Published online Apr 6, 2021. doi: 10.12998/wjcc.v9.i10.2373
Peer-review started: November 24, 2020
First decision: December 21, 2020
Revised: December 27, 2020
Accepted: January 27, 2021
Article in press: January 27, 2021
Published online: April 6, 2021
Processing time: 126 Days and 0.2 Hours
Melanoma brain metastasis is a common cause of death in melanoma patients and is associated with a poor prognosis. There are relatively few reports on intracranial infections after brain metastasis resection.
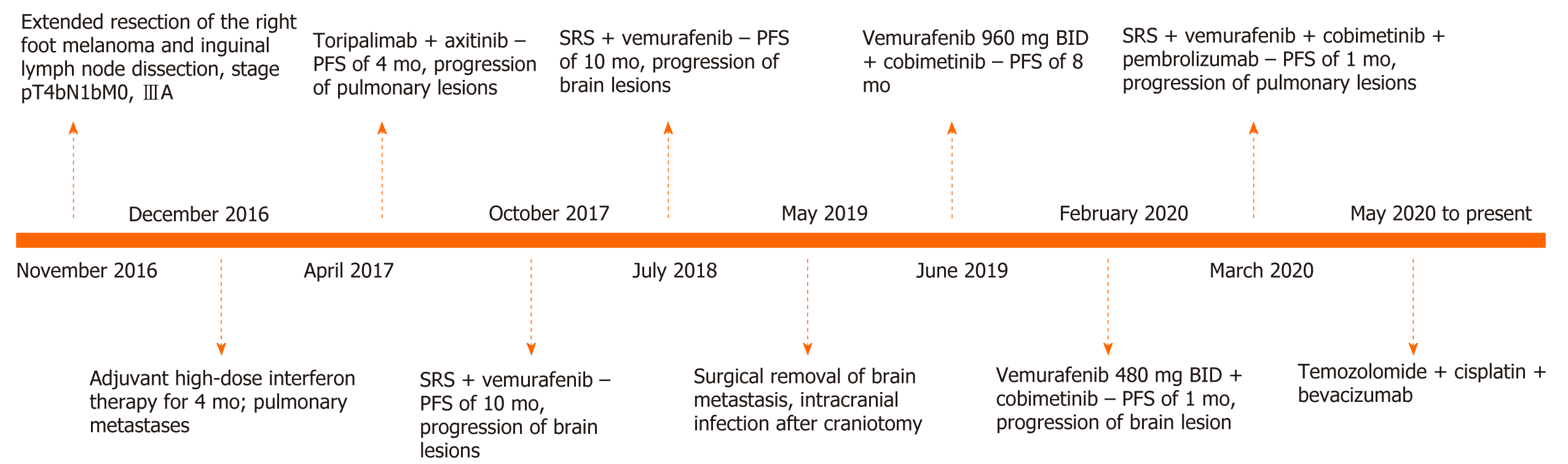
Here we report a case of melanoma brain metastases in a patient harboring a BRAF V600E mutation, who experienced intracranial tumor progression despite previous combined treatment with a programmed death (PD)-1 inhibitor, axitinib, and vemurafenib. She repeatedly underwent local therapy, including stereotactic radiosurgery and intracranial surgery, and developed central nervous system infection. Treatment with vemurafenib combined with cobimetinib resulted in an intracranial progression-free survival of 10 mo. During the coronavirus disease 2019 (COVID-19) pandemic, the patient did not visit the hospital for regular vemurafenib treatment, and experienced intracranial progression after involuntary drug reduction for 1 mo. The patient subsequently received various systemic treatments including vemurafenib, PD-1 inhibitor, and chemotherapy, with an overall survival of 29 mo as of September 2020.
We report the first case of melanoma brain metastases with co-occurring intracranial infection and unintended drug reduction during the COVID-19 outbreak. Long-term control of the intracranial lesions was achieved with systemic and local therapies.
Core Tip: We report a melanoma patient with brain metastases who had long-term control of intracranial lesions with the combination of local therapy and BRAF/MEK inhibitor. During the treatment course, the patient experienced intracranial infection and unwanted drug reduction during the coronavirus disease 2019 outbreak.
- Citation: Wang Y, Lian B, Cui CL. Long-term control of melanoma brain metastases with co-occurring intracranial infection and involuntary drug reduction during COVID-19 pandemic: A case report. World J Clin Cases 2021; 9(10): 2373-2379
- URL: https://www.wjgnet.com/2307-8960/full/v9/i10/2373.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i10.2373
Melanoma is a rare, aggressive tumor and the brain is a common metastatic site. Melanoma brain metastasis is associated with a poor prognosis, with a median overall survival (OS) of 3-5 mo[1,2]. In patients harboring BRAF mutations, BRAF and MEK inhibitors significantly increase the intracranial control rate and OS for brain metastases[3]. However, compared to extracranial lesions, the duration of response is short; progression of intracranial lesions is the main reason for treatment failure[4]. Here we report a case of melanoma brain metastases in a patient harboring a BRAF V600E mutation; although the patient experienced unexpected intracranial infection and dose reduction, long-term control of intracranial metastases was achieved with a combination of BRAF/MEK inhibitor and local therapies.
A 46-year-old Asian woman presented with a fever and headache.
The patient had a seizure and the convulsion localized to the right limbs. Magnetic resonance imaging (MRI) of the brain revealed lesions in the left frontal and temporal regions. Surgical removal of the suspected brain metastases was performed on May 20, 2019, but the postoperative pathologic assessment showed only necrotic tissue without tumor cells. On postoperative day 7, the patient presented with a fever and headache.
The patient was diagnosed with acral melanoma with a Breslow depth of 10 mm in 2016 (Figure 1). Metastasis to inguinal lymph nodes was suspected based on Positron emission tomography/computed tomography examination. The patient later underwent extended resection of the primary lesion and inguinal lymph node dissection, with one nodal metastasis in six dissected lymph nodes. Genetic testing revealed the presence of the BRAF V600E mutation. Her initial pathologic stage was pT4bN1bM0 (American Joint Committee on Cancer/Union for International Cancer Control, 8th Edition).
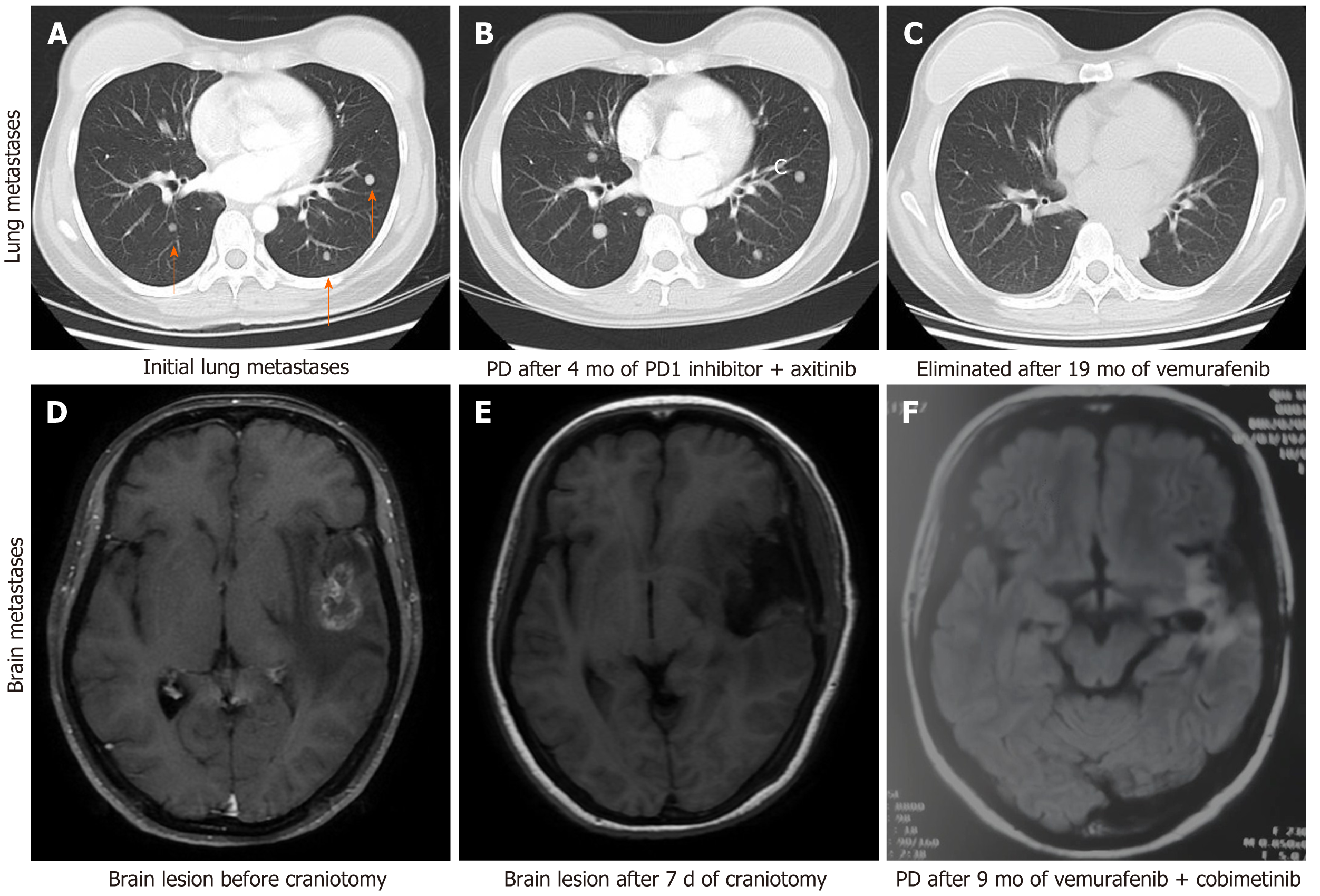
The patient received adjuvant high-dose interferon therapy and during a comprehensive review 3 mo later, pulmonary metastasis was detected. She was started on toripalimab [a programmed death (PD)-1 inhibitor that has been approved for the treatment of melanoma in China] combined with axitinib [an oral inhibitor of vascular endothelial growth factor (VEGF) receptors 1, 2, and 3] and had a progression-free survival (PFS) of 4 mo, at which point she experienced pulmonary progression. The treatment was switched to vemurafenib and after 9 mo, brain MRI revealed left frontal lobe metastasis. The patient underwent stereotactic radiosurgery (SRS) for the metastasis (24 Gy in 3 fractions) and continued on vemurafenib. Thereafter, she was examined every 6 wk for 10 mo.
