overcome the stenosis (Figure 2). Kidney function tests (serum creatinine 369 µmol/L), liver enzymes (AST 1.9 µkat/L, ALT 3.67 µkat/L, GGT 3.87 µkat/L, AP 9.86 µkat/L) and bilirubin levels (149/124 µmol/L) improved, however, levels of haemoglobin remained low (86 g/L). His case was discussed at a multidisciplinary cancer team meeting and surgical treatment was proposed.
His background medical history was remarkable for arterial hypertension, hypercholesterolemia and stage 3 chronic kidney disease.
Upon admission the patient was alert, oriented and cooperative. He was febrile with body temperature of 38.1 °C, blood pressure of 135/72 mmHg, heart rate of 71/min and oxygen saturation of 100%. Physical examination revealed swelling and large suffusion on his left thigh.
Laboratory results showed severe normocytic anaemia (haemoglobin 65 g/L, mean corpuscular volume 93.0 fL), elevated liver enzymes (AST 1.08 µkat/L, ALT 1.68 µkat/L, GGT 10.86 µkat/L, AP 10.72 µkat/L) and bilirubin levels (52/33 µmol/L) and moderately raised inflammatory parameters (C-reactive protein 63 mg/L, white blood cell 9.4 × 109/L, procalcitonin 0.31 ng/mL). Coagulation tests showed normal prothrombin time/international normalized ratio (1.03). Laboratory values upon admission are summarized in Table 1.
| Value | Unit | Normal range | ||
| Glucose | 5.5 | mmol/L | 3.6-6.1 | |
| Urea | H | 8.9 | mmol/L | 2.8-7.5 |
| Sodium | 138 | mmol/L | 135-145 | |
| Potassium | 4.6 | mmol/L | 3.7-4.9 | |
| Creatinine | H | 149 | µmol/L | 44-97 |
| eGFR (CDK-EPI) | 41 | mL/min per 1.73 m2 | ||
| Bilirubin, total | H | 52 | µmol/L | < 17 |
| Bilirubin, direct | H | 33 | µmol/L | < 5 |
| AP | H | 10.72 | µkat/L | < 2.15 |
| AST | H | 1.08 | µkat/L | < 0.58 |
| ALT | H | 1.68 | µkat/L | < 0.74 |
| GGT | H | 10.86 | µkat/L | < 0.94 |
| Amylase | 1.15 | µkat/L | < 1.67 | |
| Lipase | 0.61 | µkat/L | < 1.00 | |
| LDH | H | 5.50 | µkat/L | < 4.13 |
| CRP | H | 63 | mg/L | 0-5 |
| Procalcitonin | H | 0.31 | µg/mL | < 0.24 |
| WBC | 9.4 | 109/L | 4.0-10.0 | |
| RBC | L | 2.04 | 1012/L | 4.50-5.50 |
| Haemoglobin | L | 65 | g/L | 130-170 |
| Haematocrit | L | 0.190 | 1 | 0.400-0.500 |
| MCV | 93.0 | fL | 83.0-101.0 | |
| Platelet count | 349 | 109/L | 150-410 | |
| Prothrombin time | 1.00 | 1 | 0.70-1.30 | |
| INR | 1.00 | < 1.30 | ||
| D-dimer | H | 7904 | µg/L | < 500 |
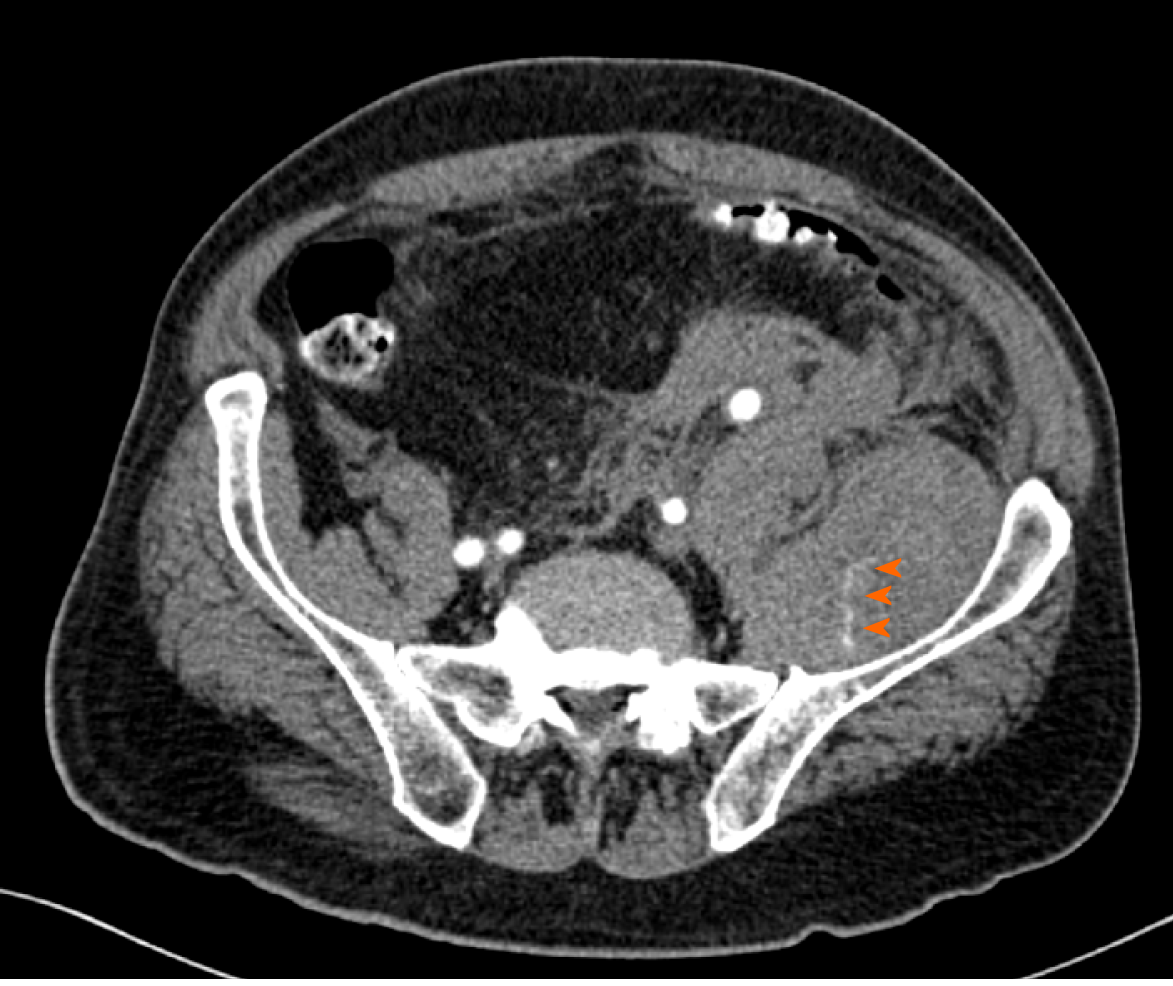
Deep vein thrombosis was excluded with a venous point-of-care ultrasound. Ultrasound of the soft tissues of the left thigh revealed a large hematoma (13 cm × 5 cm × 3 cm) in one of the muscles of the anterior/extensor compartment. Later on, when no significant improvement in haemoglobin level (73 g/L) was observed despite treatment with multiple erythrocyte transfusions, a CT angiography of the abdomen and lower limbs was performed. Here, hematoperitoneum with a large haematoma in the left iliacus muscle (5 cm × 9 cm × 29 cm) and left femoral rectus muscle (7 cm × 5 cm × 30 cm; previously described) was discovered, both with multiple active bleeding sites (Figure 3).
After presentation, the patient received three units of packed red blood cells and was admitted for further treatment and evaluation. Antibiotic treatment due to cholangitis, confirmed upon abdominal ultrasound, was initiated and another endoscopic retrograde cholangiopancreatography was performed to replace the poorly functioning choledochal stent. A haematologist was consulted and ordered additional coagulation tests that revealed elevated activated partial thromboplastin time (aPTT; 92 s), normal prothrombin time (1.00) and international normalized ratio (1.00), solely reduced FVIII activity (1%) and appearance of anti-FVIII antibodies, with titre of 30 Bethesda units (BU)/mL (Table 2). Von Willebrand factor (vWF) antigen, vWF activity and vWF ristocetin cofactor activity were normal.
| Value | Unit | Normal range | ||
| Prothrombin time | 1.00 | 1 | 0.70-1.30 | |
| INR | 1.00 | < 1.30 | ||
| aPTT | H | 92 | s | 25.9-36.6 |
| Fibrinogen | 2.67 | g/L | 1.8-3.5 | |
| Thrombin time | 15.7 | s | 14.0-21.0 | |
| D-dimer | H | 4040 | µg/L | < 500 |
| Factor VIII:C | L | 0.01 | IU/mL | 0.50-1.50 |
| Factor VIII antibodies | H | 30 | BU/mL | 0 |
Due to reduced FVIII activity (1%) and the appearance of anti-FVIII antibodies, with titre of 30 BU/mL, AH was diagnosed, likely secondary to bile duct cancer. Other coagulation disorders were excluded as specific bleeding-related clinical picture with prolonged aPTT, in combination with low FVIII activity and the appearance of anti-FVIII antibodies, is a specific finding in AH.
Bleeding was treated with recombinant activated FVII (rFVIIa; NovoSeven®), transfusions of packed red blood cells, fresh frozen plasma and platelet plasma. The dosage of rFVIIa was adjusted clinically, based on vital haemodynamic parameters indicating haemorrhage control. On a repeated abdominal CT scan, no signs of active blood extravasation were observed. At the same time, as inhibitor eradication treatment, methylprednisolone (80 mg daily) and cyclophosphamide (100 mg daily) were instituted. The dosage of methylprednisolone was later adjusted from 80 mg/d to 30 mg/d in light of the upcoming surgery with the purpose of lowering the risk of infection.
The patient underwent total pancreatectomy with splenectomy with the aim of tumour removal. During the procedure, he was treated with rFVIIa 8 mg/2 h intravenously and continuous infusion of tranexamic acid, receiving 1000 mg/8 h. Estimated intraoperative blood loss was 1000 mL, considered as expected. A R0 resection of distal choledochal adenocarcinoma (pT3N0Mx) was confirmed upon histological species examination. After the procedure, the patient was admitted to the intensive care unit. Haematologists were consulted daily, and the rFVIIa therapy was adjusted accordingly.
During the following days the patient’s condition gradually deteriorated. Inflammatory parameters increased, and sanguineous drainage from his postoperative drains as well as melena were observed. Abdominal CT scan showed active bleeding from the territory of inferior mesenteric artery that was embolised by interventional radiologists. Despite intervention, repeated revision surgeries were required thereafter. They revealed a large amount of blood and coagula in the abdominal cavity, but no active bleeding sites were found. Correction of hepato-jejunal and gastro-enteral anastomotic dehiscence was carried out. The dosage of rFVIIa and tranexamic acid were adjusted based on laboratory findings and clinical status. Prothrombin time, aPTT, thromboplastin time, fibrinogen and D-dimer were checked daily, while FVIII activity and anti-FVIII antibody titre were checked two times per week. Due to continuing sanguineous abdominal drainage, the patient required multiple transfusions of packed red blood cells, platelets and fresh frozen plasma. With corresponding therapy, the patient’s status transiently stabilised and the interval of rFVIIa application was gradually lengthened. However, the titre of FVIII antibody did not decrease (55 BU/mL). Anastomosis dehiscence was suspected again upon abdominal ultrasound. Due to lack of clinical response and failure of controlling the paraneoplastic bleeding syndrome, all active treatments were abolished in consensus with the treating intensivist, haematologist and abdominal surgeon. Palliative care was instituted, and the patient died 32 d after tumour resection. Autopsy revealed no residual malignant disease.
AH typically presents with extensive subcutaneous, mucosal and deep soft tissue haemorrhages, described by doctors as “never seen before”[8]. Soft tissue haemorrhages may be so extensive that they can cause compartment syndrome as well as a severe drop in haemoglobin levels, leading to haemorrhagic shock and death. In some cases, the initial sign of a haemostatic disorder may be prolonged oozing and later on overt bleeding from sites of intravenous/intramuscular injections or similar invasive procedures[8,9]. Occasionally, clinical presentation in AH may be silent, and the disease may only be detected due to abnormally prolonged aPTT[3]. Due to the rarity of the disease, AH is often unrecognised or is discovered too late. Importantly, AH should be suspected in patients without personal or family history of bleeding who present with abrupt onset of bleeding diathesis and an unexplained prolonged aPTT when other, more frequent causes of prolonged aPTT, such as von Willebrand disease, haemophilia A and B, deficiency of FXI and FXII are ruled out[10].
After a suggestive clinical presentation, with unusual overt bleeding pattern and reduced haemoglobin levels, a hemostasis screening should be performed[8]. In patients with cancer, it might be difficult to discriminate AH from other coagulo-pathies causing abnormal bleeding. These are disseminated intravascular coagulation (DIC), thrombotic microangiopathies (TMA), immune thrombocytopenia, heparin-induced thrombocytopenia, concomitant use of oral anticoagulants, decreased synthesis of coagulation factors due to liver dysfunction (e.g., cirrhosis), inherited non-severe form and acquired form of von Willebrand disease, haemophilia C, deficiency of FXII and acquired inhibitors to other coagulation factors[7,11,12]. DIC in malignancy results from activation of coagulation in damaged blood vessels endothelia related to tissue factor and vascular endothelial growth factor expressed by solid tumours. Gradual consumption of platelets and coagulation factors results in bleeding, which may be the first clinical symptom indicating the presence of DIC[11,12].
Similar clinical presentation of cancer-related coagulopathy is seen in TMA syndrome in the form of secondary thrombotic thrombocytopenic purpura. The latter should always be considered in case of concomitant microangiopathic haemolytic anaemia and thrombocytopenia. As an entity among TMA syndromes, haemolytic uremic syndrome is rare in advanced cancer[12]. Immune thrombocytopenia should always be considered in isolated and cancer-related thrombocytopenia. The formation of antibodies directed against the complex of heparin and platelet factor 4 is named heparin-induced thrombocytopenia and may occur more often in cancer patients[7]. Furthermore, thrombocytopenia may also be a consequence of chemotherapy and radiation[13]. Among other haematologic diseases, myelodysplastic syndrome should be recognized, as it also can present with bleeding diathesis and is common in the elderly population[14]. The clinical presentation ranging from bleedings to thrombosis can also be seen in acute traumatic coagulopathy, where a sterile injury provokes a cellular response and changes coagulation[15]. Patients with AH present with isolated prolonged aPTT, while prothrombin time, thrombin time and platelet count are within normal range. Isolated prolongation of aPTT may be caused by heparin use, single coagulation factor deficiency (FVIII, FIX, FXI, FXII) or the presence of an inhibitor[8,9]. True factor deficiency as in haemophilia A should be ruled out with a mixing study. Patient plasma is mixed with normal pool plasma in 1:1 ratio[16]. Since FVIII inhibitors are time- and temperature-dependent, the mixture is then incubated for two hours at 37 °C. If after incubation aPTT fails to correct within the normal range, the presence of an inhibitor is suspected. At this point, the presence of lupus anticoagulant and heparin presence should be excluded[8,9]. FVIII activity assay should be performed which shows reduced activity of FVIII below 50%. The inhibitor titre is then determined using Bethesda assay[10,17]. In our case the FVIII activity was 1% and anti-FVIII antibodies titre was 30 BU/mL, both clearly suggestive of AH diagnosis.
As soon as the diagnosis of AH was established in our patient, the treatment was initiated. Being a rare disease, current recommendations are based on data from patient registries and expert consensus opinions[3]. Generally, patients should be treated at a reference haemophilia centre or at least consultation with such centre should be established[8]. The treatment of AH consists of bleeding control and eradication of the inhibitor[10]. For bleeding control, either of the two available bypassing agents is recommended, rFVIIa (Novo Seven®) and activated prothrombin complex concentrate (aPCC; FEIBA®), which contains activated FII, FVII, FIX and FX. Both agents are safe and effective with reported rates of haemostasis achievement being above 90%[3]. Insufficient data exist to support the preference of one over the other[4,18]. The lack of adequate laboratory test for measuring over- and underdosing is a major setback for both agents[10]. It is also important to consider the risk of thrombogenic potential in both agents, especially in elderly patients[4,8,18].
rFVIIa is reported to be relatively safe combined with tranexamic acid in case of severe bleeding[18]. The dosage regimen is standard as in other rFVIIa approved clinical indications, 90-120 μg/kg/2-3 h until the bleeding stops[8]. In Slovenia, rFVIIa is mostly used for haemostasis control in AH due to the safety and efficacy profile and good clinical experience with the drug[19]. Bleeding and its severity cannot be predicted based on FVIII level or inhibitor titre, so the treatment is directed according to the clinical manifestation of the disease[4]. The dosages of rFVIIa and intervals of administration in our patient were adjusted towards clinical and laboratory signs of active bleeding (90 µg/kg every 2-3 h with the extension of the interval when bleeding control was established). Tranexamic acid was added as an additional haemostatic agent[10].
In order to eradicate the inhibitors and prevent recurrent bleeding, immuno-suppressive therapy should be initiated at diagnosis and the underlying cause of AH should be identified and treated[2,5,8]. Corticosteroids are used, often in combination with cyclophosphamide[5]. Complete response with a combination of both agents may be more frequent than with corticosteroids alone[5], although some other authors showed no such difference[1,4]. In current literature, reported remission rates were 61%-72% when treated with either of the two regimens in a median time of 5-6 wk[4,5,10,20]. This may, however, take longer for patients with lower FVIII activity levels at presentation[20]. The use of immunosuppressive therapy is weighted against its side effects and should be applied carefully since mortality secondary to sepsis might be higher than the bleeding-related mortality in those patients[4]. Rituximab can be added to corticosteroids and/or cyclophosphamide if no response is seen after 2-3 wk of treatment[8,10,20].
In patients with underlying malignancy, higher rates in inhibitor eradication were observed when the tumour was effectively treated with either surgery or chemotherapy, reaching a success rate of 88.8% in a systematic review conducted by Napolitano et al[2]. In their report, this trend was particularly strong for B-cell lymphoproliferative malignancies, prostate cancer and lung cancer. Furthermore, they observed that if the tumour was not removed, the inhibitor eradication was not successful. Another smaller study by Sallah et al[21] showed complete response of AH in 70% of cases (especially in patients with an early-stage tumour) with a median time to remission of 20 wk. The majority of patients where the disappearance of the inhibitor was not achieved had advanced, uncurable malignancy.
In our patient, the treatment with high dose methylprednisolone (80 mg daily) in combination with cyclophosphamide (100 mg daily) was immediately started. An initial response was observed but was transient. Decrease of antibody titre to 13 BU/mL and increase in FVIII levels to 0.06 IE/mL were followed by worsening of both parameters during the next month. To lower the risk of postoperative infection, immunosuppressive regimen was adapted perioperatively. Furthermore, total pancreatectomy was performed instead of pancreatoduodenectomy to avoid possible complications associated with a formation of pancreatoenterostomy (anastomotic dehiscence, fistula, bleeding). However, despite tumour removal achieving R0 resection and concomitant immunosuppressive therapy, multiple surgical and radiologic interventions were needed due to constant bleeding. The patient also suffered from serious infectious complications with anastomotic dehiscence and abdominal sepsis.
There are only few reports describing AH in the setting of bile duct carcinoma. One such case was reported by Onishi et al[22] where abnormal bleeding occurred after surgical removal of the tumour. The patient was treated with both prednisolone and rituximab, and successful inhibitor eradication took place in postoperative week 18. In our case, there was no long-lasting improvement in haemostasis, and remission was not achieved after more than 5 wk of intensive treatment. However, considering the time to remission reported in literature, the duration of treatment in our patient was too short to determine whether the treatment was effective. The other explanation could be the presence of micrometastases. It has been observed that such loci of cancer cells, however small and undetectable, still have the ability to generate the production of antibodies[23]. The use of rituximab, was considered[8,10]. Due to worsening of the clinical status, the medication was not prescribed.
Mortality in patients with AH due to fatal haemorrhage is 9%-33%[1,3,4] with an even higher overall mortality rate in the subgroup of cancer patients reaching 44%[24]. Napolitano et al[2] observed a death rate of 28%. This can be attributed to selection bias since cases with favourable outcomes are generally reported in literature. Complete remission in patients with malignancy is less frequent and death more often occurs because of bleeding or complications associated with underlying cancer or chemotherapy.
In patients with malignancy with concomitant unusual bleeding patterns, it is important to consider widely all the options. Among these are DIC, TMA, immune thrombocytopenia, heparin-induced thrombocytopenia, coagulation factor or vitamin K deficiency. In this report, we presented a rare manifestation of coagulopathy as a consequence of newly developed autoantibodies against FVIII secondary to adenocarcinoma of the distal bile duct. Despite R0 resection of the tumour and immunosuppressive treatment, the eradication of the inhibitor was not achieved, likely due to lack of time, shorter than 4 wk. Therefore, the outcome of AH in cases of longer administration of immunosuppressive treatment cannot be predicted. The possibility of persisting micrometastases could also pose a cause of lingering disease. AH should be considered as a haematological emergency and should be recognised and treated promptly in order to achieve a favourable outcome.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Slovenia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fu TL, Hamada Y S-Editor: Gao CC L-Editor: Filipodia P-Editor: Xing YX
| 1. | Collins PW, Hirsch S, Baglin TP, Dolan G, Hanley J, Makris M, Keeling DM, Liesner R, Brown SA, Hay CR; UK Haemophilia Centre Doctors' Organisation. Acquired hemophilia A in the United Kingdom: a 2-year national surveillance study by the United Kingdom Haemophilia Centre Doctors' Organisation. Blood. 2007;109:1870-1877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 500] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 2. | Napolitano M, Siragusa S, Mancuso S, Kessler CM. Acquired haemophilia in cancer: A systematic and critical literature review. Haemophilia. 2018;24:43-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Knoebl P, Marco P, Baudo F, Collins P, Huth-Kühne A, Nemes L, Pellegrini F, Tengborn L, Lévesque H; EACH2 Registry Contributors. Demographic and clinical data in acquired hemophilia A: results from the European Acquired Haemophilia Registry (EACH2). J Thromb Haemost. 2012;10:622-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 363] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 4. | Borg JY, Guillet B, Le Cam-Duchez V, Goudemand J, Lévesque H; SACHA Study Group. Outcome of acquired haemophilia in France: the prospective SACHA (Surveillance des Auto antiCorps au cours de l'Hémophilie Acquise) registry. Haemophilia. 2013;19:564-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 5. | Collins P, Baudo F, Knoebl P, Lévesque H, Nemes L, Pellegrini F, Marco P, Tengborn L, Huth-Kühne A; EACH2 registry collaborators. Immunosuppression for acquired hemophilia A: results from the European Acquired Haemophilia Registry (EACH2). Blood. 2012;120:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 240] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 6. | Reeves BN, Key NS. Acquired hemophilia in malignancy. Thromb Res. 2012;129 Suppl 1:S66-S68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | DeLoughery TG. Management of acquired bleeding problems in cancer patients. Emerg Med Clin North Am. 2009;27:423-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Collins P, Baudo F, Huth-Kühne A, Ingerslev J, Kessler CM, Castellano ME, Shima M, St-Louis J, Lévesque H. Consensus recommendations for the diagnosis and treatment of acquired hemophilia A. BMC Res Notes. 2010;3:161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 9. | Franchini M, Castaman G, Coppola A, Santoro C, Zanon E, Di Minno G, Morfini M, Santagostino E, Rocino A; AICE Working Group. Acquired inhibitors of clotting factors: AICE recommendations for diagnosis and management. Blood Transfus. 2015;13:498-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 10. | Kruse-Jarres R, Kempton CL, Baudo F, Collins PW, Knoebl P, Leissinger CA, Tiede A, Kessler CM. Acquired hemophilia A: Updated review of evidence and treatment guidance. Am J Hematol. 2017;92:695-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 258] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 11. | Kvolik S, Jukic M, Matijevic M, Marjanovic K, Glavas-Obrovac L. An overview of coagulation disorders in cancer patients. Surg Oncol. 2010;19:e33-e46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Falanga A, Marchetti M, Vignoli A. Coagulation and cancer: biological and clinical aspects. J Thromb Haemost. 2013;11:223-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 359] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 13. | Ayesh Haj Yousef MH, Alawneh K, Zahran D, Aldaoud NH, Khader Y. Immune thrombocytopenia among patients with cancer and its response to treatment. Eur J Haematol. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Byrne M, Liu X, Carter CM, Zumberg MS. Myelodysplastic syndrome and associated coagulopathy: a case report and review. Blood Coagul Fibrinolysis. 2014;25:172-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Moore HB, Winfield RD, Aibiki M, Neal MD. Is Coagulopathy an Appropriate Therapeutic Target During Critical Illness Such as Trauma or Sepsis? Shock. 2017;48:159-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Miller CH. Mixing Studies. In: Shaz BH, Hillyer CD, Reyes Gil M. Transfusion Medicine and Hemostasis: Clinical and Laboratory Aspects. 3rd ed. Elsevier; 2019: 783-784. |
| 17. | Duncan E, Collecutt M, Street A. Nijmegen-Bethesda assay to measure factor VIII inhibitors. Methods Mol Biol. 2013;992:321-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Baudo F, Collins P, Huth-Kühne A, Lévesque H, Marco P, Nemes L, Pellegrini F, Tengborn L, Knoebl P; EACH2 registry contributors. Management of bleeding in acquired hemophilia A: results from the European Acquired Haemophilia (EACH2) Registry. Blood. 2012;120:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 273] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 19. | Zver S, Škerget M. Acquired hemophilia: overview and case report. Anaesthesiol Res Med. 2011;5:181-186. |
| 20. | Tiede A, Klamroth R, Scharf RE, Trappe RU, Holstein K, Huth-Kühne A, Gottstein S, Geisen U, Schenk J, Scholz U, Schilling K, Neumeister P, Miesbach W, Manner D, Greil R, von Auer C, Krause M, Leimkühler K, Kalus U, Blumtritt JM, Werwitzke S, Budde E, Koch A, Knöbl P. Prognostic factors for remission of and survival in acquired hemophilia A (AHA): results from the GTH-AH 01/2010 study. Blood. 2015;125:1091-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 197] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 21. | Sallah S, Wan JY. Inhibitors against factor VIII in patients with cancer. Analysis of 41 patients. Cancer. 2001;91:1067-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 22. | Onishi I, Kayahara M, Munemoto M, Sakai S, Makino I, Hayashi H, Nakagawara H, Tajima H, Takamura H, Kitagawa H, Tani T, Ohta T. Management of postoperative hemorrhage associated with factor VIII inhibitor: report of a case. Surg Today. 2013;43:1058-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Kreuter M, Retzlaff S, Enser-Weis U, Berdel WE, Mesters RM. Acquired haemophilia in a patient with gram-negative urosepsis and bladder cancer. Haemophilia. 2005;11:181-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Bitting RL, Bent S, Li Y, Kohlwes J. The prognosis and treatment of acquired hemophilia: a systematic review and meta-analysis. Blood Coagul Fibrinolysis. 2009;20:517-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |