Published online Apr 6, 2021. doi: 10.12998/wjcc.v9.i10.2289
Peer-review started: September 19, 2020
First decision: January 7, 2021
Revised: January 19, 2021
Accepted: February 1, 2021
Article in press: February 1, 2021
Published online: April 6, 2021
Processing time: 192 Days and 2.6 Hours
Familial hemophagocytic lymphohistiocytosis (FHL) is a primary immunodefici-ency disease caused by gene defects. The onset of FHL in adolescents and adults may lead clinicians to ignore or even misdiagnose the disease. To the best of our knowledge, this is the first report to detail the clinical features of type 2 FHL (FHL2) with compound heterozygous perforin (PRF1) defects involving the c.163C>T mutation, in addition to correlation analysis and a literature review.
We report a case of a 27-year-old male patient with FHL2, who was admitted with a persistent fever and pancytopenia. Through next-generation sequencing technology of hemophagocytic lymphohistiocytosis (HLH)-related genes, we found compound heterozygous mutations of PRF1: c.65delC (p.Pro22Argfs*29) (frameshift mutation, paternal) and c.163C>T (p.Arg55Cys) (missense mutation, maternal). Although he did not receive hematopoietic stem cell transplantation, the patient achieved complete remission after receiving HLH-2004 treatment protocol. To date, the patient has stopped taking drugs for 15 mo, is in a stable condition, and is under follow-up observation.
The delayed onset of FHL2 may be related to the PRF1 mutation type, pathogenic variation pattern, triggering factors, and the temperature sensitivity of some PRF1 mutations. For individual, the detailed reason for the delay in the onset of FHL warrants further investigation.
Core Tip: In familial hemophagocytic lymphohistiocytosis (FHL), the perforin (PRF1): c.163C>T mutation is rare and the clinical features have not been reported. We discuss a case of adult onset type 2 FHL with PRF1 c.65delC/c.163C>T compound heterozygous mutations, and conduct a predictive analysis of the effects of the two mutations on PRF1 function and disease onset.
- Citation: Liu XY, Nie YB, Chen XJ, Gao XH, Zhai LJ, Min FL. Adult onset type 2 familial hemophagocytic lymphohistiocytosis with PRF1 c.65delC/c.163C>T compound heterozygous mutations: A case report. World J Clin Cases 2021; 9(10): 2289-2295
- URL: https://www.wjgnet.com/2307-8960/full/v9/i10/2289.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i10.2289
Hemophagocytic lymphohistiocytosis (HLH) can be divided into primary (familial) form and secondary form. Familial HLH (FHL) is a rare autosomal recessive genetic disease, which is more common in children under 2-year-old, with a high fatality rate. Secondary HLH is more common in adolescents and adults, and can be induced by some specific factors. In recent years, there have been some reports of late-onset FHL in adolescents or adults[1-3], so age is no longer the restriction for FHL diagnosis. To date, five subtypes of FHL (FHL1-5) and their pathogenic genes have been reported: Perforin (PRF1, FHL2), unc-13 homolog D (UNC13D, FHL3), syntaxin 11 (STX11, FHL4), and syntaxin-binding protein 2 (STXBP2, FHL5). FHL1 is caused by an unidentified gene defect on chromosome 9[4]. FHL2 is a genetic disease related to PRF1 deficiency. PRF1 is located on chromosome 10 q21-22 and contains three exons. Exons 2 and 3 encode 555 amino acid residues to form PRF1. Human PRF1 is composed of three major domains: The membrane attack complex PRF1-like/cholesterol-dependent cytolysin (MACPF/CDC) domain, the epidermal growth factor domain, and the C terminus domain[5]. When human congenital defects lead to impaired PRF1 synthesis or function, or PRF1 release disorders, activated cytotoxic T cells (CTLs) cannot clear antigen presentation targets, resulting in the uncontrolled expansion of T cells and macrophage system in the body, which eventually leads to a storm of cytokines in the blood-the onset of FHL2[6].
In this study, we report the case of a Chinese male patient with a typical clinical FHL2 phenotype carrying compound heterozygous PRF1 defects involving a PRF1 mutation, c.163C>T.
A 27-year-old Chinese man was admitted to our institution after half a month, with persistent fever and a cough without sputum with no obvious inducement.
His temperature fluctuated to about 39.5 °C. He had no chest tightness, no shortness of breath, and no headache.
His past medical history was unremarkable.
No specific personal history of disease was recorded.
His vital signs were as follows: Temperature, 39.3 °C; heart rate, 90 beats per min; respiratory rate, 16 breaths per min; blood pressure, 121/60 mmHg; and oxygen saturation in room air, 97%. His mental state was apathetic. He presented with petechiae and ecchymoses on the skin of neck and limbs, along with conjunctival hemorrhage of the right eyeball. Spleen was 1.0 cm below the left costal margin and the liver was not palpable. Superficial lymph nodes were not affected, heart and lung auscultation were normal, and neither lower limb had edema. No obvious abnormalities were found during nervous system examination.
Laboratory examinations revealed pancytopenia (leukocytes 0.46 × 109/L, hemoglobin 54.0 g/L, and platelets 5 × 109/L), hypertriglyceridemia (2.40 mmol/L, reference interval [RI]: 0.23-1.70 mmol/L), hypofibrinogenemia (1.290 g/L, RI: 2.0-4.5 g/L), hyperferritinemia (> 1500 μg/L, RI: 21.81-274.66 μg/L), soluble cluster of differentiation 25 (CD25) (26095.13 pg/mL, RI: 410-2623 pg/mL), total protein (61.3 g/L, RI: 65-85 g/L), albumin (33.9 g/L, RI: 40-55 g/L), aspartate aminotransferase (27.5 U/L, RI: 10-40 U/L), alanine aminotransferase (29.0 U/L, RI: 9-50 U/L), and lactate dehydrogenase (291.1 U/L, RI: 109-245 U/L). Blood tumor biomarkers, autoimmune antibody, and blood culture were all within normal limits. No obvious virus infection (include Epstein-Barr virus [EBV] and cytomegalovirus) was detected.
The plain computed tomography (CT) scan and contrast enhancement of his chest and abdomen showed evidence of a small amount of pleural effusion on both sides; splenomegaly, about 13.6 cm × 6.7 cm; slightly low density shadow of liver and spleen; and enlarged lymph nodes in the hilar region and abdominal aorta, of which the larger one in the hilar region measured about 1.8 cm × 1.4 cm.
Bone marrow smear displayed hemophagocytosis with evidence of 2.5% phagocytic cells in the whole slide. Bone marrow biopsy showed obviously active bone marrow hyperplasia, erythroblastic clusters, few mature granulocytes, and atypical small clusters of lymphocyte-like cells. Further immunohistochemistry showed myeloperoxidase (-), PG-M1 (-), CD20 (-), CD3 (+), paired box 5 (-), CD56 (-), S100 (-), T-cell intracellular antigen 1 (+), Ki-67 (50%), and CD30 (-). Bone marrow gene rearrangement: T-cell receptor beta (TCRβ), TCRγ (-). Bone marrow flow cytometry: Natural killer (NK) cells showed no abnormal phenotype, B lymphocytes showed no clonal abnormalities, T lymphocytes accounted for 54.66% of lymphocytes; the value of CD3+CD4+/CD3+CD8+ decreased, and CD5 and CD7 expression decreased. Chromosome karyotype analysis: 46, XY (20). Labial gland biopsy: (Lower lip) revealed a small number of lymphocytes and plasma cells between the labial acini and one lymphocyte nest with more than 50 lymphocytes.
After admission, the patient was treated with cefepime and imipenem successively, but the curative effect was not satisfactory and he still had a fever, with the highest temperature at 40.2℃, accompanied by persistent pancytopenia. He then underwent splenectomy. Postoperative pathology showed the presence of spleen tissue structure, small focal extramedullary hematopoietic cells and no obvious tumor. During the operation, the surrounding mesenteric root lymph nodes were removed, and the pathology showed lymphoid tissue hyperplasia.
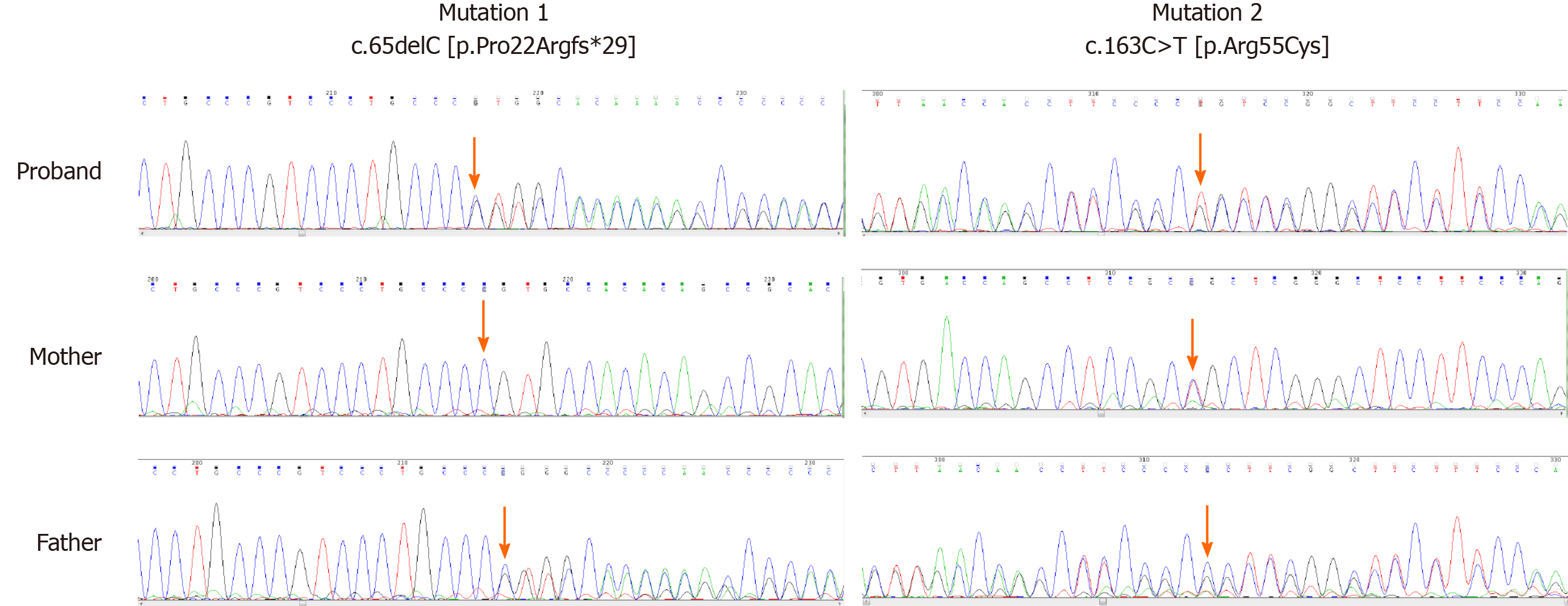
According to the HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis[7], the patient was diagnosed with HLH. To further clarify the etiology of HLH, the next-generation sequencing technology (NGS) of HLH-related genes (Table 1) was performed in the peripheral blood of our patient. We found two mutations of human PRF1: c.65delC (p.Pro22Argfs*29) (frameshift mutation) and c.163C>T (p.Arg55Cys) (missense mutation) (Table 2). The expression of PRF1 and granzyme in the NK cells of our patient and his parents was measured by flow cytometry. The results are shown in Table 3.
| HLH-related genes | ||||
| AP3B1 | BLOC1S6 | CD27 | IL2RG | ITK |
| LYST | MAGT1 | PRF1 | RAB27A | SH2D1A |
| STX11 | STXBP2 | TCN2 | UNC13D | XIAP |
| Gene | Chromosome position | Transcript | Mutation position | Nucleotide change | Amino acid change | dbSNP | Homozygous/ Heterozygous | Disease/ Phenotype | Genetic mode | Source |
| PRF1 | chr10:72360593 | NM_001083116 | Exon2 | c.65delC | p.P22Rfs*29 | - | Heterozygous | FHL2 | AR | Father |
| PRF1 | chr10:72360496 | NM_001083116 | Exon2 | c.163C>T | p.R55C | rs201032696 | Heterozygous | FHL2 | AR | Mother |
| Patient | Father | Mother | |
| Perforin positive rate of NK cells | 80.44% | 95.89% | 94.61% |
| Granzyme positive rate of NK cells | 90.49% | 75.45% | 84.07% |
According to the latest 2017 version of the American College of Medical Genetics and Genomics guidelines[8-9], it is inferred that the c.65delC mutation is a pathogenic mutation (PVS1 + PM1 + PM2 + PP4), while the c.163C>T mutation is a likely pathogenic mutation (PM1 + PM2 + PM3 + PP3 + PP4). The specific bases are as follows: The two mutations are all located in the MACPF/CDC domain (PM1), and based on the results of flow cytometry analysis and the clinical manifestations of our patient, the patient’s phenotype is highly consistent with clinical features of FHL2 (PP4). The ExAC03 Database records that the proportion of these mutations in the population is 0.0001 while in 1000 Genomes Database is 0.000199681 (c.163C>T) (PM2). Meanwhile, the c.65delC mutation is a frameshift mutation, which can cause a truncation mutation and lead to a change in the downstream coding region (PVS1). According to the pathogenicity of c.65delC and his parents' Sanger Sequencing (Figure 1), the c.163C>T mutation is in trans (PM3) and is predicted by SIFT, PolyPhen-2 and MutationTaster Database that the mutation has a great effect on the protein structure and may affect the protein function (PP3).
The final diagnosis was FHL2.
The patient was treated according to the HLH-2004 treatment protocol[7].
After 8 wk of initial chemotherapy of HLH-2004 treatment protocol, the signs, symptoms and auxiliary examination results of the patient were close to normal. Because there was no suitable donor, the hematopoietic stem cell transplantation could not be completed, and the patient continued to receive maintenance chemotherapy for 32 wk and achieved complete remission. To date, the patient has stopped taking drugs for 15 mo, and his condition is stable and under follow-up observation.
In this patient, the two mutations of PRF1 are both located in the MACPF/CDC domain and recorded in 460 single-nucleotide variants of PRF1 reported by Willenbring et al[5]. The c.65delC mutation has been reported in both China and South Korea[1,2,10]. Except for only one case of FHL2 caused by single allele heterozygous mutation[1], all others were caused by a compound heterozygous mutation. Among them, Korean cases mainly occur in infancy and Chinese cases are reported in both children and adults, which suggests that the c.65delC mutation might be ethnically and geographically specific and has no direct correlation with the onset time of FHL2. In the Clinvar database, there is only one record of PRF1: c.163C>T mutation in FHL2, and the specific clinical features have not been described in detail.
According to our literature review, the delayed onset of FHL2 may be related to the mutation type[11-13], triggering factors[14-15], and pathogenic variation pattern of PRF1[16-18]. Additionally, the temperature sensitivity of some PRF1 mutations can also explain this[19].
There are mainly the following forms of PRF1 mutations: Missense mutations, frameshift mutations, nonsense mutations and inframe insertion/deletion mutations, among which the first three are more common[11]. Through sequencing HLH-related genes, Miao et al[12] found that most of the variants are nonsense mutations and frameshift mutations in pediatric HLH cases, which is different from that in adult cases, where missense mutations are much more prevalent and suggest that most gene mutations in adult HLH may partially damage rather than completely eliminate the function of the affected proteins. Trizzino et al[11] found that the expression of PRF1 in NK cells decreases in almost all FHL2 with biallelic mutations of PRF1, and the onset time of FHL2 might depend on the effect of different types of PRF1 mutations on protein function. The onset age of patients with two destructive mutations (frameshift mutation or nonsense mutation) is significantly earlier than that of patients with a destructive mutation and a missense mutation. Using whole-exome sequencing (WES), Feng et al[13] advanced that FHL2 patients with compound heterozygous mutations of PRF1 have delayed onset; a possible explanation for this phenomenon is that these patients carry a heterozygous missense mutation, which encodes part of the PRF1 activity. Ueda et al[20] reported an 11-year-old late-onset FHL2 with compound heterozygous missense mutations of PRF1. Two siblings of the patient died in early infancy because of fatal FHL, although they carried the same pathogenic gene mutations.
It is generally believed that there is always a triggering factor in the onset of HLH. The most common triggering factors are infections and malignancies[14]. While in FHL, the most prevalent trigger is the viral infection that cannot be eliminated by genetically defective CTLs. In terms of triggering factors, EBV or other herpesviruses are the most frequently reported, there are also some findings about arthritis driven inflammation, macrophage activation syndrome and leishmaniasis[15]. Our patient has a compound heterozygous state of frameshift mutation and missense mutation and developed FHL2 at the age of 27. The above inducing factors have been excluded. Combined with the literature about c.65delC mutations, it is found that FHL2 with this kind of mutation occurs in both infants and adults[1,2,10]. We propose that the mutation type of the pathogenic gene is not a single determinant of the age of onset.
Gao et al[16] analyzed the potential "second-hit" mechanism of FHL2 by WES, suggesting that another gene mutation (homozygous missense mutation S1006L in PCDH18) destroyed the stability of the protein structure and cooperated with PRF1 mutation to lessen the PCDH18-induced inhibition of CTLs activation and reduce their apoptosis, which resulted in the "second-hit" of FHL2. By contrast, Zhang et al[17-18] found that the onset age of heterozygotes with variants in PRF1 and one gene involved in cytotoxic lymphocyte degranulation pathway (UNC13D (MUNC13-4), STX11, STXBP2, and RAB27A) was later than that of PRF1 single heterozygotes, and PRF1 homozygotes or compound heterozygotes. Heterozygous mutations of degranulation genes seemed to partially offset the adverse effects of PRF1 mutations and delay the onset time. These studies revealed that more potential FHL-related susceptibility genes and their pathogenic mechanism could be found through in-depth study with advanced technologies.
Flow cytometry analysis has confirmed that the decreased expression of PRF1 has a connection with PRF1 mutations[20]. Voskoboinik et al[6] found that the onset of FHL2 shows a bimodal phenomenon, patients carrying missense mutations with a complete loss of cytotoxicity generally develop FHL2 within 6 mo of birth, while disease tends to occur after 24 mo of age in patients with missense mutations retaining partial cytotoxicity. Chia et al[19] put forward that there is a significant relationship between the onset age of FHL2 and the degree to which cytotoxicity can be recovered at 30°C. PRF1 mutations with temperature sensitivity enable carriers to retain sufficient PRF1 activity, eventually preventing the early-onset of FHL2 in childhood. Gao et al[16] also suggested that the discrepancy in the onset age of FHL2 is related to the degree of loss of PRF1-dependent cytotoxicity caused by various PRF1 mutations. In our patient, the two allele mutations of PRF1 are frameshift mutation and missense mutation, and the expression of PRF1 in NK cells is slightly lower than normal, suggesting that the activity of NK cells is slightly lower. It is speculated that these PRF1 mutations may retain part of the cytotoxicity and delay the onset of the disease. However, there are also some cases in which NK cell activity is very low but clinical onset is late, and there are some cases in which NK cell activity is normal, but the patient develops FHL2 within 1 year after birth[1,11].
In this late-onset case of FHL2 with compound heterozygous PRF1 defects, the c.163C>T mutation is rare and there is no detailed description of this mutation till now. We conducted a predictive analysis of the two mutations’ effects on the function of PRF1 and onset of FHL2. However, the relationship between the changes of protein function caused by these mutations and the onset time of FHL needs to be verified by more advanced technology.
Manuscript source: Unsolicited manuscript
Specialty type: Hematology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Moschovi MA S-Editor: Zhang H L-Editor: Filipodia P-Editor: Li JH
| 1. | Wang Y, Wang Z, Zhang J, Wei Q, Tang R, Qi J, Li L, Ye L, Wang J, Ye L. Genetic features of late onset primary hemophagocytic lymphohistiocytosis in adolescence or adulthood. PLoS One. 2014;9:e107386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 2. | Chen X, Wang F, Zhang Y, Teng W, Wang M, Nie D, Zhou X, Wang D, Zhao H, Zhu P, Liu H. Genetic variant spectrum in 265 Chinese patients with hemophagocytic lymphohistiocytosis: Molecular analyses of PRF1, UNC13D, STX11, STXBP2, SH2D1A, and XIAP. Clin Genet. 2018;94:200-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 3. | Jin Z, Wang Y, Wang J, Zhang J, Wu L, Gao Z, Lai W, Wang Z. Primary hemophagocytic lymphohistiocytosis in adults: the utility of family surveys in a single-center study from China. Orphanet J Rare Dis. 2018;13:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Gholam C, Grigoriadou S, Gilmour KC, Gaspar HB. Familial haemophagocytic lymphohistiocytosis: advances in the genetic basis, diagnosis and management. Clin Exp Immunol. 2011;163:271-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 5. | Willenbring RC, Ikeda Y, Pease LR, Johnson AJ. Human perforin gene variation is geographically distributed. Mol Genet Genomic Med. 2018;6:44-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Voskoboinik I, Smyth MJ, Trapani JA. Perforin-mediated target-cell death and immune homeostasis. Nat Rev Immunol. 2006;6:940-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 450] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 7. | Henter JI, Horne A, Aricó M, Egeler RM, Filipovich AH, Imashuku S, Ladisch S, McClain K, Webb D, Winiarski J, Janka G. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3075] [Cited by in RCA: 3601] [Article Influence: 200.1] [Reference Citation Analysis (1)] |
| 8. | Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL; ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19696] [Cited by in RCA: 22544] [Article Influence: 2254.4] [Reference Citation Analysis (0)] |
| 9. | Abou Tayoun AN, Pesaran T, DiStefano MT, Oza A, Rehm HL, Biesecker LG, Harrison SM; ClinGen Sequence Variant Interpretation Working Group (ClinGen SVI). Recommendations for interpreting the loss of function PVS1 ACMG/AMP variant criterion. Hum Mutat. 2018;39:1517-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 566] [Article Influence: 80.9] [Reference Citation Analysis (0)] |
| 10. | Kim MS, Cho YU, Jang S, Seo EJ, Im HJ, Park CJ. Familial Hemophagocytic Lymphohistiocytosis Type 2 in a Korean Infant With Compound Heterozygous PRF1 Defects Involving a PRF1 Mutation, c.1091T>G. Ann Lab Med. 2017;37:162-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Trizzino A, zur Stadt U, Ueda I, Risma K, Janka G, Ishii E, Beutel K, Sumegi J, Cannella S, Pende D, Mian A, Henter JI, Griffiths G, Santoro A, Filipovich A, Aricò M; Histiocyte Society HLH Study group. Genotype-phenotype study of familial haemophagocytic lymphohistiocytosis due to perforin mutations. J Med Genet. 2008;45:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 12. | Miao Y, Zhu HY, Qiao C, Xia Y, Kong Y, Zou YX, Miao YQ, Chen X, Cao L, Wu W, Liang JH, Wu JZ, Wang L, Fan L, Xu W, Li JY. Pathogenic Gene Mutations or Variants Identified by Targeted Gene Sequencing in Adults With Hemophagocytic Lymphohistiocytosis. Front Immunol. 2019;10:395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | Feng WX, Yang XY, Li JW, Gong S, Wu Y, Zhang WH, Han TL, Zhuo XW, Ding CH, Fang F. Neurologic Manifestations as Initial Clinical Presentation of Familial Hemophagocytic Lymphohistiocytosis Type2 Due to PRF1 Mutation in Chinese Pediatric Patients. Front Genet. 2020;11:126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Birndt S, Schenk T, Heinevetter B, Brunkhorst FM, Maschmeyer G, Rothmann F, Weber T, Müller M, Panse J, Penack O, Schroers R, Braess J, Frickhofen N, Ehl S, Janka G, Lehmberg K, Pletz MW, Hochhaus A, Ernst T, La Rosée P. Hemophagocytic lymphohistiocytosis in adults: collaborative analysis of 137 cases of a nationwide German registry. J Cancer Res Clin Oncol. 2020;146:1065-1077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 15. | Cetica V, Sieni E, Pende D, Danesino C, De Fusco C, Locatelli F, Micalizzi C, Putti MC, Biondi A, Fagioli F, Moretta L, Griffiths GM, Luzzatto L, Aricò M. Genetic predisposition to hemophagocytic lymphohistiocytosis: Report on 500 patients from the Italian registry. J Allergy Clin Immunol 2016; 137: 188-196. e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 126] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 16. | Gao L, Dang X, Huang L, Zhu L, Fang M, Zhang J, Xu X, Zhu L, Li T, Zhao L, Wei J, Zhou J. Search for the potential "second-hit" mechanism underlying the onset of familial hemophagocytic lymphohistiocytosis type 2 by whole-exome sequencing analysis. Transl Res. 2016;170:26-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Zhang K, Jordan MB, Marsh RA, Johnson JA, Kissell D, Meller J, Villanueva J, Risma KA, Wei Q, Klein PS, Filipovich AH. Hypomorphic mutations in PRF1, MUNC13-4, and STXBP2 are associated with adult-onset familial HLH. Blood. 2011;118:5794-5798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 309] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 18. | Zhang K, Chandrakasan S, Chapman H, Valencia CA, Husami A, Kissell D, Johnson JA, Filipovich AH. Synergistic defects of different molecules in the cytotoxic pathway lead to clinical familial hemophagocytic lymphohistiocytosis. Blood. 2014;124:1331-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 19. | Chia J, Yeo KP, Whisstock JC, Dunstone MA, Trapani JA, Voskoboinik I. Temperature sensitivity of human perforin mutants unmasks subtotal loss of cytotoxicity, delayed FHL, and a predisposition to cancer. Proc Natl Acad Sci USA. 2009;106:9809-9814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 20. | Ueda I, Morimoto A, Inaba T, Yagi T, Hibi S, Sugimoto T, Sako M, Yanai F, Fukushima T, Nakayama M, Ishii E, Imashuku S. Characteristic perforin gene mutations of haemophagocytic lymphohistiocytosis patients in Japan. Br J Haematol. 2003;121:503-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 3.0] [Reference Citation Analysis (0)] |