Physical examination revealed signs of meningeal irritation. Vital signs were stable.
A lumbar puncture was performed and the cerebrospinal fluid (CSF) had a white blood cell count of 842/μL, with an elevated lactate level (2.5 mmol/L) and reduced sugar level (2.4 mmol/L).
Brain MRI demonstrated left frontotemporal alterations following craniotomy (Figure 2).
Intracranial infection.
The patient was treated with meropenem. In the re-examination 3 d later, the CSF test results were normal. A week later, the patient discontinued the antibiotic and was discharged. A combined treatment regimen of vemurafenib + cobimetinib was initiated on the 20th postoperative day.
The patient continued the treatment of vemurafenib + cobimetinib with monthly follow-up. During the coronavirus disease 2019 (COVID-19) pandemic period in February 2020, the patient was unable to visit specialized hospitals that were not in her city of residence to receive vemurafenib treatment because of travel restrictions, and she self-administered a reduced dose of vemurafenib (from 960 to 480 mg, BID) for 1 mo. In March 2020, the patient was re-examined by brain MRI and a new intracranial metastatic lesion was detected. The patient again underwent SRS with sequential vemurafenib, cobimetinib, and pembrolizumab treatments. After one cycle of combined therapy, imaging examination showed the progression of pulmonary metastases; the patient also presented with thrombocytopenia. The treatment was switched to chemotherapy with temozolomide + cisplatin + bevacizumab. As of September 2020, the patient had completed five cycles of combined chemotherapy and had stable disease.
Melanoma is highly malignant and often has a poor prognosis. In recent years, advances in immunotherapy and targeted therapy have significantly improved the survival rate[3,5-7]. For patients harboring BRAF mutations, the combination of BRAF and MEK inhibitors yields a high response rate with a median survival of 1 year[5]; immunotherapies such as PD-1, programmed death ligand (PD-L) 1, or cytotoxic T lymphocyte antigen (CTLA) 4 inhibitors have a lower initial response rate but longer response duration. Although in preclinical models BRAF and MEK inhibitors enhanced the antitumor efficacy of immunotherapy[8,9], clinical studies[10,11] have shown that combining BRAF and MEK inhibitors with PD-1 or PD-L1 inhibitor was associated with a higher risk of grade 3/4 treatment-related adverse events necessitating dose reduction or treatment discontinuation in a large number of cases. The optimal treatment regimen for patients with advanced melanoma with BRAF mutations has yet to be established.
Our patient preferred the PD-1 inhibitor toripalimab combined with the VEGF inhibitor axitinib as the initial systemic treatment. Axitinib combined with PD-1 blockade has shown promising antitumor activity in patients with metastatic mucosal melanoma, with a median PFS of 7.5 mo[12]. PFS in our patient was only 4 mo on this treatment, indicating that it was not very effective. Although the patient experienced intracranial progression several times after switching to BRAF inhibitor and the combination of BRAF and MEK inhibitors, on the latter regimen the disease has been controlled for nearly 30 mo until treatment failure occurred when the patient undertook dose reduction on her own.
This case also illustrates that the combination of local therapy and BRAF/MEK inhibitor offers a survival benefit for melanoma patients with brain metastasis. It was previously reported that SRS combined with BRAF/MEK inhibitor treatment had a 1-year local intracranial control rate of 72%[13], and concurrent or post-SRS BRAF/MEK inhibitors increased intracranial tumor control and improved OS in patients[14]. SRS may affect blood–brain barrier permeability and increase the intracranial delivery of BRAF/MEK inhibitors[15]. The strategy of combining local and BRAF/MEK inhibitor therapies warrants more detailed investigation in order to determine the optimal modality and sequence of local and BRAF/MEK inhibitor therapies, along with the associated risks.
Our patient had an intracranial infection after craniotomy for tumor resection. Intracranial infection is among the most common perioperative complications of craniotomy, with a reported incidence of 1.4%-9.5%[16-19] and high rates of long-term complications and mortality. There are relatively few reports on intracranial infections after brain metastasis resection, which has an estimated incidence of 4%[20]. To our knowledge, secondary intracranial infection after resection of melanoma brain metastasis has not been previously reported. Longer operation time, external drainage, and contamination of surgical wounds increase the risk of post-craniotomy intracranial infection[20-22]. None of these risk factors were present in our case, except for a long operation time (4 h). The infection was quickly controlled after antibiotic treatment, allowing the systematic antitumor treatment to proceed. Our experience with this case also demonstrates that when selecting the local treatment modality for patients with melanoma brain metastasis, severe complications such as intracranial infection should be considered.
Antitumor treatments can be lifesaving and improve patients’ prognosis. However, in the context of COVID-19, physicians have become more cautious when administering antitumor therapy. At the same time, because of travel restrictions and lockdown, many patients from small cities or the countryside are unable to visit cancer specialists in major cities for treatment. In this type of public emergency situation, diagnosis and treatment as well as drug distribution via the internet are an option. In fact, since the COVID-19 pandemic, our hospital and many others in China and worldwide have established efficient telemedicine and remote counseling systems[23-25] for the convenience of patients to diminish the possibility of adverse events or disease progression as a result of involuntary dose reduction or treatment discontinuation.
Melanoma brain metastasis is a major challenge in the treatment of melanoma. Intracranial infection after craniotomy for resection of melanoma brain metastasis is a very rare event and has not been specifically reported in the literature. Based on our case, patients with melanoma brain metastases can achieve long-term control of intracranial lesions with a combination of BRAF/MEK inhibitors. Our experience also highlights the importance of considering severe complications of local therapy and establishing internet-based diagnosis and treatment procedures.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sarti D S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Zhang YL
| 1. | Fife KM, Colman MH, Stevens GN, Firth IC, Moon D, Shannon KF, Harman R, Petersen-Schaefer K, Zacest AC, Besser M, Milton GW, McCarthy WH, Thompson JF. Determinants of outcome in melanoma patients with cerebral metastases. J Clin Oncol. 2004;22:1293-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 315] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 2. | Staudt M, Lasithiotakis K, Leiter U, Meier F, Eigentler T, Bamberg M, Tatagiba M, Brossart P, Garbe C. Determinants of survival in patients with brain metastases from cutaneous melanoma. Br J Cancer. 2010;102:1213-1218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 3. | Davies MA, Saiag P, Robert C, Grob JJ, Flaherty KT, Arance A, Chiarion-Sileni V, Thomas L, Lesimple T, Mortier L, Moschos SJ, Hogg D, Márquez-Rodas I, Del Vecchio M, Lebbé C, Meyer N, Zhang Y, Huang Y, Mookerjee B, Long GV. Dabrafenib plus trametinib in patients with BRAFV600-mutant melanoma brain metastases (COMBI-MB): a multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol. 2017;18:863-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 554] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 4. | Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, Garbe C, Jouary T, Hauschild A, Grob JJ, Chiarion-Sileni V, Lebbe C, Mandalà M, Millward M, Arance A, Bondarenko I, Haanen JB, Hansson J, Utikal J, Ferraresi V, Kovalenko N, Mohr P, Probachai V, Schadendorf D, Nathan P, Robert C, Ribas A, DeMarini DJ, Irani JG, Swann S, Legos JJ, Jin F, Mookerjee B, Flaherty K. Dabrafenib and trametinib vs dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet. 2015;386:444-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 977] [Cited by in RCA: 1037] [Article Influence: 103.7] [Reference Citation Analysis (0)] |
| 5. | Long GV, Flaherty KT, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, Garbe C, Jouary T, Hauschild A, Chiarion-Sileni V, Lebbe C, Mandalá M, Millward M, Arance A, Bondarenko I, Haanen JBAG, Hansson J, Utikal J, Ferraresi V, Mohr P, Probachai V, Schadendorf D, Nathan P, Robert C, Ribas A, Davies MA, Lane SR, Legos JJ, Mookerjee B, Grob JJ. Dabrafenib plus trametinib vs dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol. 2019;30:1848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Robert C, Ribas A, Schachter J, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil CM, Lotem M, Larkin JMG, Lorigan P, Neyns B, Blank CU, Petrella TM, Hamid O, Su SC, Krepler C, Ibrahim N, Long GV. Pembrolizumab vs ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019;20:1239-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 837] [Article Influence: 139.5] [Reference Citation Analysis (0)] |
| 7. | Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, Cowey CL, Schadendorf D, Wagstaff J, Dummer R, Ferrucci PF, Smylie M, Hogg D, Hill A, Márquez-Rodas I, Haanen J, Guidoboni M, Maio M, Schöffski P, Carlino MS, Lebbé C, McArthur G, Ascierto PA, Daniels GA, Long GV, Bastholt L, Rizzo JI, Balogh A, Moshyk A, Hodi FS, Wolchok JD. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2019;381:1535-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1826] [Cited by in RCA: 2640] [Article Influence: 440.0] [Reference Citation Analysis (0)] |
| 8. | Homet Moreno B, Mok S, Comin-Anduix B, Hu-Lieskovan S, Ribas A. Combined treatment with dabrafenib and trametinib with immune-stimulating antibodies for BRAF mutant melanoma. Oncoimmunology. 2016;5:e1052212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 9. | Hu-Lieskovan S, Mok S, Homet Moreno B, Tsoi J, Robert L, Goedert L, Pinheiro EM, Koya RC, Graeber TG, Comin-Anduix B, Ribas A. Improved antitumor activity of immunotherapy with BRAF and MEK inhibitors in BRAF(V600E) melanoma. Sci Transl Med. 2015;7:279ra41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 452] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 10. | Ribas A, Lawrence D, Atkinson V, Agarwal S, Miller WH Jr, Carlino MS, Fisher R, Long GV, Hodi FS, Tsoi J, Grasso CS, Mookerjee B, Zhao Q, Ghori R, Moreno BH, Ibrahim N, Hamid O. Combined BRAF and MEK inhibition with PD-1 blockade immunotherapy in BRAF-mutant melanoma. Nat Med. 2019;25:936-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 249] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 11. | Gutzmer R, Stroyakovskiy D, Gogas H, Robert C, Lewis K, Protsenko S, Pereira RP, Eigentler T, Rutkowski P, Demidov L, Manikhas GM, Yan Y, Huang KC, Uyei A, McNally V, McArthur GA, Ascierto PA. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAFV600 mutation-positive melanoma (IMspire150): primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020;395:1835-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 462] [Article Influence: 92.4] [Reference Citation Analysis (0)] |
| 12. | Sheng X, Yan X, Chi Z, Si L, Cui C, Tang B, Li S, Mao L, Lian B, Wang X, Bai X, Zhou L, Kong Y, Dai J, Wang K, Tang X, Zhou H, Wu H, Feng H, Yao S, Flaherty KT, Guo J. Axitinib in Combination With Toripalimab, a Humanized Immunoglobulin G4 Monoclonal Antibody Against Programmed Cell Death-1, in Patients With Metastatic Mucosal Melanoma: An Open-Label Phase IB Trial. J Clin Oncol. 2019;37:2987-2999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 130] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 13. | Acharya S, Mahmood M, Mullen D, Yang D, Tsien CI, Huang J, Perkins SM, Rich K, Chicoine M, Leuthardt E, Dowling J, Dunn G, Keller J, Robinson CG, Abraham C. Distant intracranial failure in melanoma brain metastases treated with stereotactic radiosurgery in the era of immunotherapy and targeted agents. Adv Radiat Oncol. 2017;2:572-580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 14. | Ahmed KA, Abuodeh YA, Echevarria MI, Arrington JA, Stallworth DG, Hogue C, Naghavi AO, Kim S, Kim Y, Patel BG, Sarangkasiri S, Johnstone PA, Sahebjam S, Khushalani NI, Forsyth PA, Harrison LB, Yu M, Etame AB, Caudell JJ. Clinical outcomes of melanoma brain metastases treated with stereotactic radiosurgery and anti-PD-1 therapy, anti-CTLA-4 therapy, BRAF/MEK inhibitors, BRAF inhibitor, or conventional chemotherapy. Ann Oncol. 2016;27:2288-2294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 110] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 15. | Mastorakos P, Xu Z, Yu J, Hess J, Qian J, Chatrath A, Taylor DG, Kondziolka D, Warnick R, Chiang V, Sheehan J. BRAF V600 Mutation and BRAF Kinase Inhibitors in Conjunction With Stereotactic Radiosurgery for Intracranial Melanoma Metastases: A Multicenter Retrospective Study. Neurosurgery. 2019;84:868-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Wang LY, Cao XH, Shi LK, Ma ZZ, Wang Y, Liu Y. Risk factors for intracranial infection after craniotomy: A case-control study. Brain Behav. 2020;10:e01658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 17. | McClelland S 3rd. Postoperative intracranial neurosurgery infection rates in North America vs Europe: a systematic analysis. Am J Infect Control. 2008;36:570-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Kourbeti IS, Vakis AF, Ziakas P, Karabetsos D, Potolidis E, Christou S, Samonis G. Infections in patients undergoing craniotomy: risk factors associated with post-craniotomy meningitis. J Neurosurg. 2015;122:1113-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 106] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 19. | Davies BM, Jones A, Patel HC. Implementation of a care bundle and evaluation of risk factors for surgical site infection in cranial neurosurgery. Clin Neurol Neurosurg. 2016;144:121-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Shi ZH, Xu M, Wang YZ, Luo XY, Chen GQ, Wang X, Wang T, Tang MZ, Zhou JX. Post-craniotomy intracranial infection in patients with brain tumors: a retrospective analysis of 5723 consecutive patients. Br J Neurosurg. 2017;31:5-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 21. | Elward A, Yegge J, Recktenwald A, Jadwisiak L, Kieffer P, Hohrein M, Hopkins-Broyles D, Woeltje KF. Risk Factors for Craniotomy or Spinal Fusion Surgical Site Infection. Pediatr Infect Dis J. 2015;34:1323-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Inoue T, Shimizu H, Fujimura M, Sato K, Endo H, Niizuma K, Sakata H, Tominaga T. Risk factors for meningitis after craniotomy in patients with subarachnoid hemorrhage due to anterior circulation aneurysms rupture. Clin Neurol Neurosurg. 2015;139:302-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Gambardella C, Pagliuca R, Pomilla G, Gambardella A. COVID-19 risk contagion: Organization and procedures in a South Italy geriatric oncology ward. J Geriatr Oncol. 2020;11:1187-1188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Li YX, He CZ, Liu YC, Zhao PY, Xu XL, Wang YF, Xia SY, Du XH. The impact of COVID-19 on gastric cancer surgery: a single-center retrospective study. BMC Surg. 2020;20:222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Tolone S, Gambardella C, Brusciano L, Del Genio G, Lucido FS, Docimo L. Telephonic triage before surgical ward admission and telemedicine during COVID-19 outbreak in Italy. Effective and easy procedures to reduce in-hospital positivity. Int J Surg. 2020;78:123-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |